Abstract
Purpose
Hospital-acquired Burkholderia cepacia (B. cepacia) infection are not commonly recorded in patients without underlying lung disease, such as cystic fibrosis and chronic granulomatous disease. However, in 2014, B. cepacia appeared more frequently in pediatric blood samples than in any other year. In order to access this situation, we analyzed the clinical characteristics of B. cepacia infections in pediatric patients at our hospital.
Materials and Methods
We conducted a retrospective study of blood isolates of B. cepacia taken at our hospital between January 2004 and December 2014. Patient clinical data were obtained by retrospective review of electronic medical records. We constructed a dendrogram for B. cepacia isolates from two children and five adult patients.
Results
A total of 14 pediatric patients and 69 adult patients were identified as having B. cepacia bacteremia. In 2014, higher rates of B. cepacia bacteremia were observed in children. Most of them required Intensive Care Unit (ICU) care (12/14). In eleven children, sputum cultures were examined, and five of these children had the same strain of B. cepacia that grew out from their blood samples. Antibiotics were administered based on antibiotic sensitivity results. Four children expired despite treatment. Compared to children, there were no demonstrative differences in adults, except for history of ICU care.
Conclusion
Although there were not many pediatric cases at our hospital, awareness of colonization through hospital-acquired infection and effective therapy for infection of B. cepacia is needed, as it can cause mortality and morbidity.
Keywords: Burkholderia cepacia, sepsis, hospital infection, child
INTRODUCTION
Knowledge of infection, hygiene, and nutrition has improved tremendously in comparison to the past, and antibiotics have developed remarkably. Nevertheless, hospital-acquired infections remain an issue, especially for patients in the Intensive Care Unit (ICU) and those under prolonged hospitalization. Pathogens resistant to antimicrobial agents are another emerging issue. According to the Centers for Disease Control and Prevention, gram negative bacteria accounted for the most urgent and serious microorganisms, such as carbapenem-resistant Enterobacteriaceae, Acinetobacter, and Pseudomonas aeruginosa.1,2,3
Burkholdria cepacia (B. cepacia) has emerged as another important bacterium cause of hospital-acquired infections. The bacterium is known formerly as Pseudomonas cepacia, a gram negative aerobic, glucose, non-fermenting, motile bacillus. Immunocompromised and hospitalized patients are especially susceptible to this infection, leading to severe bacteremia that results in death.4 Studies also report its growth in endocarditis among drug addicts, in patients with prosthetic cardiac valves, in eye infections following surgical procedures, and in infections of the central nervous system. Once colonized with a specific strain, replacement of the original strain with another is infrequent.5 B. cepacia can cause severe disease in humans with underlying disease, such as cystic fibrosis and chronic granulomatous disease (CGD).5,6 Fortunately, since cystic fibrosis and CGD are very rare in Korea, B. cepacia infection is not usually a great concern.
B. cepacia infection is not a common source of infection in adult and pediatric patients. However, in 2014, B. cepacia growth was confirmed more frequently in pediatric blood samples than in any other year. Therefore, to assess the possibility that B. cepacia has become another important source of nosocomial infection, we analyzed clinical characteristics of B. cepacia infection in pediatric patients in our hospital over the course of the past ten years.
MATERIALS AND METHODS
A retrospective study was performed in patients with blood isolates of B. cepacia from January 2004 to December 2014 in our hospital.
The BacT/ALERT 3D (bioMérieux, Inc., Durham, NC, USA) blood culture system was used. Isolates were identified using the VITEK 2 system with GP and GN cards. An antimicrobial susceptibility test (AST) was performed via a broth microdilution test using the VITEK 2 system with P625, N211, and N212 cards. AST interpretation was determined using Clinical and Laboratory Standard Institute (CLSI) guidelines; CLSI recommended testing with ceftazidime, minocycline, meropenem, and cotrimoxazole in 2010.7
Since not all samples were available, we constructed a dendrogram for B. cepacia from the sputum of three children and four adults only. The main spectrum profile (MSP) showing the highest score was selected from each isolate and was included to construct the dendrogram using the statistical toolbox in MATLAB 7.1 integrated in the matrix-assisted laser desorption/ionization (MALDI) Biotyper 2.0 software (Bruker Daltonics GmbH, Leipzig, Germany). Based on the principle that identification score reflects the agreement of the spectra with the standard Acinetobacter baumannii database entry, the MSP profile showing the highest score implies that the specific spectra represents the most typical aspects of a certain strain from the database. This selection of the highest score marking spectra was necessary, especially when highly similar strains were studied because several mass spectral features related to limited reproducibility of the method might eclipse mass spectral differences between the strains. Test strain clonality was determined with cut-off values at a distance of 250.8 Instead of pulsed-field gel electrophoresis or whole genome sequencing, the gold standard for confirming clonality, we used the MALDI TOF Mass Spectrometry method.9
Patient clinical data were obtained retrospectively from electronic medical records. This study was approved by the Institutional Review Board (IRB) in our hospital (IRB No. 4-2015-0540).
RESULTS
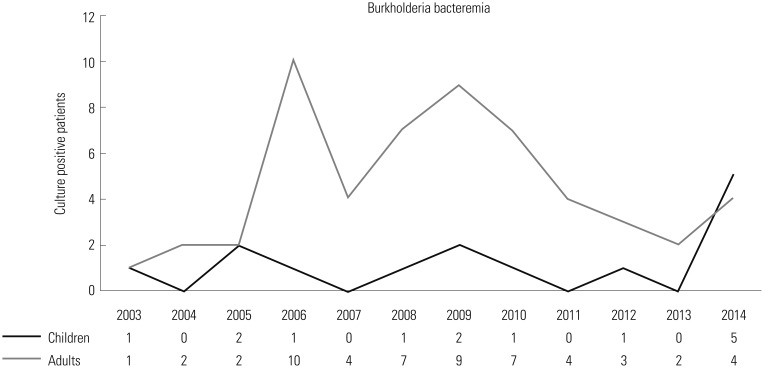
Over the 10-year period, a total of 14 pediatric patients and 55 adult patients were identified as having B. cepacia bacteremia (Table 1). In the years 2005, 2009 and especially in 2014, higher rates of B. cepacia bacteremia were observed in pediatric patients (Fig. 1). Although the increasing patterns in 2009, 2013, and 2014 were similar, there were few differences in annual incidence between adults and children. The overall incidence of B. cepacia bacteremia in pediatric patients was lower than that in adult patients.
Table 1. Clinical Characteristics of B. Cepacia Infection in Pediatric Patients and Adult Patients.
| Patient ID | Year isolated | Age | Sex | Underlying disease | Isolation site | ICU care | B. cepacia in the sputum culture | Outcome | Used antibiotics |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2003 | 0 | M | None | Blood | Yes | Yes | Treated | Imipenem |
| IV catheter tip | |||||||||
| 2 | 2005 | 0 | M | Neuroblastoma | Blood | Yes | No | Treated | Meropenem |
| IV catheter tip | |||||||||
| 3 | 2005 | 1 | F | Large VSD | Blood | Yes | Sputum culture not done | Treated | Ceftriaxone |
| 4 | 2006 | 1 | F | Core pulmonale, vegetation on aortic valve | Blood | Yes | Sputum culture not done | Treated | Ceftazidime |
| Meropenem | |||||||||
| Levofloxacin | |||||||||
| 5 | 2008 | 0 | M | Prematurity, panperitonitis | Blood | Yes | No | Treated | Cefepime |
| 6 | 2009 | 10 | F | Medulloblastoma | Blood | Yes | Sputum culture not done | Treated | Carbenin |
| Cefepime | |||||||||
| 7 | 2009 | 0 | M | Atresia of pulmonary artery | Blood | Yes | No | Expired | Meropenem |
| 8 | 2010 | 0 | F | Prematurity, cystic hygroma | Blood | Yes | Yes | Treated | Meropenem |
| Urine | |||||||||
| 9 | 2012 | 7 | F | Chronic granulomatous disease | Blood | Yes | Yes | Expired | Meropenem |
| 10 | 2014 | 6 | M | Medulloblastoma | Blood | Yes | No | Expired | Meropenem |
| Colistin | |||||||||
| 11 | 2014 | 0 | M | HLHS | Blood | Yes | Yes | Expired | Meropenem |
| IV catheter tip | Cotrimoxazole | ||||||||
| 12 | 2014 | 0 | F | Coarctation of aorta, DORV | Blood | Yes | Yes | Treated | Certazidime |
| Meropenem | |||||||||
| 13 | 2014 | 12 | M | ALL | Blood | No | No | Treated | Meropenem |
| 14 | 2014 | 6 | F | ATRT | Blood | No | No | Treated | Meropenem |
| Adult patients (55 cases) |
2003-2014 | 18-87 (62.1) |
M:F (1.72:1) |
Oncology patients: 40 cases | Blood: 55 cases | 26 cases | 5 cases | Treated: 35 cases | |
| Patients with cardiovascular | Catheter tip: 4 cases | ||||||||
| problems: 13 cases | Urine: 4 cases | ||||||||
| Simple surgery: 2 cases | Pleural fluid: 1 case |
ICU, Intensive Care Unit; B. cepacia, Burkholderia cepacia; VSD, ventricular septal defect; HLHS, hypoplastic left heart syndrome; ALL, acute lymphoblastic leukemia; ATRT, atypical teratoid rhabdoid tumor; IV, intravenous.
Fig. 1. Annual incidence of B. cepacia infection in our hospital. B. cepacia, Burkholderia cepacia.
There was no difference between genders in children. Most children had underlying disease except for one patient. Underlying diseases included cancer (4/14), congenital heart disease (4/14), immunodeficiency, such as CGD (1/14), and prematurity (2/14). One previously healthy pediatric patient who had pneumonia and a history of treatment in the ICU also had B. cepacia bacteremia. Three out of 14 pediatric patients had positive B. cepacia intravenous catheter tip cultures simultaneously with blood culture.
Among adults, more male than female patients had B. cepacia bacteremia (1.72:1). Almost all adult patients had underlying disease. They were mostly oncology patients (40/55) and patients with cardiovascular problems (13/55). Only two had a history of simple surgery.
Most of the pediatric patients had undergone ICU care (12/14). Twelve of the patients with ICU care were initially due to underlying disease or right after cardiac surgery, not due to B. cepacia infection. In adult patients, 47% underwent ICU care (26/29), fewer that for the children.
Eleven pediatric patients had their sputum cultures examined, of which five children had the same strain of B. cepacia grown from the blood samples; the other six children had other strains but not B. cepacia. Three did not get sputum cultures because there was no pneumonia. Among the adult patients, only five patients had the same strain of B. cepacia grown from the blood samples. Two patients had multidrug resistance who stayed in the oncologic general ward without history of ICU care. Though they are isolated in the same month, the sensitivity patterns of antibiotics were different, and thus, they were thought to be different pathogens. Antibiotics were used properly based on the antibiotics sensitivity results (Table 2). Only one patient was administered co-timoxazole for treatment, and 11 patients including the previously mentioned patients used meropenem. Four children expired despite treatment. Only two of the patients were thought to have expired by direct effect of B. cepacia bacteremia. Another one patient had multiple gram negative bacterial growth in their blood samples, and could not predicate B. cepacia as the direct cause of death. The remaining patient expired due to underlying cardiac disease after cardiac surgery. Among the adults, 20 patients expired, 13 due to the direct effect of B. cepacia.
Table 2. Microbial Sensitivity of B. Cepacia Isolated from 14 Patients.
| Patient ID | Ceftazidime | Levofloxacin | Meropenem | Minocycline | Tetracycline | Cotrimoxazole |
|---|---|---|---|---|---|---|
| 1 | S | R | S | Not done | R | I |
| 2 | S | R | S | Not done | R | I |
| 3 | S | S | S | Not done | R | S |
| 4 | I | I | R | Not done | R | S |
| 5 | S | S | S | Not done | S | S |
| 6 | S | S | S | S | Not done | S |
| 7 | I | I | S | I | Not done | S |
| 8 | S | I | S | S | Not done | R |
| 9 | S | S | S | S | Not done | S |
| 10 | S | S | S | S | Not done | S |
| 11 | S | S | S | S | Not done | S |
| 12 | I | S | S | S | Not done | S |
| 13 | R | I | R | R | Not done | S |
| 14 | I | I | R | R | Not done | R |
B. cepacia, Burkholderia cepacia; S, susceptible; I, intermediate; R, resistant.
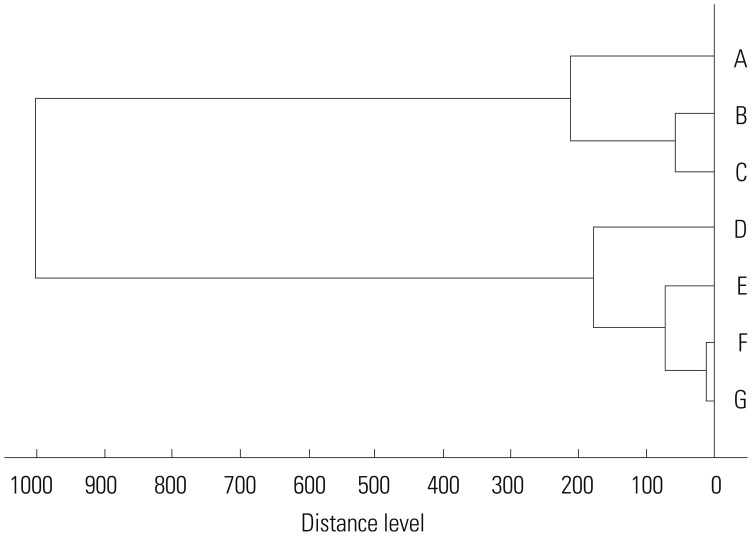
Since not all samples were available for study, we only gathered seven samples of B. cepacia from sputum, three from children and four from adults. Patients B, E, and F were children. Patients A, B, C, and G had a history of ICU care in March 2014, and patient D had a history of ICU care in August 2014. Patients E and F had a history of pediatric cardiovascular ICU (PCCU) care in March and August (Fig. 2). We were able to find two clonalities; those were each isolated in March and August in the ICU and PCCU.
Fig. 2. Dendrogram of B. cepacia isolated in seven patients. Patients B, E, and F are pediatric patients; the others are adult patients. Patients A, B, C, D, and G were from the ICU, and Patients E and F were from the pediatric cardiovascular ICU. B. cepacia, Burkholderia cepacia; ICU, Intensive Care Unit.
DISCUSSION
Our hospital data showed that B. cepacia infection was not common over the past ten years. The total number of cases with B. cepacia bacteremia was 69, and of these, about 20% were pediatric cases with low incidence. As in adult patients, pediatric patients continually showed B. cepacia infection in our hospital. We do not think B. cepacia infection is increasing in pediatric populations. However, in 2014, there were five cases of B. cepacia bacteremia in pediatric patients, which was unlike the rate in other years. We are unable to explain the reason for the increased incidence of B. cepacia in year 2014. However, physicians should be aware of this pathogen as a cause of the hospital infection in immunocompromised patients and in previously healthy pediatric patients with a history of ICU care.
According to other reports and studies, B. cepacia mainly causes problems in patients with cystic fibrosis or immunodeficient patients, such as CGD, rather than in immunocompetent patients. In our case, there was one patient with CGD and expired due to B. cepacia sepsis. It can cause mortality in over 90% of pediatric cystic fibrosis patients who have pulmonary damage, after colonization and being transferred to the blood stream. In the ICU setting and in treatment with catheters or similar devices, this infection can lead to morbidity and mortality. In our study, mortality cases were suspected to have expired due to infection with B. cepacia. Fortunately, in Korea, as cystic fibrosis and CGD are rare conditions, B. cepacia is not much of a concern to physicians. However, as ICU care and catheter or device treatment is increasing, the importance of B. cepacia infection is also increasing. In 14 cases of B. cepacia bacteremia, 13 cases had underlying disease, and 12 had a history of ICU care. This is consistent with other reports.10,11,12,13,14 Although immune status was not assessed during the study period, cardiac surgery, chemotherapy, and prematurity can cause immunosuppressive conditions, and this may lead to sepsis.
When comparing data for the children and the adults, there were no significant differences, except for ICU care. As many of the adult patients had signed do not resuscitate (DNR) documents, patients were not admitted to the ICU and maintained treatment in the general ward.
Five of the 11 cases with sputum culture results showed the same strain of B. cepacia grown from blood samples. This suggests that colonization with B. cepacia in the airway can cause bacteremia and lead to morbidity and mortality, although there is no exact evidence that B. cepacia in the airway is a pathogen or not. Nevertheless, there is no effective method for eradication of colonized B. cepacia. Therefore, hospital environment and health care hygiene are still the most important issues.
Therapeutic options for B. cepacia are unfortunately limited because many strains of this organism exhibit high levels of resistance to many antimicrobial agents in vitro, which may be intrinsic to certain cases. Studies report up to 50.4% resistance to every antibiotic tested, indicating that multidrug resistant isolates are not uncommon.5,15 In our study, B. cepacia isolated from five cases all reported sensitivity to antibiotic agents. Only one patient showed resistance to ceftazidime, three to meropenem, and two to co-trimoxazole.
The antimicrobial option most commonly used in B. cepacia infection is co-trimoxazole (trimethoprim/sulfamethoxazole). Co-trimoxazole is also the prophylactic drug of choice for CGD. There is some hurdle to the use of co-trimoxazole, since allergic or hypersensitivity reactions, intolerance, and resistance may be observed in patients receiving co-trimoxazole.15 Our CGD case was also using cefixime for prophylactic measure instead of co-trimoxazole due to a history of allergic reaction to co-trimoxazole.
In this study, many different antimicrobials were tested. Interestingly, patients who survived responded efficiently to the prescribed antibiotics. Even after removal of the catheter or device, we could not isolate B. cepacia from the blood of patients anymore, without changing antibiotics. This might suggest that B. cepacia infection in patients without underlying immunocompromised disease may be controlled well only with removal of contaminated catheters or devices. The prevailing thought has been that removal of a contaminated catheter or device should be essential for the control of B. cepacia infection, which is the principal treatment for catheter infections.
As B. cepacia bacteremia was increased in both adults and children, there was no obvious association between the two groups, though the trend seemed to be similar. According to the dendrogram results, we suspect that there may be a reservoir in our hospital. We do not know whether the pathogens isolated from children are from adult patients or contrary pathogens isolated from adults are form children. As we do not have a separate pediatric ICU and share the same space with adult patients, we can say that children can share the same hospital infection. Accordingly, this suggests the importance of sustainable infection control and hygiene.
The limitation of this study is that it was a retrospective study from a single center. The number of study subjects was also too small. The strength of this study is that we reviewed data for a ten-year period and B. cepacia bacteremia in pediatric patients. Since not much data exists regarding B. cepacia in Korea, especially in pediatric patients, more data should be collected and analyzed.
Although there were not many cases in our hospital, awareness of colonization and effective therapy of B. cepacia infection is needed, as such an infection can cause mortality and morbidity. Also, infection control and monitoring are important, especially in patients under prolonged hospital admission.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Mehrad B, Clark NM, Zhanel GG, Lynch JP., 3rd Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest. 2015;147:1413–1421. doi: 10.1378/chest.14-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunz AN, Brook I. Emerging resistant Gram-negative aerobic bacilli in hospital-acquired infections. Chemotherapy. 2010;56:492–500. doi: 10.1159/000321018. [DOI] [PubMed] [Google Scholar]
- 3.Solomon SL, Oliver KB. Antibiotic resistance threats in the United States: stepping back from the brink. Am Fam Physician. 2014;89:938–941. [PubMed] [Google Scholar]
- 4.Segonds C, Chabanon G. [Burkholderia cepacia: dangers of a phytopathogen organism for patients with cystic fibrosis] Ann Biol Clin (Paris) 2001;59:259–269. [PubMed] [Google Scholar]
- 5.Brady MT, Marcon MJ. Pseudomonas and realated genera. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, editors. Textbook of Pediatric Infectious Disease. 7th ed. Philadelphia: Sunders Elsevier; 2014. pp. 1582–1609. [Google Scholar]
- 6.Govan JR, Hughes JE, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 7.Gautam V, Singhal L, Ray P. Burkholderia cepacia complex: beyond pseudomonas and acinetobacter. Indian J Med Microbiol. 2011;29:4–12. doi: 10.4103/0255-0857.76516. [DOI] [PubMed] [Google Scholar]
- 8.Griffin PM, Price GR, Schooneveldt JM, Schlebusch S, Tilse MH, Urbanski T, et al. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry to identify vancomycin-resistant enterococci and investigate the epidemiology of an outbreak. J Clin Microbiol. 2012;50:2918–2931. doi: 10.1128/JCM.01000-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinali S, van Belkum A, Goering RV, Girard V, Welker M, Van Nuenen M, et al. Microbial typing by matrix-assisted laser desorption ionization-time of flight mass spectrometry: do we need guidance for data interpretation? J Clin Microbiol. 2015;53:760–765. doi: 10.1128/JCM.01635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graindorge A, Menard A, Neto M, Bouvet C, Miollan R, Gaillard S, et al. Epidemiology and molecular characterization of a clone of Burkholderia cenocepacia responsible for nosocomial pulmonary tract infections in a French intensive care unit. Diagn Microbiol Infect Dis. 2010;66:29–40. doi: 10.1016/j.diagmicrobio.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Hua CN, Tokeshi J. Emergence of Burkholderia cepacia in Honolulu: a case of nursing home-acquired B. cepacia sepsis. Hawaii J Med Public Health. 2013;72:308–309. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaitwatcharachai C, Silpapojakul K, Jitsurong S, Kalnauwakul S. An outbreak of Burkholderia cepacia bacteremia in hemodialysis patients: an epidemiologic and molecular study. Am J Kidney Dis. 2000;36:199–204. doi: 10.1053/ajkd.2000.8295. [DOI] [PubMed] [Google Scholar]
- 13.Liao CH, Chang HT, Lai CC, Huang YT, Hsu MS, Liu CY, et al. Clinical characteristics and outcomes of patients with Burkholderia cepacia bacteremia in an intensive care unit. Diagn Microbiol Infect Dis. 2011;70:260–266. doi: 10.1016/j.diagmicrobio.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Lo Cascio G, Bonora MG, Zorzi A, Mortani E, Tessitore N, Loschiavo C, et al. A napkin-associated outbreak of Burkholderia cenocepacia bacteraemia in haemodialysis patients. J Hosp Infect. 2006;64:56–62. doi: 10.1016/j.jhin.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Agents. 2009;33:394–404. doi: 10.1016/j.ijantimicag.2008.09.010. [DOI] [PubMed] [Google Scholar]