Abstract
Background
An oral glucose tolerance test (OGTT) is the current method used for screening and diagnosis of gestational diabetes mellitus (GDM). OGTT is a relatively complicated procedure and is expensive. Thus, new strategies that do not require fasting or more than a single blood draw may improve the diagnosis of GDM and increase the rate of GDM testing. We investigated the utility of monitoring glycosylated hemoglobin (HbA1c) levels for the diagnosis of GDM.
Methods
The data from 992 pregnant women with estimated gestational ages ranging from 24 to 28 weeks were retrospectively reviewed. There were 367 women with plasma glucose levels ≥140 mg/dL 1 hour after a 50-g OGTT. GDM was diagnosed according to the Carpenter-Coustan criteria for a 3-hour 100 g OGTT. A HbA1c assessment was performed at the same time.
Results
We enrolled 343 women in this study, and there were 109 women with GDM. The area under the curve the receiver operating characteristic curve for HbA1c detection of GDM was 0.852 (95% confidence interval, 0.808 to 0.897). A HbA1c cutoff value ≥5.35% had maximal points on the Youden index (0.581). The sensitivity was 87.2% and the specificity was 70.9% for diagnosing GDM. A threshold value ≥5.35% indicated that 163 patients had GDM and that 68 (41.7%) were false positive. The positive predictive value was 58.3% at this threshold value.
Conclusion
Despite substantial progress in methodology, HbA1c values cannot replace OGTT for the diagnosis of GDM.
Keywords: Diabetes, gestational; Diagnosis; Hemoglobin A, glycosylated
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as a carbohydrate intolerance of varying severity that is first recognized during pregnancy and is independent of glycemic status after delivery [1].
GDM is associated with adverse pregnancy outcomes including macrosomia, birth trauma, and metabolic complications in the newborn. Furthermore, women with GDM have a significantly increased risk for the subsequent development of overt diabetes. The majority of clinical complications caused by GDM can be prevented by controlling blood glucose levels. Thus, early GDM diagnosis and treatment are important.
An acceptable screening test for GDM should meet the following requirements: high precision, high reproducibility, convenience, and low cost [2]. The International Workshop Conference on GDM recommends performing an oral glucose tolerance test (OGTT) between 24 and 28 weeks of pregnancy for GDM screening. However, it is a relatively complicated procedure and is expensive. Therefore, alternative strategies that do not require fasting or more than a single blood draw may increase the rate of GDM testing.
Glycosylated hemoglobin (HbA1c) levels have been proposed as a diagnostic tool for identifying patients with undiagnosed diabetes or have a risk of developing diabetes [3]. In 2011, the World Health Organization (WHO) and the American Diabetic Association (ADA) accepted HbA1c levels as a diagnostic tool for diabetes mellitus [4,5]. However, there are no recommendations available for the use of HbA1c as a diagnostic tool for GDM. Rajput et al. [6] previously suggested the use of HbA1c levels for the diagnosis of GDM in India. Therefore, we investigated the utility of HbA1c for the diagnosis of GDM in Korea.
METHODS
The subjects included pregnant women attending the local obstetric center in Cheonan from September 1, 2011, to September 30, 2012. The data from 992 pregnant women with estimated gestational ages ranging from 24 to 28 weeks were retrospectively reviewed. There were 367 women with plasma glucose levels ≥140 mg/dL 1 hour after the 50 g OGTT. We excluded women who had known diabetes or were suffering from anemia, chronic renal disease, pancreatic disease or other severe illnesses. The WHO defines anemia in pregnancy as a hemoglobin concentration <11 g/dL. Therefore, 24 women were excluded from this study due to anemia (hemoglobin [Hb] <11 g/dL). The subjects were advised to fast overnight and were subjected to OGTT in the morning. The 100 g OGTT was performed as a diagnostic test 2 to 4 weeks after the screening test. The HbA1c levels were analyzed simultaneously.
GDM was diagnosed according to the Carpenter-Coustan criteria for a 3-hour 100 g OGTT. The criteria indicate GDM if two or more plasma glucose levels met or exceeded the following thresholds: fasting glucose concentration of 95 mg/dL, 1-hour glucose concentration of 180 mg/dL, 2-hour glucose concentration of 155 mg/dL, and 3-hour glucose concentration of 140 mg/dL.
A venous blood sample was collected in ethylenediamine tetraacetic acid (EDTA) tubes and sent for HbA1c estimation. The HbA1c was measured using a turbidimetric immunoassay (COBAS Integra 800; Roche, Mannheim, Germany).
The t-test was performed to determine the difference between means. The Pearson correlation coefficient is also known as r and is a measure of the strength and direction of the linear relationship between HbA1c and each value of the 100 g OGTT. A receiver operating characteristic (ROC) curve was used to assess the sensitivity and specificity. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The level of significance was 0.05.
RESULTS
Among 992 subjects, a total of 367 women (37.0%) had a positive screening test result (plasma glucose ≥140 mg/dL 1 hour after 50 g OGTT). Twenty-four women were excluded from this study. The remaining 343 women were finally enrolled in this study. There were 109 women with GDM in the study group.
The mean maternal age was 31.36 years (median, 31.0 years; SD, 3.82; range, 21 to 44 years). The mean Hb was 11.83 g/dL (median, 11.70 g/dL; SD, 0.64; range, 11.0 to 14.4 g/dL), and the mean HbA1c level was 5.39% (median, 5.30%; SD, 0.31; range, 4.5% to 7.2%). The chronological age, Hb, and HbA1c values for the 50 and 100 g OGTT segregated by diagnosis are shown in Table 1. The women with GDM were older than the women without GDM (P=0.001). There were no significant differences in Hb levels between groups (P=0.305).
Table 1. Comparison of women with and without GDM.
| Variable | GDM (n=109) |
Non-GDM (n=234) |
P valuea |
|---|---|---|---|
| Age, yr | 32.38±3.95 | 30.89±3.68 | 0.001 |
| Hemoglobin, g/dL | 11.89±0.74 | 11.80±0.58 | 0.305 |
| 50 g OGTT 1-hr, mg/dL | 170.34±16.77 | 155.69±12.41 | <0.001 |
| OGTT fasting, mg/dL | 98.30±9.43 | 88.85±6.22 | <0.001 |
| OGTT 1-hr, mg/dL | 193.97±28.26 | 150.53±23.19 | <0.001 |
| OGTT 2-hr, mg/dL | 172.29±31.72 | 133.28±17.91 | <0.001 |
| OGTT 3-hr, mg/dL | 133.09±36.29 | 108.92±19.88 | <0.001 |
| Glycosylated hemoglobin, % | 5.64±0.33 | 5.27±0.21 | <0.001 |
Values are presented as mean±standard deviation.
GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test.
aP values are for independent t-test.
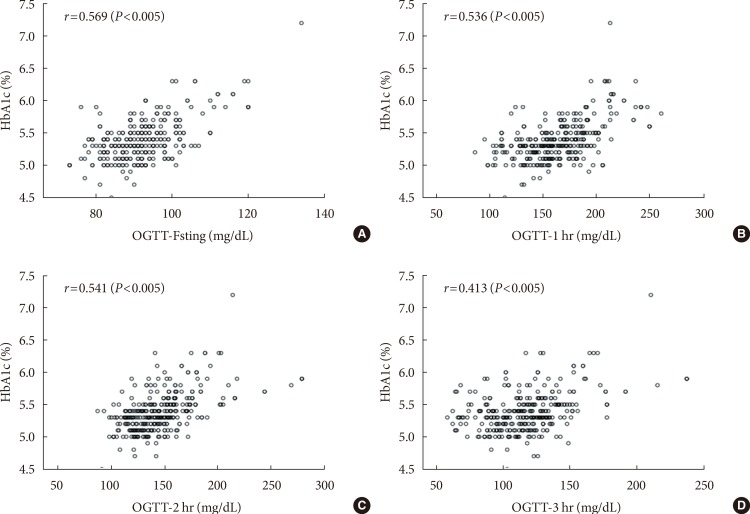
The Pearson's correlation coefficients (r) for HbA1c levels at each time point during the 100 g OGTT (fasting, 1-, 2-, and 3-hour) were 0.569, 0.536, 0.541, and 0.413, respectively (P< 0.005) (Fig. 1).
Fig. 1. Correlation of glycosylated hemoglobin (HbA1c) with each value of 100 g oral glucose tolerance test (OGTT) (A, fasting; B, 1-hour; C, 2-hour; and D, 3-hour). r=Pearson's correlation coefficient.
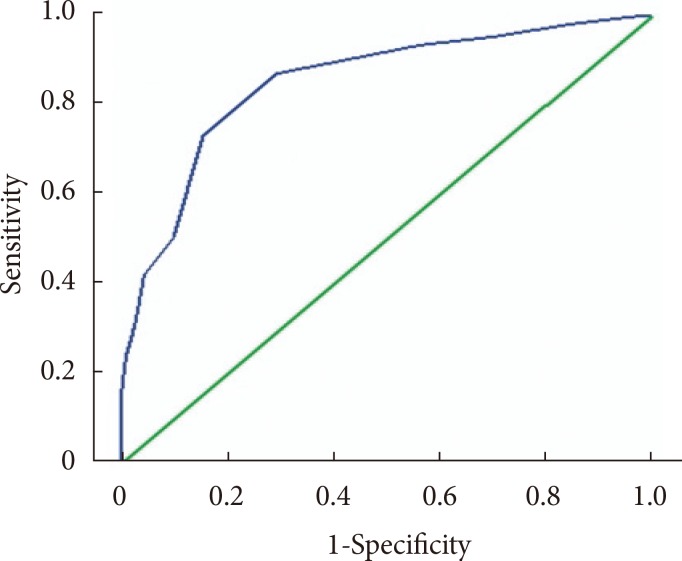
A ROC curve was constructed to determine the sensitivity and specificity of HbA1c in detecting GDM (Fig. 2). The area under the curve the ROC curve for the detection of GDM was 0.852 (95% confidence interval, 0.808 to 0.897).
Fig. 2. Receiver operating characteristic curve showing the sensitivity and specificity of glycosylated hemoglobin in detecting gestational diabetes mellitus.
Table 2 lists selected threshold values for HbA1c and the associated sensitivity, specificity, false positive, and false negative rates in addition to the likelihood ratios of positive and negative tests. A HbA1c cutoff value ≥5.35% had maximal points on the Youden index (0.581). The sensitivity was 87.2% and the specificity was 70.9% in diagnosing GDM. Using a threshold value ≥5.35% to determine disease showed 163 patients would have GDM and 68 (41.7%) would be false positives. The positive predictive value was 58.3% at this threshold.
Table 2. Selected threshold values of glycosylated hemoglobin and associated data.
| Variable | Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Threshold percentage, % | 5.05 | 5.15 | 5.25 | 5.35 | 5.45 | 5.55 | 5.65 | 5.75 | 5.85 |
| No. of patients ≥threshold (TP+FP) | 304 | 268 | 234 | 163 | 116 | 78 | 56 | 40 | 28 |
| % of patients ≥threshold | 88.6 | 78.1 | 68.2 | 47.5 | 33.8 | 22.7 | 16.3 | 11.7 | 8.2 |
| Sensitivity, % | 98.2 | 95.4 | 93.6 | 87.2 | 73.4 | 50.5 | 42.2 | 31.2 | 23.9 |
| Specificity, % | 15.8 | 29.9 | 43.6 | 70.9 | 84.6 | 90.2 | 95.7 | 97.4 | 99.1 |
| Positive predictive value, % | 35.2 | 38.8 | 43.6 | 58.3 | 69.0 | 70.5 | 82.1 | 85.0 | 92.9 |
| Negative predictive value, % | 94.9 | 93.3 | 93.6 | 92.2 | 87.2 | 79.6 | 78.0 | 75.2 | 73.7 |
| False positive rate, % | 84.2 | 70.1 | 56.4 | 29.1 | 15.4 | 9.8 | 4.3 | 2.6 | 0.9 |
| False negative rate, % | 1.8 | 4.6 | 6.4 | 12.8 | 26.6 | 49.5 | 57.8 | 68.8 | 76.1 |
| Positive likelihood ratio | 1.17 | 1.36 | 1.66 | 3.00 | 4.77 | 5.15 | 9.81 | 12.00 | 26.56 |
| Negative likelihood ratio | 0.11 | 0.15 | 0.15 | 0.18 | 0.31 | 0.55 | 0.60 | 0.71 | 0.77 |
| TN+FN | 39 | 75 | 109 | 180 | 227 | 265 | 287 | 303 | 315 |
| Youden index | 0.140 | 0.253 | 0.372 | 0.581 | 0.580 | 0.407 | 0.379 | 0.286 | 0.230 |
TP, true positive; FP, false positive; TN, true negative; FN, false negative.
DISCUSSION
In 2011, the ADA recommended that all pregnant women not known to have prior diabetes undergo a 75 g OGTT at 24 to 28 weeks of gestation based on the findings of the International Association of Diabetes and Pregnancy Study Group (IADPSG). The National Institutes of Health consensus panel recommended continuation of the "two-step" screening approach with a 1-hour 50 g glucose load test followed by a 3-hour 100 g OGTT for those who screened positive.
GDM screening can be accomplished with either of two strategies. However, there is no study evaluating the "one-step" approach by the IADPSG. The current conventional "two-step" approach and Carpenter-Coustan diagnostic criteria are mainly used in Korea.
Although OGTT is the gold standard test, it is a cumbersome procedure for both the participant and health care providers. It requires the participant to fast and requires at least 2 hours for sample collections because a minimum of two blood samples must be collected. The ADA recommended the use of HbA1c for diagnosing diabetes because the HbA1c test does not require fasting. Although HbA1c has the advantage of convenience and good intraindividual reliability, it is important to note that HbA1c measurements are affected by underlying hemoglobinopathies and anemia associated with accelerated red cell turn over. A prior study observed that HbA1c levels were lower in pregnant women than in control women [7]. This result is likely due to the normal decrease in fasting plasma glucose in early pregnancy, which is caused by glucose being diverted to the developing fetus. This is sustained throughout the pregnancy by increasing insulin resistance, which is most prominent in the third trimester of pregnancy. Additionally, the life span of erythrocytes is likely reduced during pregnancy. The result is shorter exposure times to plasma glucose and reduced glycation for new erythrocytes [8,9].
The majority of studies that evaluated HbA1c as a possible screening test for GDM were published between 1980 and 1990. These studies concluded that HbA1c was not a suitable screening test. The conclusions were similar despite the variation in methodology of the HbA1c assays and lack of standardized diagnostic criteria for GDM. Frisoli et al. [10] found that the mean HbA1c was higher in pregnancy but it was unreliable for the GDM screening. Artal et al. [11] reported the high incidence of false negatives and false positives make HbA1c 'inadequate' for GDM screening. The study by Morris et al. [12] measured HbA1c by specific affinity exchange chromatography. The authors noted that HbA1c may be a sensitive predictor of GDM [12]. The study by Rajput et al. [6] showed that HbA1c levels cannot replace OGTT for the diagnosis of GDM. However, it can be used in combination with OGTT to obviate the need for further OGTT.
Test sensitivity is the proportion of true positives that are correctly identified by the test, whereas specificity is the proportion of true negatives that are correctly identified by the test. A diagnostic test with higher specificity will have fewer false positives than tests with lower specificity.
In the present study, a HbA1c cut-off value ≥5.35% had the highest Youden index (0.581) and high sensitivity (87.2%) in detecting GDM (Table 2). However, the specificity was low (70.9%) and the false positive rate was 29.1%. In practical terms, this means to identify 87.2% of the diseased patients then 29.1% of patients without GDM would be misdiagnosed. Conversely, 12.8% of the GDM patients would be missed.
The Pearson's correlation coefficient (r) at each time point of the 100 g OGTT was statistically significant (P<0.001). The most significant value was the fasting plasma glucose level after the 100 g OGTT (r=0.569) and was followed by the 2-hour plasma glucose level during the 100 g OGTT (r=0.536). The 3-hour plasma glucose level during the 100 g OGTT was not significant (r=0.413) (Fig. 1). Despite the positive correlations between HbA1c and GDM diagnosis, the utility of HbA1c levels as a diagnostic test for GDM remains controversial.
This study has several limitations. The number of study subjects was relatively small, and the data were obtained in a retrospective manner. Therefore, medications or co-existing diseases that affect HbA1c may not be excluded. Additionally, the study subjects were candidates for further testing after the 50 g OGTT. This result suggests the study subjects do not reflect the real population at late pregnancy. It is also possible that HbA1c levels are not available for all normal subjects because this is a retrospective study. An ideal study would include a large cohort of normal subjects who had glucose levels less than 140 mg/dL at 1 hour after the 50 g OGTT.
In conclusion, this study confirms that despite technological developments, such as improved standardization, automation, and the wide-availability the HbA1c level, cannot replace OGTT for the diagnosis of GDM. This test can be used in combination with OGTT to obtain supplementary data for diagnosing GDM. Further large and prospective studies must be performed to evaluate simple, patient-friendly, and sensitive tests for diagnosing GDM.
ACKNOWLEDGMENTS
This study was supported by Soonchunhyang University.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–B167. [PubMed] [Google Scholar]
- 2.Maegawa Y, Sugiyama T, Kusaka H, Mitao M, Toyoda N. Screening tests for gestational diabetes in Japan in the 1st and 2nd trimester of pregnancy. Diabetes Res Clin Pract. 2003;62:47–53. doi: 10.1016/s0168-8227(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 3.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus. Geneva: World Health Organization; 2011. [Google Scholar]
- 6.Rajput R, Yogesh Y, Rajput M, Nanda S. Utility of HbA1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;98:104–107. doi: 10.1016/j.diabres.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen LR, Ekbom P, Damm P, Glumer C, Frandsen MM, Jensen DM, Mathiesen ER. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200–1201. doi: 10.2337/diacare.27.5.1200. [DOI] [PubMed] [Google Scholar]
- 8.Lind T, Cheyne GA. Effect of normal pregnancy upon the glycosylated haemoglobins. Br J Obstet Gynaecol. 1979;86:210–213. doi: 10.1111/j.1471-0528.1979.tb10595.x. [DOI] [PubMed] [Google Scholar]
- 9.Lurie S, Danon D. Life span of erythrocytes in late pregnancy. Obstet Gynecol. 1992;80:123–126. [PubMed] [Google Scholar]
- 10.Frisoli G, Naranjo L, Shehab N. Glycohemoglobins in normal and diabetic pregnancy. Am J Perinatol. 1985;2:183–188. doi: 10.1055/s-2007-999945. [DOI] [PubMed] [Google Scholar]
- 11.Artal R, Mosley GM, Dorey FJ. Glycohemoglobin as a screening test for gestational diabetes. Am J Obstet Gynecol. 1984;148:412–414. doi: 10.1016/0002-9378(84)90717-8. [DOI] [PubMed] [Google Scholar]
- 12.Morris MA, Grandis AS, Litton J. Glycosylated hemoglobin: a sensitive indicator of gestational diabetes. Obstet Gynecol. 1986;68:357–361. doi: 10.1097/00006250-198609000-00013. [DOI] [PubMed] [Google Scholar]