Abstract
Introduction
Acute pancreatits (AP) still reqiures better diagnostic and therapeutic options to be introduced in order to decrease its morbidity and mortality. It appears that the assessment of serum levels of interleukin 18 (IL-18) and its correlation with C-reactive protein (CRP) may provide adequate prognostic value.
Aim
To measure serum concentrations of IL-18 and inflammation markers such as CRP in patients with AP during subsequent hospital stay days and to assess the role of IL-18 as an early AP marker and prognostic factor.
Material and methods
Thirty-two patients aged 47 ±16.7 years were included into the study (17 males and 15 females), in whom AP was diagnosed based on ultrasound and computer aided tomography imaging and amylase. Serum amylase, CRP, and IL-18 levels were measured on the 1st, 2nd, 3rd, and 5th days of hospital stay. All patients were scored “B” according to Balthazar and mild AP based on Ranson criteria. The control group consisted of 30 healthy volunteers aged 50.7 ±12.4 years (15 males and 15 females).
Results
The average IL-18 serum level in the control group was 86.91 ±4.94 pg/ml. Mean IL-18 study group levels were 128.4 ±7.6 pg/ml on the 1st, 112.0 ±4.4 pg/ml on the 3rd, and 122.8 ±6.8 pg/ml on the 5th day of AP, and were significantly higher than those in the control group, accordingly: p < 0.001, p < 0.005, p < 0.001. A positive correlation between IL-18 and CRP serum concentrations was observed. A slight increase in correlation was observed as the days went by.
Conclusions
We concluded that the serum IL-18 level increases in the initial phase of AP, and it may be used as an inflammatory reaction marker in patients with AP, and it is correlated with CRP, which may indicate its prognostic role in AP.
Keywords: acute pancreatitis, interleukin 18, C-reactive protein
Introduction
Acute pancreatitis (AP) is an inflammatory reaction either confined to the pancreatic gland itself, or sometimes encompassing distant tissue and glands. The annual incidence of AP ranges from 5 to 80 cases per 100,000 population [1]. In about 75% of patients AP appears as a benign, self-limiting inflammatory reaction accompanied by pancreatic oedema. The remaining 25% suffer from severe necrosis, which is sometimes caused by infection [2]. It may consequently result in sepsis, as well as systemic inflammatory reaction syndrome (SIRS), disseminated intravascular coagulation (DIC), and multiorgan failure (MOFS) and, less often, in an adjacent intestinal loop lesion in the aftermath [3]. These complications render mortality, which, according to the literature, amounts to 19% for infected necrosis, 67.5% for sepsis, 62.5% for DIC, and 50–91% for MOFS, respectively [1, 4, 5]. Total mortality, regardless of AP form, according to various sources, ranges from 2.1% to 7.8% [6–8].
The most frequent aetiological factors are alcohol abuse and biliary tract diseases, which constitute over 80% of causes of AP, regardless of the geographical distribution of the study population [9]. The remaining causes are iatrogenic measures, usually retrograde cholangiopancreatography (ERCP) in 1.6–6.7% [10, 11] and applied medications in about 1.5% [8, 12, 13].
Acute pancreatitis may develop as a benign inflammatory reaction with predominance of oedema and other typical symptoms of inflammation as well as a severe form of AP with predominance of necrotic process in the gland itself and its surroundings. In both forms of AP: benign and severe, trypsin activation not only evokes pancreatic proteolytic enzyme secretion, but also initiates activation processes of complement, coagulation, and fibrinolytic systems and production of several cytokines [14]. In the initial phase of AP there is also an increase in the number and activation of microphages, lymphocytes, and monocytes in the pancreatic parenchyma. They release substances such as interleukin 1 (IL-1), tumor necrosis factor α (TNF-α), IL-18, IL-19, etc. These cytokines, together with reactive oxygen species (ROS) and other substances secreted by the damaged pancreatic cells, stimulate the further stages of inflammatory processes in the gland [15, 16]. Their action, especially that of IL-1β, may be profoundly downregulated by heparin [17]. In order to apply the proper treatment, a profound need for accurate and reliable prognostic factors exists, and markers such as widely acknowledged C-reactive protein (CRP), but also procalcitonin, serum amyloid A, IL-6, and others, have been successfully used. However, their sensitivity and specificity is still unsatisfactory, which stimulates research on other, more suitable AP prognostic factors.
Interleukin 18 was first described in 1999 [18]. It had been named interferon γ inducing factor – IGIF or interleukin-1γ, when that it emerged that it did not bind to the IL-1 receptor [9, 19]. The main source of IL-18 in humans are mononuclear cells, such as monocytes, macrophages, dendritic cells, lymphocytes B [20], Langerhans cells, Kupffer cells, keratinocytes, synoviocytes, osteoblasts [21], and respiratory and intestinal epithelial cells [22].
Biologically active IL-18 is a cytokine with a molecular weight of 18 kD. Activation of the gene encoding pro-IL-18 is induced by an increase in the expression of two pro-IL-18 promoters in response to direct stimulation of the cell by microbial products, such as lipopolysaccharide (LPS), or interferons α, β, and γ (IFN-α, IFN-β, and IFN-γ). Biologically active IL-18 is produced by an inactive precursor with a molecular weight of 24 kD, called pro-IL 18, as a result of the activity of IL-1β converting enzyme (ICE), also named caspase 1.
Membrane receptor for IL-18 (IL-18R) is a heterodimer comprising two α and β subunits. IL-18Rα binds IL-18 and IL-18Rβ signalling unit. IL-18Rβ subunit activates the signal pathway of this receptor.
Interleukin-18 has pleiotropic activity. Its activity as an anti-inflammatory cytokine consists of the stimulation of non-CD14+ mononuclear cells, mostly monocytes, macrophages, and peripheral blood basophils. It has been proven that IL-18 activates the production of other interleukins, such as IL-2, IL-8, IL-12, IL-15, IL-23, and LPS-induced IFN-γ production in vivo [20, 23, 24].
Wereszczynska-Siemiatkowska et al. noticed a significant increase in IL-18 concentration in patients with AP. Moreover, they suggested using IL-18 as an early marker of AP severity. Ueda et al. [25] found a significantly increased IL-18 serum concentration level not only during the course of the disease, but also up to 4 weeks after the recovery.
Aim
The purpose of this study was to evaluate the possible diagnostic and prognostic role of IL-18 measured in early AP.
Material and methods
Thirty-two patients hospitalised at the Department of Digestive Tract Diseases of Lodz Medical University, aged 39–57 years (median age 47 ±16.7 years), 17 men and 15 women with alcoholic AP, were included in the study based on clinical symptoms and a serum amylase level increase to at least five times the upper limit. Patients with concurrent diseases such as diabetes, hypertension, cardiovascular diseases, kidney failure, inflammatory diseases, and autoaggression were excluded. Moreover, all patients underwent ultrasound examination and contrast-enhanced abdominal computed tomography scanning and were scored ‘B’ according to Balthazar [26]. The control group consisted of 30 healthy volunteers aged 33–60 years (median age 50.7 ±12.4), 15 men and 15 women. The study and control groups did not differ in terms of demographic indicators. Fasting blood samples were taken in the morning on the first, third, and fifth day of hospital stay in order to measure IL-18 and CRP concentrations. Biochemical assays were prepared in the Laboratory Diagnostics Department of Lodz Medical University. The IL-18 concentration was determined using QUANTIKINE Human Interleukin 18 Immunoassay ELISA kit (R&D Systems). The study was conducted in accordance with Bioethical Committee of Lodz Medical University approval of protocol (RNN 72/07/KE). All patients were informed about the study and gave their consent.
Results
Interleukin 18 serum level
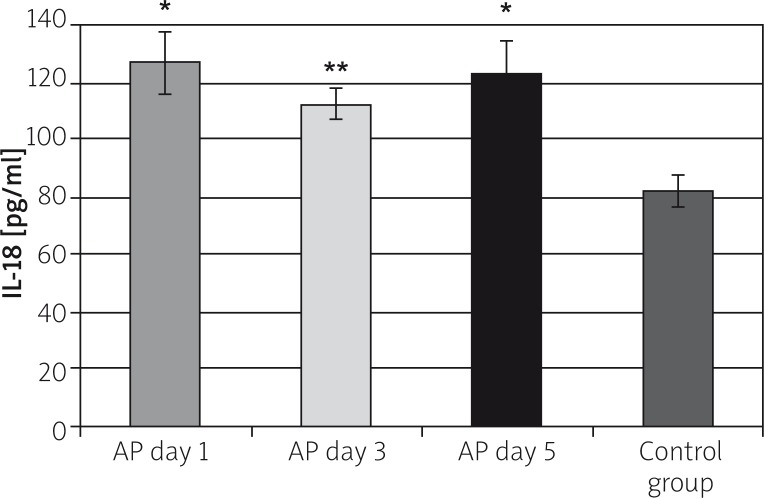
The average IL-18 serum level in the control group was 86.91 ±4.94 pg/ml. The IL-18 study-group levels were: 128.4 ±7.6 pg/ml on the first, 112.0 ±4.4 pg/ml on the third, and 122.8 ±6.8 pg/ml on the fifth day of AP, and they were significantly higher than in the control group, accordingly p < 0.001, p < 0.005, p < 0.001. The increase in IL-18 level amounted to 49% on the first, 30% on the third, and 42% on the fifth day, in comparison to the control group (Figure 1).
Figure 1.
The IL-18 serum concentration in the study groups
*p < 0.001 compared to the control group, **p < 0.005 compared to the control group.
C-reactive protein serum levels
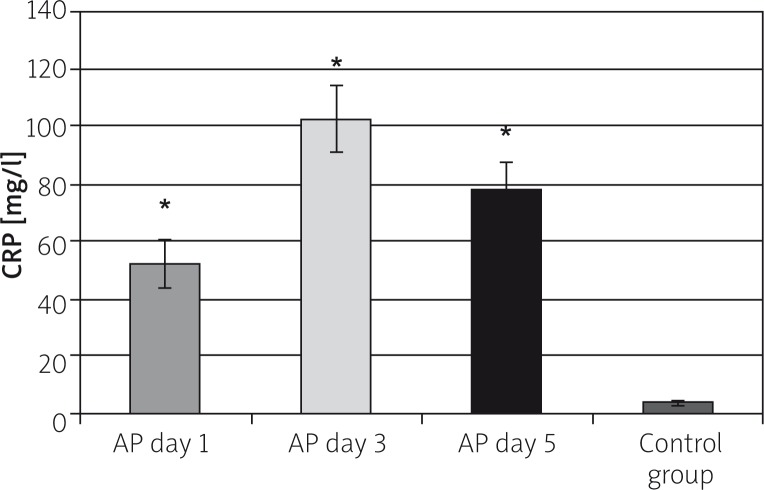
The average CRP serum level in the control group was 3.44 ±0.56 mg/l. Mean CRP study-group levels amounted to 51.94 ±8.15mg/l on the first, 102.4 ±11.7 mg/l on the third, and 78.31 ±8.57 mg/l on the fifth day of AP, and they were significantly higher in comparison to the control group, on the first, third, and fifth day of hospital stay (p < 0.001). The increase in CRP level was over 15 fold, almost 30 fold, and over 22 fold higher in the first, third, and fifth day of AP, respectively (Figure 2).
Figure 2.
The CRP serum concentration in the study groups
*p < 0.001 compared to the control group.
Analysis of the correlation between C-reactive protein and interleukin 18 levels on subsequent days of acute pancreatitis
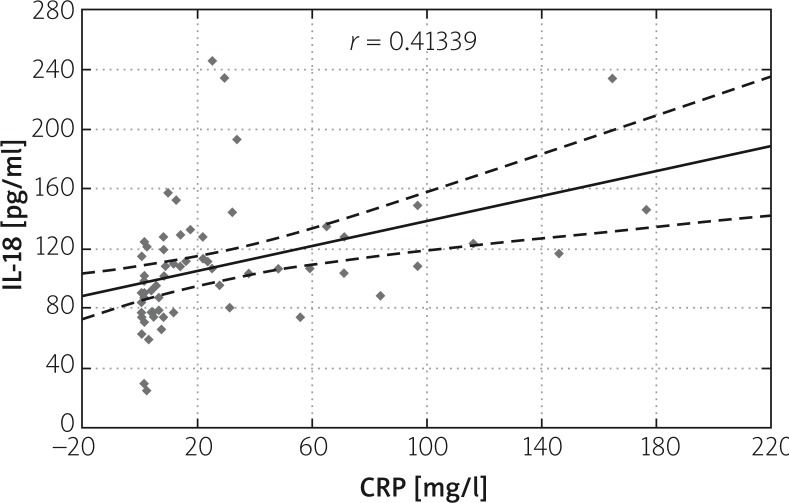
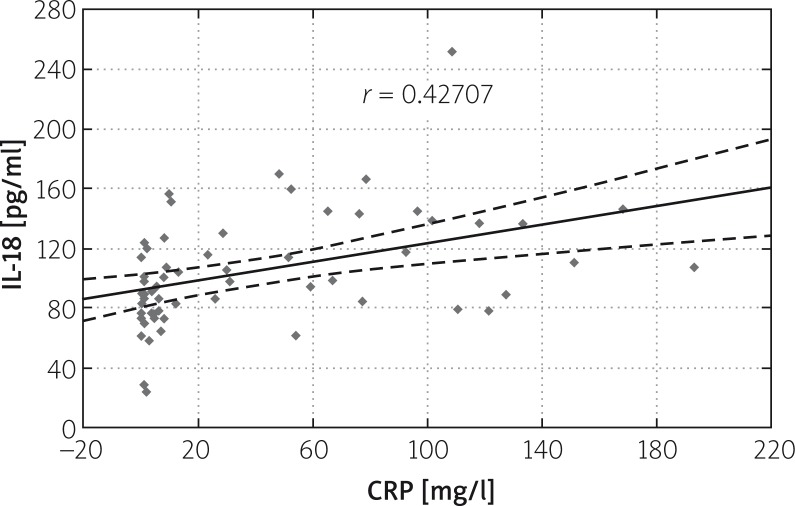
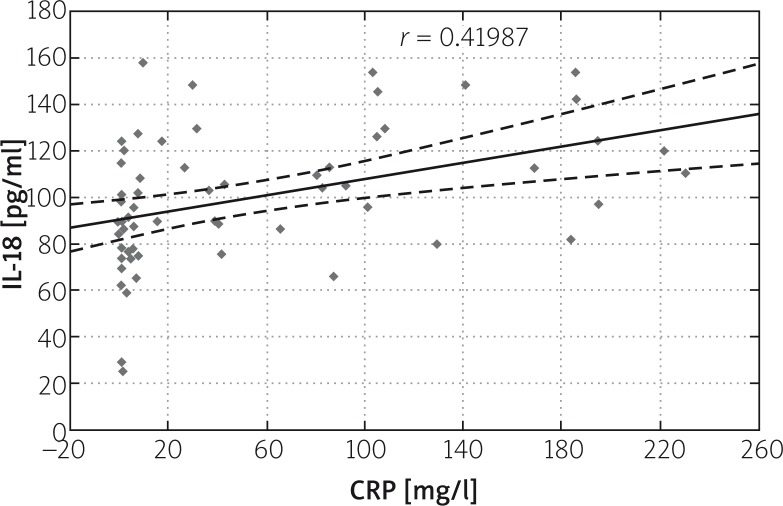
Correlation was found between IL-18 and CRP levels on subsequent days of AP. The correlation ratio in the study group amounted to r = 0.41339 on the first, r = 0.41893 on the third, and r = 0.42707 on the fifth day. A slight increase in correlation between IL-18 and CRP levels may be found in subsequent days of the disease (Figures 3–5).
Figure 3.
Correlation between IL-18 and CRP levels on the first day of AP
Figure 5.
Correlation between IL-18 and CRP levels on the fifth day of AP
Figure 4.
Correlation between IL-18 and CRP levels on the third day of AP
Discussion
The role of IL-18 as a proinflammatory cytokine has not been fully explained – literature data are unclear. Some of them, like IL-17, may be helpful in establishing the diagnosis of AP, but notin evaluating the severe form of the disease [27]. In this study we observed an increase in IL-18 and CRP levels on the first, third, and fifth day of hospital stay, which has been confirmed in the literature. Wereszczynska-Siemiatkowska et al. found a statistically significant increase in IL-18 level in 30 patients suffering from both benign and severe AP forms on the first 10 days of hospital stay. In severe AP (necrotic form), IL-18 level increased on the first, third, and fifth day of hospital stay, accordingly, by 81%, 84%, and 55%. In benign AP (oedema form) IL-18 levels were lower, similar to our results. The IL-18 levels increased on the first, third, and fifth day of hospital stay, accordingly, by 54%, 50%, and 64% in comparison to the control group, whereas in our study IL-18 levels increased by 49%, 30%, and 42% in comparison to the control group [28].
Likewise, Rau et al. [29] and Ueda et al. [25] observed increased IL-18 level in patients suffering from AP in comparison to the control group. Rau described 68 patients suffering from AP, among whom 37 were diagnosed with locoregional complications, such as pancreatic necrosis, and others with systemic complications such as renal and cardiovascular or multiorgan failure. They claimed that the increase in IL-18 level only referred to the latter, which may be related to the decrease in IL-18 secretion due to kidney insufficiency. On the other hand, Martin et al. compared IL-18 levels in patients suffering from AP with those in patients with cholecystolithiasis or common bile duct lithiasis without cholangitis [30]. Thirty-six patients were included in the study, in whom IL-18 levels were measured on the first, second, third, fifth, and seventh day of the disease. According to the literature, IL-18 levels are higher in patients suffering from both benign and severe forms of AP than in the control group. A significant difference between patients suffering from both forms of AP existed only during the first 24 h. Martin et al. assumed that IL-18 may be acknowledged as an early marker of both systemic and locoregional complications on account of its larger increase in the severe form of AP [30]. Both in patients suffering from the benign and the severe form of AP, IL-18 levels measured on the first, third, fifth, and tenth days of the disease were significantly higher than in the control group. Moreover, the IL-18 level was higher in patients diagnosed with the severe form than in those with the benign form of AP [28, 31]. Ueda et al. investigated the above-named parameters in patients both with benign and severe forms of AP. In 43 patients there was an increase in IL-18 level, which persisted for up to 4 weeks after treatment. The authors did not find any significant differences between IL-18 serum levels in patients suffering from a coexistent pancreatic necrosis and the benign form of AP [23]. Melnikov et al. proved that suppression of IL-18 production protects caspase-1-deficient mice from acute renal failure. According to these authors, suppression of IL-18 production at an early stage of inflammation may be a useful therapeutic tool in the prevention of ischaemia-induced acute renal failure [32].
We noticed a slightly strengthening correlation between IL-18 and CRP levels on subsequent days of the disease in our study, which was confirmed by Wereszczynska-Siemiatkowska et al., who presented findings concerning IL-18 serum level and its correlation with CRP in patients suffering from severe form of AP, as well as in the control group. The authors observed a correlation between IL-18 and CRP, which amounted to r = 0.41 (p < 0.05) on the first day of hospital stay and weakened over the following days to r = 0.27 (p < 0.05) [28, 30]. The difference between a constant increase in the CRP and IL-18 correlation in our study as compared to the initial rise and further decrease of the strength of this phenomenon in the mentioned paper may be attributed to the difference in disease severity in both studies, with more severe patients remaining under our observation.
Perejaslow et al. [33] obtained similar results, detecting a significant increase in IL-18 serum concentration in 87 patients suffering from AP on the fifth, seventh, and even the thirty-first day following the diagnosis. In addition, both authors discovered a correlation between AP severity and IL-18 serum concentration. The IL-18 may have an impact on the AP pathophysiology by influencing immune cells via IL-1β, TNF-α, and other chemokine secretion, and for neutrophils via an increased expression of adhesion molecules. Due to the fact that the above-named cytokines, being the earliest mediators of great significance in AP, are largely responsible for development of the disease, their regulatory mechanisms may depend on the IL-18 activity [34]. The IL-18-induced neutrophil activation may result in a persistent inflammatory reaction leading to multiorgan failure and ARDS [35].
On the basis of the gathered data, further investigation into the relationship between the proinflammatory action of IL-18 and AP seems advisable. Explanation of the proper and exact role of IL-18 in the prediction of the severity and course of AP requires further analysis.
Conclusions
Mean concentrations of IL-18 increases in AP and their levels are highly correlated with CRP value. These data suggest that IL-18 may be potentially useful as an AP diagnostic and prognostic marker.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.De Campos T, Cerqueira C, Kuryura L, et al. Morbimortality indicators in severe acute pancreatitis. JOP. 2008;9:690–7. [PubMed] [Google Scholar]
- 2.Kozieł D, Kozłowska M, Deneka J, et al. Retrospective analysis of clinical problems concerning acute pancreatitis in one treatment center. Prz Gastroenterol. 2013;8:320–6. doi: 10.5114/pg.2013.38736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kędra B, Myśliwiec P, Romatowski W. Descending colon stenosis as a complication of acute pancreatitis [Polish] Prz Gastroenterol. 2009;4:53–6. [Google Scholar]
- 4.Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol. 2007;13:5043–51. doi: 10.3748/wjg.v13.i38.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaurich T. Drug-induced acute pancreatitis. Proc (Bayl Univ Med Cent) 2008;21:77–81. doi: 10.1080/08998280.2008.11928366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnovale A, Rabitti PG, Manes G, et al. Mortality in acute pancreatitis: is it an early or a late event? JOP. 2005;6:438–44. [PubMed] [Google Scholar]
- 7.Gullo L, Migliori M, Oláh A, et al. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223–7. doi: 10.1097/00006676-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kimura Y, Takada T, Kawarada Y, et al. JPN Guidelines for the management of acute pancreatitis: treatment of gallstone-induced acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:56–60. doi: 10.1007/s00534-005-1052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–34. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 10.Konturek PC, Brzozowski T, Pajdo R, et al. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325–36. [PubMed] [Google Scholar]
- 11.Lee JH, Ort T, Ma K, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro osteoarthritis cartilage. Osteoarthritis Cartilage. 2009;17:613–20. doi: 10.1016/j.joca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Vege SS, Gardner TB, Chari ST, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104:710–5. doi: 10.1038/ajg.2008.77. [DOI] [PubMed] [Google Scholar]
- 13.Matykiewicz J, Głuszek S, Kozieł D. Acute pancreatitis – an endoscopic retrograde cholangiopancreatography complication. Prz Gastroenterol. 2012;7:103–7. [Google Scholar]
- 14.Saluja AK, Bhagat L, Lee HS, et al. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol. 1999;276:G835–42. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 15.Leśniowski B, Kumor A, Daniel P, et al. Evaluation of serum concentration of selected adipocytokines in acute pancreatitis. Gastroenterol Pol. 2007;14:415–7. [Google Scholar]
- 16.Leśniowski B, Kumor A, Jasińska A, et al. Is resistin may be a new laboratory marker in diagnosis acute pancreatitis? Pol Merk Lek. 2007;131:385–7. [PubMed] [Google Scholar]
- 17.Ceranowicz P, Dembiński M, Warzecha Z, et al. Healing effect of heparin in the course of acute cerulein-induced pancreatitis [Polish] Prz Gastroenterol. 2009;4:199–205. [Google Scholar]
- 18.Dinarello CA. Interleukin-18. Methods. 1999;19:121–32. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 19.Vitone LJ, Greenhalf W, Howes NR, et al. Trypsinogen mutations in pancreatic disorders. Endocrinol Metab Clin North Am. 2006;35:271–87. doi: 10.1016/j.ecl.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–24. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 21.Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167:2879–86. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 22.Wereszczynska-Siemiatkowska U, Kosel J, Siemiatkowski A. Biological properties of interleukin 18. Pol Merk Lek. 2004;16:297–81. [PubMed] [Google Scholar]
- 23.Boraschi D, Dinarello CA. IL-18 in autoimmunity: review. Eur Cytokine Netw. 2006;17:224–52. [PubMed] [Google Scholar]
- 24.Paulukat J, Bosmann M, Nold M, et al. Expression and release of IL-18 binding protein in response to IFN-gamma. J Immunol. 2001;167:7038–43. doi: 10.4049/jimmunol.167.12.7038. [DOI] [PubMed] [Google Scholar]
- 25.Ueda T, Takeyama Y, Yasuda T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41:158–65. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 26.Balthazar EJ, Ranson JH, Naidich DP, et al. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767–72. doi: 10.1148/radiology.156.3.4023241. [DOI] [PubMed] [Google Scholar]
- 27.Czarny-Działak M, Głuszek S. IL-17 serum concentration during acute pancreatitis with regard to disease severity [Polish] Prz Gastroenterol. 2009;4:31–40. [Google Scholar]
- 28.Wereszczynska-Siemiatkowska U, Mroczko B, Siemiątkowski A, et al. The importance of interleukin 18, glutathione peroxidase, and selenium concentration changes in acute pancreatitis. Dig Dis Sci. 2004;49:642–50. doi: 10.1023/b:ddas.0000026312.47460.a3. [DOI] [PubMed] [Google Scholar]
- 29.Rau B, Baumgart K, Paszkowski AS, et al. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: high correlation of serum interleukin -18 with pancreatic necrosis and systemic complications. Crit Care Med. 2001;29:1556–62. doi: 10.1097/00003246-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Martín MA, Saracíbar E, Santamaría A. Interleukin 18 (IL-18) and other immunological parameters as markers of severity in acute pancreatitis. Rev Esp Enferm Dig. 2008;100:768–73. doi: 10.4321/s1130-01082008001200006. [DOI] [PubMed] [Google Scholar]
- 31.Wereszczynska-Siemiatkowska U, Mroczko B, Siemiatkowski A. Serum profiles of interleukin-18 in different severity forms of human acute pancreatitis. Scand J Gastroenterol. 2002;37:1097–102. doi: 10.1080/003655202320378310. [DOI] [PubMed] [Google Scholar]
- 32.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perejaslov A, Chooklin S, Bihalskyy I. Implication of interleukin 18 and intercellular adhesion molecule (ICAM)-1 in acute pancreatitis. Hepatogastroenterology. 2008;55:1806–13. [PubMed] [Google Scholar]
- 34.Date Y, Nakazato M, Hashiguchi S, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–9. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 35.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]