Abstract
Introduction
Invasion is usually recognized as the main reason for the high recurrence and death rates of gliomas. Therefore, properly understanding the molecular mechanisms of migration and invasion of human gliomas has become a focus and will be helpful for the treatment of gliomas. Syntenin has been demonstrated to be implicated in the migration, invasion and metastasis of many types of malignant tumors. Therefore, we investigated the expression of syntenin in human gliomas and its relationship with glioma migration.
Material and methods
Immunohistochemistry, Western blot and real time-polymerase chain reaction (RT-PCR) were performed to detect the expression of syntenin in human gliomas. Phosphorylated FAK in human gliomas was examined by western blot.
Results
Scattered syntenin positive glioma cells were detected by immunohistochemistry in normal tissue. Syntenin expression in grade II, III and IV gliomas increased with the degree of tumor malignancy, and no syntenin expression was detected in grade I gliomas. The level of phosphorylated FAK at the tyrosine 397 site also elevated with the degree of tumor malignancy. There was a positive correlation between the syntenin level and the pathological grade of gliomas (rs = 0.896, p < 0.05). Phosphorylated FAK was also upregulated along with the stage of glioma progression and the increase of syntenin expression.
Conclusions
Our results indicate that the enhanced expression of syntenin and phosphorylated FAK may correlate with the increase of the malignancy of human gliomas. Syntenin may promote human glioma migration through interaction with FAK.
Keywords: glioma, syntenin, focal adhesion kinase, migration
Introduction
Glioma, one of the most common types of central nervous system (CNS) tumors, is characterized by malignancy, invasion and poor prognosis [1]. Invasion, which limits the efficacy of surgery and other therapies, is usually recognized as the main reason for the high recurrence and death rates of gliomas [2, 3], and migration of tumor cells plays an important role in the invasion of gliomas [4, 5]. Although many studies have shown that many factors are involved in the invasion of gliomas, including adhesion molecules, extracellular matrix (ECM), protease system and angiogenesis, the exact molecular mechanism and process of the invasive growth of gliomas have not been clearly elucidated [6–10]. Therefore, properly understanding the molecular mechanisms of migration and invasion of human gliomas has become a focus and will be helpful for the treatment of gliomas.
Syntenin is a widely expressed protein in mammals [11]. It has been demonstrated to be implicated in the migration, invasion and metastasis of many types of malignant tumors, including human melanoma, breast cancer and gastrointestinal cancer [12, 13]. Forced syntenin overexpression is able to enhance the migration of melanoma cells, and knockdown of syntenin reduces the migration/invasion of a highly metastatic melanoma variant [14]. Overexpressed syntenin could activate the downstream signaling molecules, including focal adhesion kinase (FAK), which is overexpressed in many types of tumors and has been reported as an indicator of tumor metastasis, c-Jun-NH2-kinase, and p38, and consequently promote melanoma metastasis [12, 15, 16]. Therefore, syntenin is considered as a positive regulator of melanoma migration, invasion and metastasis [14]. In our previous study, we found that overexpression of syntenin could activate FAK-JNK and FAK-Akt signaling in human brain glioma cells and then enhance the migration capacity [17]. Although syntenin has been identified as a novel cell signaling and metastasis-associated gene for many years, our knowledge about the expression of syntenin in human gliomas and the correlation between syntenin and gliomas remains deficient. Therefore, we aimed to determine the expression of syntenin in human gliomas and analyze the correlation between syntenin and the malignancy grade of gliomas in the present study. Our results may provide a new viewpoint to understand the mechanism of recurrence and metastasis of human brain gliomas.
Material and methods
Patients
Between January 2008 and January 2010, human glioma tissue samples were collected from 84 patients (45 men and 39 women; range: 17–68 years) who had undergone brain surgery at the Department of Neurosurgery, First Affiliated Hospital of Chongqing Medical University. All the samples used in the present study were confirmed by pathological examination. The samples were classified according to the guidelines of the World Health Organization (WHO) 2007 classification of tumors of the CNS [18]. We observed 10 grade I, 29 grade II, 25 grade III, and 20 grade IV tumors. Normal brain tissues that had to be excised in the resection of intracranial deep tumors were used as a control. The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Immunohistochemistry
The expression of syntenin was examined by immunohistochemical (IHC) staining. Brain glioma tissues were fixed with 4% paraformaldehyde and embedded with paraffin using standard methods. IHC was carried out using a specific rabbit polyclonal anti-syntenin antibody (1: 500, Abcam, Cambridge, MA, USA). Counterstaining was performed with hematoxylin. Control staining was conducted by omitting the primary antibody. The images were obtained with an Olympus DP70 optical microscope and analyzed by the image analysis system (Beihang, CM-2000B).
Western blot
Total protein was extracted as previously described [19]. Briefly, the brain tissue was homogenized with RIPA lysis buffer (Beyotime, Haimen, Jiangsu, China). Then the homogenate was centrifuged at 12000 g for 30 min at 4°C and the supernatants were collected for the next western blot. Protein concentration was determined with the BCA method. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) (Millipore, Billerica, MA, US). The blot was then probed with primary antibody followed by reaction with horseradish peroxidase-conjugated secondary antibody. The signal was detected using enhanced chemiluminescence and recorded on X-ray film.
Total RNA extraction and real time-polymerase chain reaction
Total RNA was extracted from brain tissue with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manual. The concentration and purity of total RNA were determined by a spectrophotometer (Eppendorf, Hamburg, Germany). Reverse transcription was performed using a first strand cDNA synthesis kit (Sangon, Shanghai, China). cDNA was stored at –20°C. Primers for target genes were as follows: syntenin forward, 5′-ATG TCT CTC TAT CCA TCT CTC G-3′, reverse, 5′-TTA AAC CTC AGG AAT GGT GTG G-3′; β-actin forward, 5′-GGA CGT GGA CAT CCG CAA AG-3′, reverse, 5′-CTG GAA GGT GGA CAG CGA GG-3′. Polymerase chain reaction was performed with a GeneAmp PCR System 9700 (ABI, Foster City, CA, USA) and started with a polymerase activation step at 94°C for 5 min followed by 30 cycles of 94°C for 30 s, 55°C (for β-actin, the anneal temperature was 50°C) for 30 s and 72°C for 60 s, and a final extension at 72°C for 10 min. The relative expression of the target gene was normalized to β-actin.
Statistical analysis
Data are expressed as means ± SD. Statistical significance was determined using SPSS 11.0 for Windows (SPSS Inc., Chicago, IL, USA). Data distribution was tested by the Anderson Darling method, and the homogeneity of variance test was performed by calculating Levene's statistic. One-way ANOVA was performed for multiple comparisons followed by Fisher LSD post-hoc comparisons. The Spearman rank correlation was used to validate the correlation between the syntenin level and the pathological grade of gliomas. Differences were deemed significant if p < 0.05.
Results
Syntenin expression in gliomas
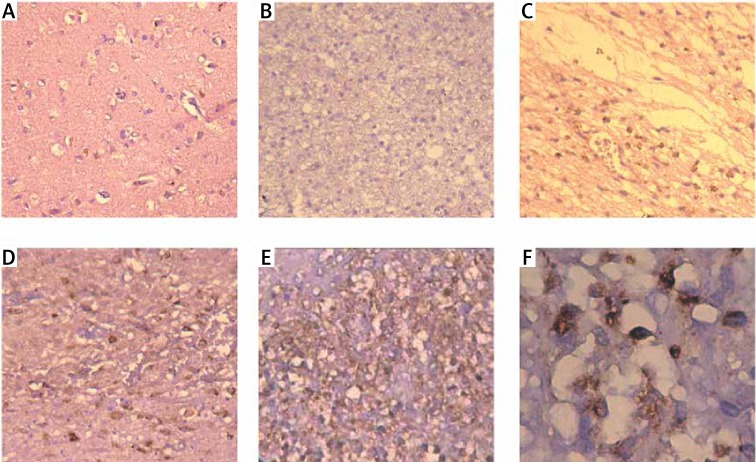
In view of the importance of syntenin in melanoma, we examined the syntenin expression in different pathological grade gliomas by immunohistochemistry. Immunostaining of syntenin showed that syntenin was located in the cytoplasm of normal and glioma cells. In normal brain tissues and grade I gliomas, the expression of syntenin was undetectable or very low (Figures 1 A and 1 B). In grade II, grade III and grade IV gliomas, there was mild to robust syntenin protein expression. As shown in Table I, there is a positive correlation between the syntenin expression and the tumor pathological grades (rs = 0.896, p < 0.05).
Figure 1.
Expression of syntenin in normal brain and glioma tissues determined by immunohistochemistry. A – Normal brain, B – grade I, C – grade II, D – grade III, E – grade IV, F – grade IV. A–E 200×, F 400×
Table I.
Expression of syntenin in different grades of gliomas
| Grade | Optical density |
|---|---|
| 0 (Normal brain) | 0.408 ±0.035 |
| I | 0.396 ±0.016 |
| II | 0.588 ±0.007 |
| III | 0.636 ±0.014 |
| IV | 0.747 ±0.027 |
Data are expressed as mean ± SD.
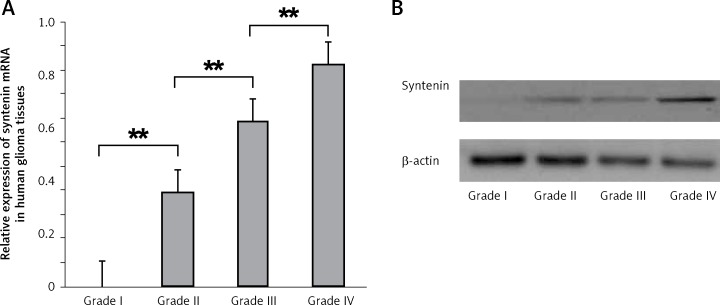
To further confirm this result, we examined the syntenin levels in gliomas of different grades by RT-PCR and western blot. The mRNA level of syntenin in gliomas is shown in Figure 2 A. Syntenin mRNA expression was significantly increased along with the rising of the grade of gliomas. Significant differences were found among the four glioma grades (grade I vs. grade II, p < 0.01; grade II vs. grade III, p < 0.01; grade III vs. grade IV, p < 0.01). In addition, western blot also showed that syntenin expression was elevated with the increase of glioma grade (Figure 2 B).
Figure 2.
Expression of syntenin mRNA and protein in glioma tissues determined by RT-PCR and western blot. A – Relative syntenin mRNA levels in different grades of glioma tissues. Total RNA was extracted from glioma tissues and semi RT-PCR was performed. Syntenin mRNA levels were calculated as the ratios of optical density of syntenin PCR products to that of the β-actin PCR product. Data are expressed as mean ± SD. B – Syntenin levels in different grades of glioma tissues determined by western blot
**p < 0.01.
Phosphorylated FAK expression in gliomas
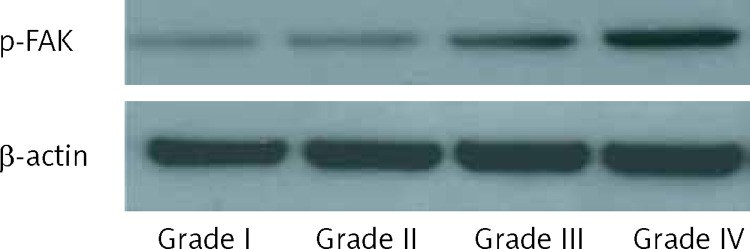
Activated FAK has been indicated as a positive modulator of cell motility and tumor migration and invasion, including gliomas [17, 20]. Therefore, we investigated the phosphorylation level of FAK in gliomas of different grades using western blot. In high-grade gliomas, the phosphorylation of FAK at Try397 was increased compared with that in low-grade gliomas (Figure 3).
Figure 3.
Phosphorylation of FAK in different grades of glioma tissues. Protein was extracted from glioma tissues and western blot was performed using antibodies against p-FAK and β-actin
Discussion
In this study, the results provide the first evidence that syntenin is expressed in human glioma tissues. The syntenin level positively correlates with the glioma grade, implicating that a high level of syntenin expression indicates higher risk of metastatic recurrence. In addition, our study also showed an increase of phosphorylated FAK in glioma tissues.
Syntenin is increasingly being studied in tumor metastasis since it may affect cell shape and promote migration and invasion [21–23]. Recent studies have confirmed that syntenin is overexpressed in melanoma, metastatic breast cancer and gastrointestinal tumors, and overexpression of syntenin is closely correlated with the tumor metastatic potential [13, 14, 24]. In the current study, we demonstrated that syntenin is present in gliomas and positively correlated with the pathological grade of gliomas, suggesting a higher metastatic possibility of high-grade gliomas. These findings have potential implications for the role of syntenin in the invasive and metastatic progression of human gliomas.
FAK plays a central role in syntenin-related cell migration and invasion pathways [14, 20, 25]. Upon integrin engagement, FAK forms a dual-kinase complex together with another factor, Src [26]. Syntenin is able to interact with c-Src and thereby lead to an increase in FAK/c-Src complex formation, c-Src activation and enhanced tumor cell invasion and metastasis [12]. In uveal melanoma cells, inhibition of syntenin expression attenuates the activation of FAK, Src and AKT, whereas its overexpression shows opposite effects [22]. In our previous study, we demonstrated that syntenin is able to induce FAK phosphorylation and thereby promote the migration of glioma cells [17]. In this study, not only syntenin but also phosphorylated FAK was elevated in glioma tissues along with the pathological grade. Since both syntenin and activation of FAK have been recognized as positive regulators of tumor cell migration and invasion [14, 27, 28], these data suggest that upregulated syntenin-FAK signaling may promote the metastasis of gliomas, especially high-grade gliomas. Although the present data only provide indirect evidence for the correlation between the presence of syntenin and metastasis of gliomas in the patients, it may also help us understand the mechanisms of glioma metastasis more completely and provide a new target in the treatment of glioma metastasis.
In conclusion, syntenin expression is increased in gliomas and positively correlates with the pathological grade of gliomas. The activated FAK in glioma tissues is also elevated along with the increase of syntenin expression and the stage of glioma progression. These results may provide a potential underlying mechanism and a therapeutic target for glioma metastasis. However, there are also some limitations of the present study. First, the number of patients included is relatively small, which limits the credibility of the study. Second, the long-term outcome of the patients and the relationship between the long-term outcome and syntenin level were not investigated. Therefore, further studies should be performed to evaluate the value of syntenin in predicting the prognosis and the probability of metastasis in post-surgery glioma patients.
Acknowledgments
This work was supported by the Natural Science Foundation of Chongqing (cstc2011JJA10091). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Nakada M, Nakada S, Demuth T, et al. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–78. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Ma L, Li J, et al. Effects of sema3g on migration and invasion of glioma cells. Oncol Rep. 2012;28:269–75. doi: 10.3892/or.2012.1796. [DOI] [PubMed] [Google Scholar]
- 3.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–28. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 4.Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 2008;233:779–91. doi: 10.3181/0711-MR-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onishi M, Ichikawa T, Kurozumi K, Date I. Angiogenesis and invasion in glioma. Brain Tumor Pathol. 2011;28:13–24. doi: 10.1007/s10014-010-0007-z. [DOI] [PubMed] [Google Scholar]
- 6.Zhai H, Acharya S, Gravanis I, et al. Annexin a2 promotes glioma cell invasion and tumor progression. J Neurosci. 2011;31:14346–60. doi: 10.1523/JNEUROSCI.3299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SK, Bhardwaj R, Wilczynska KM, et al. A complex of nuclear factor i-x3 and stat3 regulates astrocyte and glioma migration through the secreted glycoprotein ykl-40. J Biol Chem. 2011;286:39893–903. doi: 10.1074/jbc.M111.257451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Li H, Han L, et al. Expression and function of mir-27b in human glioma. Oncol Rep. 2011;26:1617–21. doi: 10.3892/or.2011.1458. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Yang L, Jin M, et al. Regulation of the invasion and metastasis of human glioma cells by polypeptide n-acetylgalactosaminyltransferase 2. Mol Med Report. 2011;4:1299–305. doi: 10.3892/mmr.2011.569. [DOI] [PubMed] [Google Scholar]
- 10.Zhao WJ, Zhang W, Li GL, et al. Differential expression of mmp-9 and aqp4 in human glioma samples. Folia Neuropathol. 2012;50:176–86. [PubMed] [Google Scholar]
- 11.Zimmermann P, Tomatis D, Rosas M, et al. Characterization of syntenin, a syndecan-binding pdz protein, as a component of cell adhesion sites and microfilaments. Mol Biol Cell. 2001;12:339–50. doi: 10.1091/mbc.12.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boukerche H, Su ZZ, Prevot C, et al. Mda-9/syntenin promotes metastasis in human melanoma cells by activating c-src. Proc Natl Acad Sci U S A. 2008;105:15914–9. doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo TH, Lee JJ, Kim EM, et al. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene. 2002;21:4080–8. doi: 10.1038/sj.onc.1205514. [DOI] [PubMed] [Google Scholar]
- 14.Boukerche H, Su ZZ, Emdad L, et al. Mda-9/syntenin: a positive regulator of melanoma metastasis. Cancer Res. 2005;65:10901–11. doi: 10.1158/0008-5472.CAN-05-1614. [DOI] [PubMed] [Google Scholar]
- 15.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 16.Hwangbo C, Kim J, Lee JJ, Lee JH. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase calpha and the pdz adapter protein mda-9/syntenin. Cancer Res. 2010;70:1645–55. doi: 10.1158/0008-5472.CAN-09-2447. [DOI] [PubMed] [Google Scholar]
- 17.Zhong D, Ran JH, Tang WY, et al. Mda-9/syntenin promotes human brain glioma migration through focal adhesion kinase (fak)-jnk and fak-akt signaling. Asian Pac J Cancer Prev. 2012;13:2897–901. doi: 10.7314/apjcp.2012.13.6.2897. [DOI] [PubMed] [Google Scholar]
- 18.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 who classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binbin C, Yinglu F, Juan D, Changquan L. Upregulation effect of ginsenosides on glucocorticoid receptor in rat liver. Horm Metab Res. 2009;41:531–6. doi: 10.1055/s-0029-1216373. [DOI] [PubMed] [Google Scholar]
- 20.Lawson C, Lim ST, Uryu S, et al. Fak promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–32. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das SK, Bhutia SK, Sokhi UK, et al. Raf kinase inhibitor rkip inhibits mda-9/syntenin-mediated metastasis in melanoma. Cancer Res. 2012;72:6217–26. doi: 10.1158/0008-5472.CAN-12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangemi R, Mirisola V, Barisione G, et al. Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PLoS One. 2012;7:e29989. doi: 10.1371/journal.pone.0029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das SK, Bhutia SK, Kegelman TP, et al. Mda-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci. 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 24.Helmke BM, Polychronidis M, Benner A, et al. Melanoma metastasis is associated with enhanced expression of the syntenin gene. Oncol Rep. 2004;12:221–8. [PubMed] [Google Scholar]
- 25.Kwiatkowska A, Kijewska M, Lipko M, et al. Downregulation of akt and fak phosphorylation reduces invasion of glioblastoma cells by impairment of mt1-mmp shuttling to lamellipodia and downregulates mmps expression. Biochim Biophys Acta. 2011;1813:655–67. doi: 10.1016/j.bbamcr.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with src and fyn. J Cell Sci. 1996;109:1787–94. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 27.Megison ML, Stewart JE, Nabers HC, et al. Fak inhibition decreases cell invasion, migration and metastasis in mycn amplified neuroblastoma. Clin Exp Metastasis. 2013;30:555–68. doi: 10.1007/s10585-012-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Wang B, Li W, et al. Intersectin1-s is involved in migration and invasion of human glioma cells. J Neurosci Res. 2011;89:1079–90. doi: 10.1002/jnr.22616. [DOI] [PubMed] [Google Scholar]