Abstract
Behçet's disease (BD) is a multiorgan inflammatory disease of complex and not entirely elucidated etiology, which was originally diagnosed in patients with aphthous stomatitis, genital ulcerations and ocular manifestations. The entity is endemic in countries of Eastern and Central Asia, especially Turkey and Iran, but rarely seen in Central Europe. As there are no specific diagnostic laboratory tests or histopathologic findings which confirm the preliminary diagnosis, the final diagnosis should be based on clinical criteria. Frequently a definitive diagnosis is established within several years or months after the first manifestations appear. The increased number of cases, recently described worldwide also in the Polish population, indicates that the disease could spread out of endemic areas. The aim of this manuscript is to present the clinical picture, diagnosis criteria and therapeutic approaches of this “international disease” which currently is observed not only in emigrants from Asia but also in native Polish citizens.
Keywords: Behçet's disease, classification, blood vessel inflammation
Introduction
Behçet's disease (BD) is a multiorgan inflammatory disease of complex and not entirely elucidated etiology, which originally was diagnosed in patients with aphthous stomatitis, genital ulcerations and ocular manifestations. However, the disease was first described by Hippocrates in the 5th century BC. It bears the name of its modern explorers: Benedictos Adamantiades (a Greek ophthalmologist) and Hulusi Behçet (a Turkish dermatologist) [1]. Although BD can occur worldwide, it is most prevalent in the region along the “Silk Road”, an ancient trading route between the Mediterranean and East Asia, where it is a major cause of morbidity. The entity is predominantly diagnosed in the Far and Middle East countries, mostly in Turkey, where its incidence is evaluated at 380/100,000 cases in the population. The prevalence of BD in Iran is 68/100,000, which is the second highest prevalence in the world [2]. The highest frequency of BD in the Western world is in the USA, where the prevalence is 8,6/100,000 [3]. Behçet's disease is definitely less frequently diagnosed in Europe: 0.64/100,000 for the United Kingdom, 1.2/100,000 for Sweden, 1.5/100,000 for Portugal, 3.7/100,000 for Italy, 5.6/100,000 for Spain and 7.2/100,000 for France. The prevalence is low for Germans as expected (1.47/100,000), whereas it is significantly higher among immigrant Turks living in Berlin (77/100,000) [4]. Numerous scientific reports suggest that full manifestation of symptoms occurs in genetically predisposed people, in whom infectious, probably viral agents, contribute to the dysregulation of the immune response, and finally to the development of inflammatory changes in the blood vessels.
Pathophysiology
Behçet disease is a rare disease, but familial occurrence is well known. Carriers of HLA-B51/HLA-B5 are at increased risk of developing BD compared with non-carriers. HLA-B51 was shown to be more prevalent in Turkish, Middle Eastern, and Japanese populations, corresponding with higher prevalence of BD in these populations. However, HLA-B51 was not shown to correlate with the severity of symptoms. A meta-analysis of 4,800 cases and 16,289 controls indicated that the risk (“odds”) of HLA–B51/B5 carriers developing BD is increased by a factor of 5.78, and by a factor of 5.90 when considering only the split antigen HLA–B51. However, the pooled rates of HLA–B51/B5–positive BD cases varied across geographic locations, but the relative risk increases associated with this allele appeared to be fairly equal for different ethnic populations. The pooled OR of HLA-B51/B5 allele carriers to develop BD compared with noncarriers was 5.78 (95%). Authors conclude that the strength of the association between BD and HLA-B51/B5 and its consistency across populations of various ethnicities support the thesis that this allele is a primary and causal risk determinant for BD [5].
Controversially, epidemiological data seem to indicate that not only genetic but also environmental factors might affect the occurrence of the disease. Less frequent occurrence of BD among Turkish immigrants living in Berlin when compared with residents of Turkey, as well as among Japanese people living outside of Japan in comparison to the native citizens of Japan, indicates the role of an unknown environmental factor in the pathogenesis of the disease [6].
Currently, all scientific theories on BD indicate its autoimmune etiology. However, the attempts at determining whether tissue antigens play a role in channeling the immune response have been unsuccessful so far. Elevated peripheral levels of γδ T cells (γδ+ T cells) in patients with BD in response to exposure to mycobacterial heat shock proteins (HSPs) compared with those found in healthy subjects imply an influence on the disease pathogenesis [7]. HSP-65, found in high concentrations in oral ulcers and active skin lesions in patients with BD, was also demonstrated to stimulate production of antibodies that exhibit cross-reactivity with streptococcal species present in the mouth [8].
Although the cause of BD remains unknown, epidemiologic findings suggest that the autoimmune process is triggered by an infectious or environmental agent, specific for the geographic region. However, there is no information supporting the hypothetical role of a single microorganism as a specific cause of autoimmune dysregulation. Herpes type viruses (herpes virus type 1), hepatitis C virus, parvovirus B19, Cytomegalovirus, and Epstein-Barr virus are considered to be viral factors that can induce development of the disease [9]. Bacterial agents which may be involved in the pathogenesis of the disease include Streptococcus sanguis, S. oralis, S. pyogenes, S. faecalis, S. salivarius, Staphylococcus aureus, Saccharomyces cerevisiae, Escherichia coli, Klebsiella pneumoniae, Mycoplasma fermentans, Helicobacter pylori, and Borrelia burgdorferi [9]. The infectious model is supported by the observation that development of oral ulcers precedes full manifestation of the disease by months or years and disease relapses are frequent. Some clinical observations indicate that unhygienic oral conditions such as periodontitis, decayed teeth, and chronic tonsillitis are common in BD patients [10]. An infectious agent could operate through molecular mimicry. A mimicked interaction or false signal could attract the inflammatory cells into the field of action, resulting in vasculitis. The disease could be provoked by an abnormal immune response to an autoantigen in the absence of an ongoing infection [11].
Classification
Manifestations of BD are not consistent among patients, and many authors underline that the clinical phenotype is dissimilar. Symptoms of the disease do not occur simultaneously and usually are heterogeneous and variable due to ethnic, geographical, and individual differences. As there are no specific diagnostic laboratory tests or histopathology findings which confirm a preliminary diagnosis, the final diagnosis must be based on clinical criteria. Very often it takes several years or months to establish a definitive diagnosis, after the appearance of the first manifestations. Diagnosing BD is especially difficult in non-endemic regions, such as Poland.
Although BD is a relatively new entity, first being described in 1937, numerous sets of classification criteria have been published. The most important and popular criteria were created in 1990 by the International Study Group (ISG) (Table I) [12]. The ISG group compared the clinical findings of 914 patients with a history of aphthous ulcers with controls. The ISG criteria comprise 5 items, two of which are mucous membrane manifestations, namely, oral aphthosis and genital aphthosis, and the third one is the presence of skin manifestations such as pseudofolliculitis and/or erythema nodosum like lesion. The fourth item is the presence of ocular manifestations including anterior uveitis, posterior uveitis or retinal vasculitis. The fifth item is the presence of pathergy phenomena. In the ISG criteria, the presence of oral aphthosis is obligatory, and two out of the four remaining items are necessary to establish the diagnosis.
Table I.
Diagnostic criteria of Behçet disease defined by the International Study Group (ISG)
| Criterion | Description |
|---|---|
| Recurrent oral ulceration | Minor aphthous, major aphthous, or herpetiform ulceration observed by physician or patient that recurred at least 3 times in one 12-month period |
| Recurrent genital ulceration | Aphthous ulceration or scarring observed by physician or patient |
| Eye lesions | Anterior uveitis, posterior uveitis, cells in vitreous on slit lamp examination, or retinal vasculitis observed by ophthalmologist |
| Cutaneous lesions | Erythema nodosum, pseudofolliculitis, papulopustular lesions, or acneiform nodules observed by physician in postadolescent patient not receiving corticosteroid treatment |
| Positive pathergy test | Read by physician at 24 to 48 h |
Sensitivity of the ISG criteria is low. Therefore, new International Criteria for Behcet's Disease (ICBD) were established, and were presented at the International Conference of Behcet's Disease in Lisbon in 2006 for the first time [13]. In the ICBD criteria vascular manifestations were added to the 5 items of the ISG criteria. Vascular manifestations were defined as superficial phlebitis, deep vein thrombosis, large vein thrombosis, arterial thrombosis, and aneurysm. In the ICBD criteria various clinical manifestations have different diagnostic value. Genital aphthous lesions and eye lesions are scored as 2, but oral aphthous lesions, skin and vascular manifestations and pathergy phenomenon are scored as 1. A diagnostic score for BD diagnosis is at least 3.
A new set of criteria (ICBD) was proposed not only to unify the previous criteria but also to establish best accuracy, along with an optimum sensitivity and specificity. Studies performed by di Meo et al. revealed that ICBD criteria exhibited higher sensitivity, specificity and accuracy than ISG criteria. According to the ICBD criteria, oral aphthosis is no longer a mandatory diagnostic clinical manifestation of BD, so that in the future, the diagnosis of BD without oral aphthous ulcers can be established [14]. Comparison of the two classification systems presented above revealed that the ICBD are more appropriate for diagnoses in the regions outside of the Silk Road, especially in countries with different ethnicities [13].
However, classification is a dynamic process. This year the International Team for the Revision of the International Criteria for BD (from 27 countries) submitted data from 2556 clinically diagnosed BD patients and 1163 controls with BD-mimicking diseases or presenting at least one major BD sign. In these new criteria, ocular lesions, oral aphthosis and genital aphthosis are each assigned 2 points, while skin lesions, central nervous system involvement and vascular manifestations are assigned 1 point each. The pathergy test, when used, was assigned 1 point. A patient scoring ≥ 4 points is classified as having BD (Table II). All authors claim that the new proposed criteria, derived from multinational data, exhibit much improved sensitivity over the ISG criteria while maintaining reasonable specificity [15].
Table II.
New International Criteria for Behcet's Disease – scoring ≥ 4 points indicates Behcet's diagnosis
| Sign/symptom | Points |
|---|---|
| Ocular lesions | 2 |
| Genital aphthosis | 2 |
| Oral aphthosis | 2 |
| Skin lesions | 1 |
| Neurological manifestations | 1 |
| Vascular manifestations | 1 |
| Positive pathergy test | 1 |
Site-specific manifestations
Organ-specific manifestations are characterized by exacerbations and relapsing course.
Oral aphthae
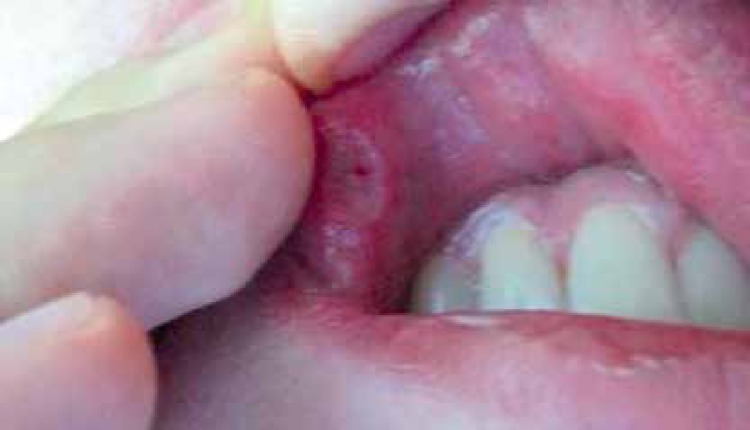
It is generally accepted that this multiorgan disease (BD) often begins with mucocutaneous involvement and oral aphthous ulceration (Figure 1), and ulcers are usually the first manifestation, which precede the onset of other manifestations by many years. The lesions may be single or multiple and frequently develop after local trauma, infection or dental intervention. To be classified as a symptoms of BD they have to relapse at least 3 times a year. The ulcers are normally covered with grayish-white pseudomembrane or present a necrotic base and have a sharp erythematous border. The ulcers are very painful. Small ulcers heal without scarring within several days. Large ulcerations cause scarring and are associated with problems such as dysphagia, dyspnea or fistulae involving the pharynx, larynx, trachea or esophagus [16]. In the ISG criteria recurrent oral aphthosis is an obligatory parameter, so in many analyses patients with BD without oral ulcers are excluded [17]. But in studies based on other classifications, oral aphthae are estimated to occur in about 98% of cases.
Figure 1.
Circular ulcer located on the oral mucosa
Genital aphthosis
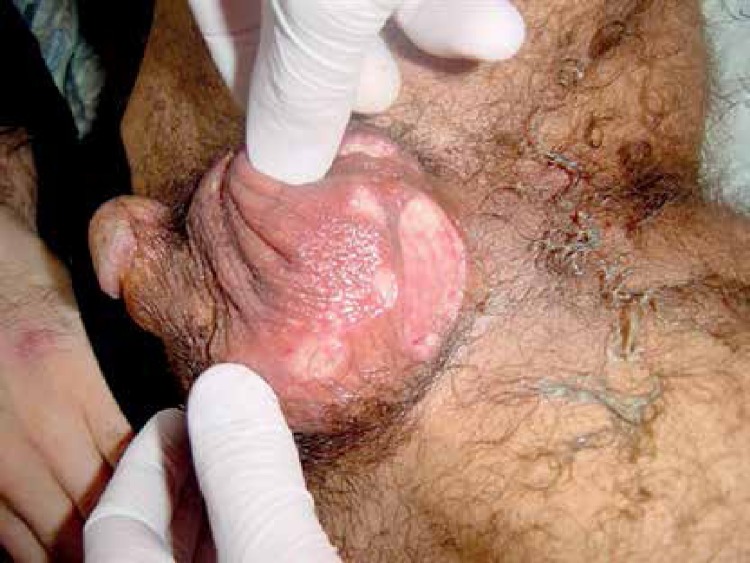
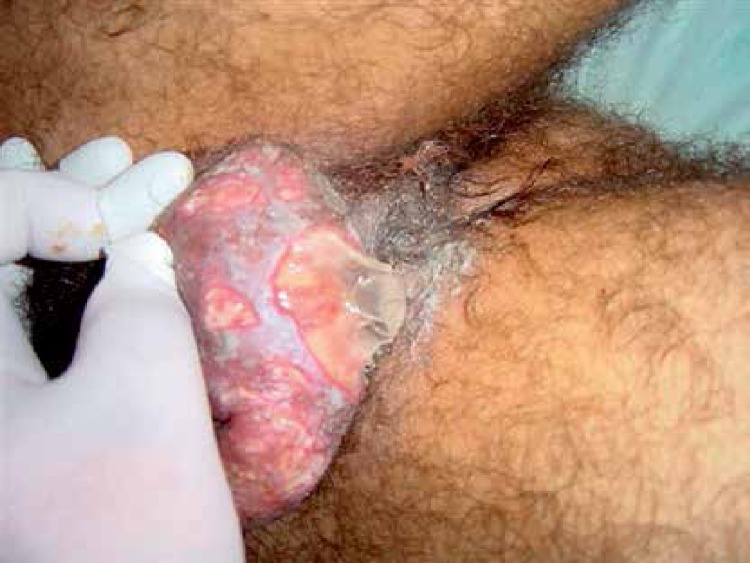
Genital aphthoses are regarded as the most frequent second symptom in the course of the disease. They appear as punched-out, painful lesions with fibrinous exudate on their base (Figures 2, 3). In males, scrotal involvement is most characteristic, but the penile shaft may also be involved. In females, the labial area is most commonly involved, but occasionally the lesions develop in the vagina and on the perineum. Genital ulcerations typically heal with scarring and are more painful in men. Presence of ulcerations in women may correlate with menstruation. Such lesions frequently negatively influence patients’ sexual functioning and psychological state [18]. The term genital ulcers is also described as genital aphthosis. Genital aphthosis frequently occurs in BD and is very suggestive of the diagnosis. According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, genital aphthoses were diagnosed respectively in 65%, 73%, 76%, 83%, and 64% [19].
Figure 2.
Genital ulcers with deeply indurated margins
Figure 3.
Genital ulcers covered with grayish-white fibrinous exudate
Ocular manifestations
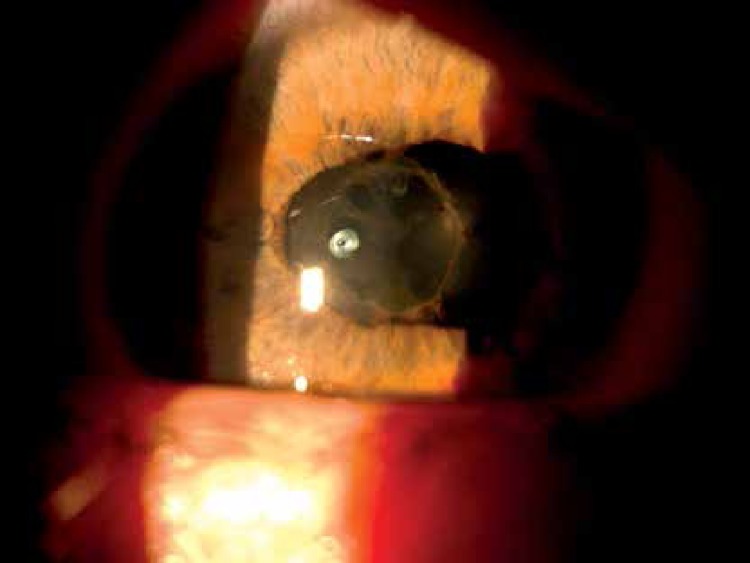
Ocular involvement is probably the most frequent extramucocutaneous manifestation (28–80%) and an important cause of morbidity. Such a great discrepancy is related to the type of center the figures come from (ophthalmology or other). According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, ocular manifestations were diagnosed respectively in 55%, 69%, 35%, 51%, and 53% [19]. This localization is more frequent in males than in females, and males usually tend to have a worse visual prognosis. Ocular involvement in BD comprises a wide spectrum of symptoms. In most cases, the ocular symptoms follow the oral and genital ulcers by 3–4 years. However, ocular disease is the initial manifestation in about 20% of cases [20]. Initially, the symptoms include periorbital pain, redness, photophobia, and blurred vision. Behçet disease is characterized by severe recurrent attacks of intraocular inflammation, i.e. episcleritis, scleritis, keratitis and anterior uveitis, posterior uveitis or panuveitis. Vitritis with large exudates due to vasculitis can lead to macular edema. A combination of optic neuritis followed by optic atrophy, exudates and retinal detachment is the cause of visual loss in some cases (Figures 4, 5). As a consequence of intermediate uveitis, a posterior capsular cataract can occur [21].
Figure 4.
Band exudates within the base of the vitreous body
Figure 5.
Posterior synechia, ovalization of the pupil and pigment on the lens
Cutaneous lesions
Skin manifestations are regarded as major manifestations of the disease. Most often papulo-pustular changes are observed. They form elevated erythema with a dome-shaped sterile pustule in the center. Erythema nodosum-like lesions occur less frequently. They are typically localized on the extremities, especially the lower legs, but they can also be situated on the face, neck, and buttocks. The lesions are painful and resolve spontaneously, although some may ulcerate or leave hyperpigmentation. A folliculitis-like rash, resembling acne vulgaris, appears not only on the face, but also on the neck, chest, back, and hairline of patients [6]. According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, pseudofolliculitis was diagnosed respectively in 57%, N/A, 31%, N/A, and 62% but erythema nodosum in 22%, N/A, 38%, N/A, and 42% [19]. Skin aphthosis is not frequent but it is the most characteristic lesion of BD [22].
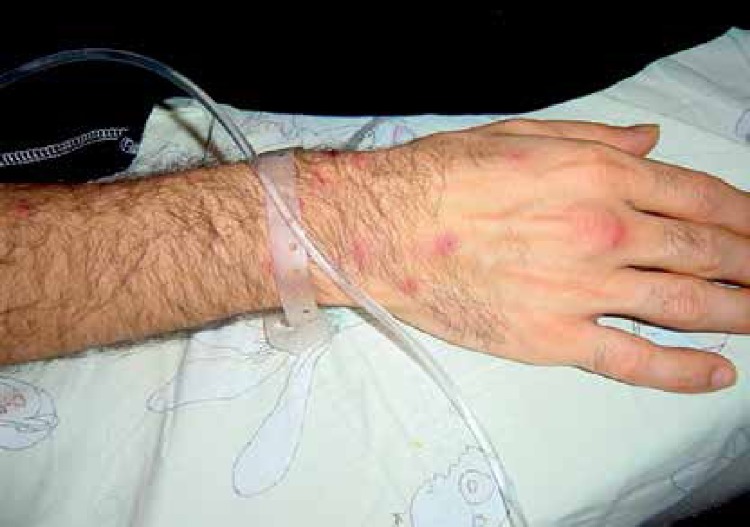
Pathergy test
A positive pathergy test is regarded as the only currently existing diagnostic test for BD. It is an important component of many of the 16 sets of classification criteria used to diagnose BD [23]. Pathergy is a cutaneous phenomenon which can be observed not only in BD but also in pyoderma gangrenosum, so a positive pathergy test is not of course synonymous with DB. In this condition a minor trauma such as an injection or surgical incisions leads to the development of skin lesions or ulcers that may be resistant to healing. Physicians looking toward a diagnosis of BD may attempt to induce a pathergy reaction with a test known as a “skin prick test”, in which they prick the skin with a small needle. After 1 to 2 days in many patients with BD a red bump may develop (Figure 6). The pathergy reaction is a unique feature of BD and, according to the ISG and also ICBD, is among the major criteria required for the diagnosis. A positive pathergy test helps to confirm the BD diagnosis, but lack of reaction does not exclude it. Presence of a positive pathergy reaction is not associated with the specific course of the disease or increased risk for specific mucocutaneous or systemic manifestations. According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, the pathergy test was positive respectively in 54%, 44%, N/A, 40%, and 34% of patients with BD [19].
Figure 6.
A positive pathergy test which manifests within 48 h as an erythematous papule at the site of a skin needle prick
Vascular manifestations
Presence of a vascular manifestation, such as superficial phlebitis, deep vein thrombosis, large vein thrombosis, arterial thrombosis or aneurysm, is regarded as a frequent and characteristic feature of BD, and was included in many diagnostic criteria before the advent of the ISG. Vascular involvement is important because of severe morbidity and increased mortality. Most of the vascular events occurred within the first 5 years of disease onset [24]. Vasculitis of small and large vessels can cause a variety of symptoms depending on the location of the lesions. Arterial disease predominantly affects males and rarely occurs in women [25]. Venous involvement, presented as superficial or deep thrombophlebitis, is more common than arterial involvement. The most frequent sites of thrombosis are lower extremity veins [24]. Patients with BD and thrombosis are significantly younger than patients with thrombosis but without BD [24]. Studies performed in the last two decades confirmed that chronic and multisystemic vasculitis play a key role in BD pathogenesis, as confirmed by immunoreactant deposits on the vessel walls and elevated serum levels of several cytokines, such as IL-1, IL-4, IL-6 and TNF-α, which are responsible for the inflammatory reaction [26]. Zhou et al. underline that the cytokine network plays a substantial role not only in the pathogenesis of the disease but also in specific organ damage [27]. According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, vascular involvement was diagnosed respectively in 8.9%, 8.9%, 7.7%, 1.8%, and 13% of patients with BD [19].
Neurological involvement
Neurological involvement is one of the most serious causes of long-term morbidity and mortality in BD. Neurological manifestations usually present with severe headache. Two types of neurological involvement are distinguished: parenchymal, which is more common, and nonparenchymal. Parenchymal brain disease mainly affects the brainstem and/or basal ganglia, but spinal cord lesions and hemisphere lesions may also occur. The classic manifestation is a meningoencephalitis [28]. Nonparenchymal involvement includes dural sinus thrombosis, arterial vasculitis, and aseptic meningitis [29]. Cerebral abscesses are rare, serious, and life-threatening complications in BD. Although there are various hypotheses regarding the cause of abscesses, including bacterial, viral or fungal infections, no microbial agent has been confirmed [30]. According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, neurological manifestations were diagnosed respectively in 9%, 11%, 6.5%, 4.6%, and 11% of patients with BD [19].
Gastrointestinal involvement
Literature data indicate that the frequency of gastrointestinal system involvement is higher in Japan and Korea than in the Middle East and the Mediterranean region [28]. Manifestations may occur throughout the whole gastrointestinal tract, from the esophagus to the anus, but ulcers are most common in the terminal ileum. Patients suffer from abdominal pain, vomiting, diarrhea, bloating or bleeding. According to nationwide surveys performed in Iran, Japan, China, Korea and Germany, vascular involvement was diagnosed respectively in 7%, 15.5%, 9%, 7.3%, and 12% of patients with BD [19].
Articular involvement
Among other organs, arthritis and arthralgia are very common occurrences and according to nationwide surveys performed in Iran, Japan, China, Korea and Germany were diagnosed respectively in 33%, 57%, 30%, 38%, and 53% of patients with BD [19]. Mainly the lower extremities, especially the knees, are affected, but the ankles, wrists and elbows can also be involved. Joint disease is usually not very severe, transient, nonerosive, and nondeforming [28].
Others
Pulmonary (vasculitis, embolism, fibrosis, pleurisy, and infection), cardiac (myocarditis, valvular lesions, pericarditis, ventricular aneurysms, intracardiac thrombosis, coronary vasculitis) and renal (hematuria, leucocyturia, casts) involvement occurs relatively rarely [28].
Treatment
Current treatment of BD involves symptomatic relief with prevention of relapse. Treatment is largely empirical, since well-controlled studies are difficult to conduct due to the heterogeneity of the disease, and its unpredictable course with exacerbations and remissions.
Topical treatment is useful for treating mucocutaneous lesions, mainly oral and genital ulcers. A combination of local anesthetic (3% lidocaine gel) and local antiseptics, such as 0.1–0.2% chlorhexidine solution, is the predominant method. Antibiotics, especially tetracycline, have been used in oral ulcers for years. Tetracycline or minocycline mouthwashes decrease pain severity and duration of oral ulcers [9]. Isolated lesions can be treated with local chemical cautery by daubing them with silver nitrate solution (1–2%) or hydrogen peroxide solution (0.5%). It is recommended not to eat hard (nuts) and spicy foods (pepper, paprika), fruit juices, citrus fruits, alcoholic or carbonated beverages, and products containing sodium lauryl sulfate [31].
Conventional systemic therapeutic approaches include corticosteroids, as a cornerstone of the anti-inflammatory agents, with or without other immunosuppressive drugs, such as cyclophosphamide, azathioprine, sulfasalazine, colchicine, methotrexate or cyclosporine A. Increased mortality is observed in the case of arterial involvement, and due to cumulative episodes of neurological involvement [32]. When steroids are used, the dose can be slowly reduced, with caution, after 4 weeks. Relapses are frequently seen after discontinuation of steroids. Immunosuppressive drugs, due to their delayed action, are prescribed initially in association with corticosteroids. They are prescribed in cases of severe organ involvement (i.e. posterior uveitis, CNS involvement, vascular involvement) [32]. Azathioprine (2.5 mg/kg/day) is a purine synthesis inhibitor which is commonly prescribed as a first- and second-line disease-modifying therapy for patients with oral or genital ulcers, skin disease or posterior uveitis [33]. Azathioprine had a beneficial effect on long-term visual outcome [34]. Cyclophosphamide orally (2 mg/kg/day) or intravenously (750 mg/m2 to 1 g/m2 every 4 weeks) was proved to be effective in BD [35]. It is a beneficial therapeutic agent for eye disease and systemic vasculitis (neurologic involvement and arterial aneurysms) [36]. In the case of multiple organ Behcet's disease, chlorambucil may also be used. Methotrexate (7.5–20 mg/1× week p.o. over 4 weeks) was reported to induce improvement of severe mucocutaneous and neurological involvement [37]. Ciclosporin (5 mg/kg/day) was found to have a beneficial effect on the ocular manifestations of Behcet's syndrome [38], but secondary nephropathy, hypertension, and hyperuricemia limit its usage. Colchicine (1–2 mg/day) is widely used for the first-line treatment for mucocutaneous manifestations. Colchicine reduced the occurrence of orogenital aphthae, pseudofollicular lesions, erythema nodosum and arthritis [39]. Thalidomide (100–300 mg/day) is a drug that selectively inhibits TNF-α synthesis. It reduced the occurrence of genital ulcers, oral ulcers and papulopustular lesions [40]. Mycophenolate mofetil (MMF) was found to be safe and effective in controlling cystoid macular edema and in reducing the relapse rate of uveitis in patients not responding to traditional immunosuppressants [41].
Nowadays new insights into BD immunopathogenesis provide novel therapeutic approaches. Clinical and laboratory observations proved the important role of TNF-α. During the last 10 years, TNF-α antagonist drugs such as infliximab, adalimumab and etanercept have been successfully used by patients with BD [42].
Plasmapheresis and intravenous immunoglobulins were efficient in anecdotal reports. Colchicine (1–2 mg/day) has beneficial effects on the mucocutaneous symptoms, decreasing the number, size and recurrence of aphthae [32].
The growing number of cases in the Polish population [43, 44] creates the need for refreshing the clinical picture, diagnostic criteria and therapeutic approaches of this rare disease, which has spread out of endemic areas.
Acknowledgments
This study was supported by a grant for scientific purposes (No. 503/1-152-01/503-01) from the Medical University of Lodz, Poland.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ustun C. A famous Turkish dermatologist, Dr Hulusi Behcet. Eur J Dermatol. 2002;12:469–70. [PubMed] [Google Scholar]
- 2.Davatchi F, Jamshidi AR, Banihashemi AT, et al. Prevalence of Behcet's disease in Iran: a WHO-ILAR COPCORD stage I study. APLAR J Rheumatol. 2007;10:239–43. [Google Scholar]
- 3.Kahl L. The Washington Manual of Rheumatology Subspecialty Consult. In: Gonzalez-Mayda MC, Atkinson JP, editors. Behcet's disease. Washington: Lippincott Williams & Wilkins; 2012. pp. 275–82. [Google Scholar]
- 4.Mahr A, Belarbi L, Wechsler B, et al. Population-based prevalence study of Behçet's disease: differences by ethnic origin and low variation by age at immigration. Arthritis Rheum. 2008;58:3951–9. doi: 10.1002/art.24149. [DOI] [PubMed] [Google Scholar]
- 5.de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behçet's disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61:1287–96. doi: 10.1002/art.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altenburg A, Papoutsis N, Orawa H, Martus P, Krause L, Zouboulis CC. Epidemiology and clinical manifestations of Adamantiades-Behcet disease in Germany – current pathogenetic concepts and therapeutic possibilities. J Dtsch Dermatol Ges. 2006;4:49–64. doi: 10.1111/j.1610-0387.2006.05841.x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Hoshi K, Matsuda T, et al. Increased peripheral blood gamma delta+ T cells and natural killer cells in Behçet's disease. J Rheumatol. 1992;19:588–92. [PubMed] [Google Scholar]
- 8.Kaneko S, Suzuki N, Yamashita N, et al. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behcet's disease (BD) in Japan. Clin Exp Immunol. 1997;108:204–12. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galeone M, Colucci R, D'Erme AM, Moretti S, Lotti T. Potential infectious etiology of Behcet's disease. Patholog Res Int. 2012;2012:595380. doi: 10.1155/2012/595380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mumcu G, Inanc N, Yavuz S, Direskeneli H. The role of infectious agents in the pathogenesis, clinical manifestations and treatment strategies in Behçet's disease. Clin Exp Rheumatol. 2007;25:S27–33. [PubMed] [Google Scholar]
- 11.Yurdakui S, Yazici H. Behçet's syndrome. Best practice and research. Clin Rheumatol. 2008;22:793–809. doi: 10.1016/j.berh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.International Study Group for Behcet's Disease. Criteria for diagnosis of Behcet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 13.Davatchi F. Diagnosis/Classification criteria for Behçet disease. Pathol Res Int. 2012;2012:607921. doi: 10.1155/2012/607921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Meo N, Bergamo S, Vidimari P, Bonin S, Trevisan G. Analysis of diagnostic criteria in Adamiantiades-Behçet disease: a retrospective study. Indian J Dermatol. 2013;58:275–7. doi: 10.4103/0019-5154.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davatchi F, Assaad-Khalil S, Calamia KT, et al. The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Derm Venereol. 2014;28:338–47. doi: 10.1111/jdv.12107. [DOI] [PubMed] [Google Scholar]
- 16.Altenburg A, Mahr A, Maldini C, et al. Epidemiology and clinical aspects of Adamantiades-Behçet disease in Germany. Current data. Ophthamologe. 2012;109:531–41. doi: 10.1007/s00347-012-2601-4. [DOI] [PubMed] [Google Scholar]
- 17.Liang MW, Neoh CY. Oral aphthosis: management gaps and recent advances. Ann Acad Med Singapore. 2012;41:463–70. [PubMed] [Google Scholar]
- 18.Hiz O, Ediz L, Gulcu E, Tekeoglu I. Effects of Behçet's disease on sexual function and psychological status of male patients. J Sex Med. 2011;8:1426–33. doi: 10.1111/j.1743-6109.2010.02040.x. [DOI] [PubMed] [Google Scholar]
- 19.Davatchi F, Shahram F, Chams-Davatchi C, et al. Behcet's disease: from East to West. Clin Rheumatol. 2010;29:823–33. doi: 10.1007/s10067-010-1430-6. [DOI] [PubMed] [Google Scholar]
- 20.Tugal-Tutkun I, Gupta V, Cunningham ET. Differential diagnosis of Behçet uveitis. Ocul Immunol Inflamm. 2013;21:337–50. doi: 10.3109/09273948.2013.795228. [DOI] [PubMed] [Google Scholar]
- 21.Kacmaz RO, Kempen JH, Newcomb C, et al. Ocular inflammation in Behçet disease: incidence of ocular complications and of loss of visual acuity. Am J Ophthalmol. 2008;146:828–36. doi: 10.1016/j.ajo.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davatchi F, Shahram F, Chams C, Chams H, Nadji A. Behçet's disease. Acta Med Iran. 2005;43:233–42. [Google Scholar]
- 23.Davatchi F, Sadeghi Abdollahi B, et al. Impact of the positive pathergy test on the performance of classification/diagnosis criteria for Behcet's disease. Mod Rheumatol. 2013;23:125–32. doi: 10.1007/s10165-012-0626-9. [DOI] [PubMed] [Google Scholar]
- 24.Erer D, Gogus F, Tumer NB, et al. Prevalence of Behçet syndrome in patients presenting with venous thrombosis: prospective study in cardiovascular outpatient clinic. Arch Med Sci. 2009;5:371–5. [Google Scholar]
- 25.Calamia KT, Schirmer M, Melikoglu M. Major vessel involvement in Behçet's disease. Curr Opin Rheumatol. 2005;17:1–8. doi: 10.1097/01.bor.0000145520.76348.dd. [DOI] [PubMed] [Google Scholar]
- 26.Kulaber A, Tugal-Tutkun I, Yentür SP, et al. Pro-inflammatory cellular immune response in Behçet's disease. Rheum Int. 2007;12:1113–8. doi: 10.1007/s00296-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhou ZY, Chen SL, Shen N, Lu Y. Cytokines and Behcet's disease. Autoimmun Rev. 2012;11:699–704. doi: 10.1016/j.autrev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behcet's disease. Patholog Res Int. 2012;2012:690390. doi: 10.1155/2012/690390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akman-Demir G, Serdaroglu P, Tasci B, et al. Clinical patterns of neurological involvement in Behçet's disease: evaluation of 200 patients. Brain. 1999;122:2171–81. doi: 10.1093/brain/122.11.2171. [DOI] [PubMed] [Google Scholar]
- 30.Togoz S, Ogmegul A, Mutluer M, Kivrak AS, Ustun ME. Cerebral abscesses in Behçet's disease. Turk Neurosurg. 2012;22:116–8. doi: 10.5137/1019-5149.JTN.3297-10.2. [DOI] [PubMed] [Google Scholar]
- 31.Zouboulis CC. Adamantiades-Behçet's disease. In: Katsambas AD, Lotti TM, editors. European Handbook of Dermatological Treatments. Berlin, Heidelberg: Springer; 2003. pp. 16–26. [Google Scholar]
- 32.Saadoun D, Wechsler B. Behçet disease. Orphanet J Rare Dis. 2012;7:20. doi: 10.1186/1750-1172-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalvi SR, Yildirim R, Yazici Y. Behcet's syndrome. Drugs. 2012;72:2223–41. doi: 10.2165/11641370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Hamuryudan V, Ozyazgan Y, Hizli N, et al. Azathioprine in Behcet's syndrome: effects on long-term prognosis. Arthritis Rheum. 1997;40:769–74. doi: 10.1002/art.1780400425. [DOI] [PubMed] [Google Scholar]
- 35.Yazici H, Pazarli H, Barnes CG, et al. A controlled trial of azathioprine in Behcet's syndrome. N Engl J Med. 1990;322:281–5. doi: 10.1056/NEJM199002013220501. [DOI] [PubMed] [Google Scholar]
- 36.Davatchi F, Shahram F, Chams H, Akbarian M. Pulse cyclophosphamide (PCP) for ocular lesions of Behcet's disease: double blind crossover study. Arthritis Rheum. 1999;42:320. [Google Scholar]
- 37.Kikuchi H, Aramaki K, Hirohata S. Low dose MTX for progressive neuro-Behcet's disease. A follow-up study for 4 years. Adv Exp Med Biol. 2003;528:575–8. doi: 10.1007/0-306-48382-3_117. [DOI] [PubMed] [Google Scholar]
- 38.Hatemi G, Silman A, Bang D, et al. EULAR recommendations for the management of Behcet disease. Ann Rheum Dis. 2008;67:1656–62. doi: 10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 39.Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, et al. Colchicine versus placebo in Behcet's disease: randomized, double-blind, controlled crossover trial. Mod Rheumatol. 2009;19:542–9. doi: 10.1007/s10165-009-0200-2. [DOI] [PubMed] [Google Scholar]
- 40.Hamuryudan V, Mat C, Saip S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behcet's syndrome: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:443–50. doi: 10.7326/0003-4819-128-6-199803150-00004. [DOI] [PubMed] [Google Scholar]
- 41.Neri P, Mariotti C, Cimino L, Mercanti L, Giovannini A. Long-term control of cystoid macular oedema in noninfectious uveitis with mycophenolate mofetil. Int Ophthalmol. 2009;29:127–33. doi: 10.1007/s10792-008-9200-z. [DOI] [PubMed] [Google Scholar]
- 42.Kose O. Development of immunopathogenesis strategies to treat Behcet's disease. Patholog Res Int. 2012;2012:261989. doi: 10.1155/2012/261989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niedzielska A, Chelminska K, Jaremin B. Behcet's disease – diagnostic difficulties [Polish] Pol Arch Med Wewn. 2007;117:427–9. [PubMed] [Google Scholar]
- 44.Romańska-Gocka K, Gocki J, Placek W, et al. Behcet's disease – case report and literature review [Polish] Postep Derm Alergol. 2009;26:224–8. [Google Scholar]