Abstract
Introduction
In normal conditions follicle-stimulating hormone receptors (FSHR) are expressed in the ovary and the testis. They can also be expressed in gonadal tumors. However, recently we have found FSHR immunostaining in pituitary adenomas, adrenal tumors and neuroendocrine tumors (carcinoids). The aim of this study was to determine whether the same occurs in thyroid tumors.
Material and methods
Thirty-six samples of surgically excised thyroids were examined. Follicle-stimulating hormone receptors immunostaining was performed on paraffin sections using the rabbit anti-human FSHR polyclonal antibody raised against a 1-190 amino acid sequence from the human FSHR (sc-13935, Santa Cruz).
Results
Normal thyroid follicles do not show immunopositivity for FSHR. The same concerns the majority of benign lesions, diagnosed as hyperplasia nodularis or thyroid adenomas. However, positive FSHR immunostaining in some follicles was observed. In all but one thyroid cancer (15 papillary, 10 follicular cancers and one case of anaplastic thyroid cancer) 10–100% of tumor cells exhibit positive FSHR immunostaining. In about 40% of samples FSHR immunoreactivity can be observed also in the endothelia of intrathyroidal blood vessels. This immunopositivity was more frequent in the samples of thyroid cancers (13/27) than in benign lesions (2/9).
Conclusions
Ectopic positive FSHR immunostaining is also present in thyroid cancers, and, to a lesser degree, in benign lesions but not in the normal thyroid epithelium.
Keywords: follicular cancer, papillary cancer, nodular hyperplasia
Introduction
In normal conditions the follicle stimulating hormone receptors (FSHR) are expressed in the gonads: in granulosa cells of the ovary and Sertoli cells of the testis (for review see Simoni et al. [1]). It is well established that FSHR can also be expressed in gonadal tumors [2]. In 2010, a group of authors reported on the expression of FSHR in endothelium of intratumoral and peritumoral blood vessels in different human cancers but not in the endothelium of vessels located in non-neoplastic tissues except the gonads [3]. Studying some endocrine tumors, including pituitary adenomas, adrenal benign and malignant tumors and neuroendocrine tumors of lungs and of the alimentary tract (carcinoids), we confirmed the expression of FSHR in the intra- and peritumoral vascular endothelia [4, 5]. However, we also observed the positive immune reaction with anti-FSHR antibody in tumor cells. These observations corroborate the earlier findings of Ben-Josef et al. [6], who reported on the FSHR expression in androgen-independent prostate cancer cells PC3 and DU145, and the recent data of Sardella et al. [7] and Renner et al. [8], who found the expression of FSHR in tumor cells of neuroendocrine pancreatic tumors and sarcomas, respectively. The mechanism of the ectopic expression of FSHR in the endothelial cells of intra- and peritumoral vessels and in the tumor cells of some extra-gonadal tumors remains unknown. The regulation of FSHR gene expression is very complex (for review see ref. [9]) and potentially could be disturbed at several points during carcinogenesis. Because so far the FSHR expression in thyroid tumors has not been studied, the aim of the present study was to determine whether it also occurs in thyroid neoplasia.
Material and methods
Thirty-six samples of surgically excised thyroids were examined. The samples included 9 benign lesions and 27 cancers (16 papillary cancers, 10 follicular cancers and 1 anaplastic cancer). Follicle stimulating hormone receptors immunostaining was performed on paraffin sections using the rabbit anti-human FSHR polyclonal antibody raised against a 1–190 amino acid sequence from the human FSHR (sc-13935, Santa Cruz). Visualization of the primary antibody was done using the Dako REAL EnVision Detection System following the procedures recommended by the manufacturer. For a positive control, a biopsy sample of the human testis was immunostained. For a negative control, the primary antibody was omitted in the staining procedure.
The study was approved by the Local Bioethical Committee, decision number RNN/537/11/KB dated 14th June 2011.
Results
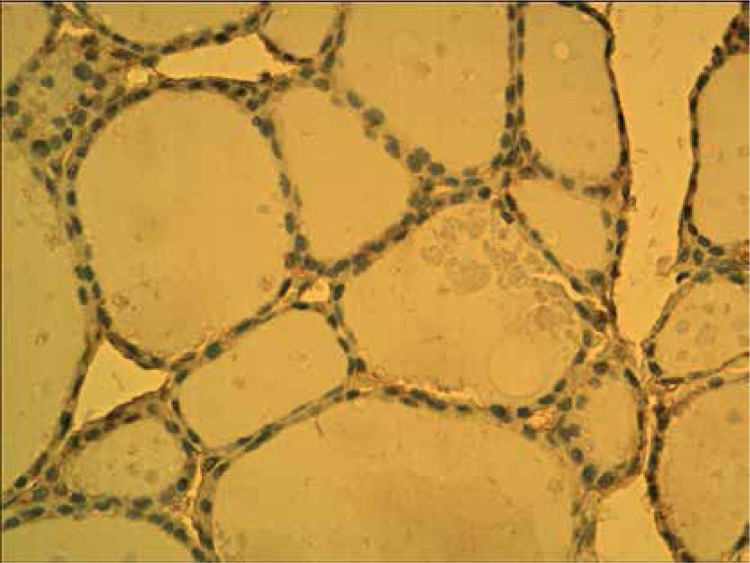
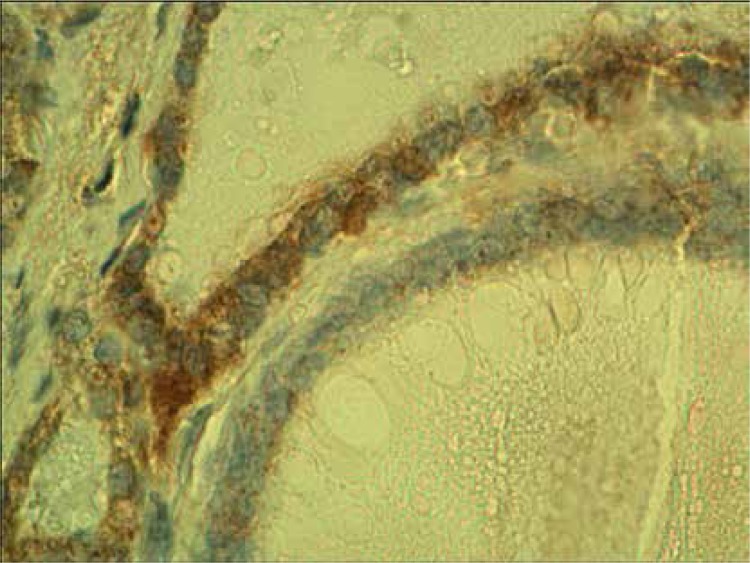
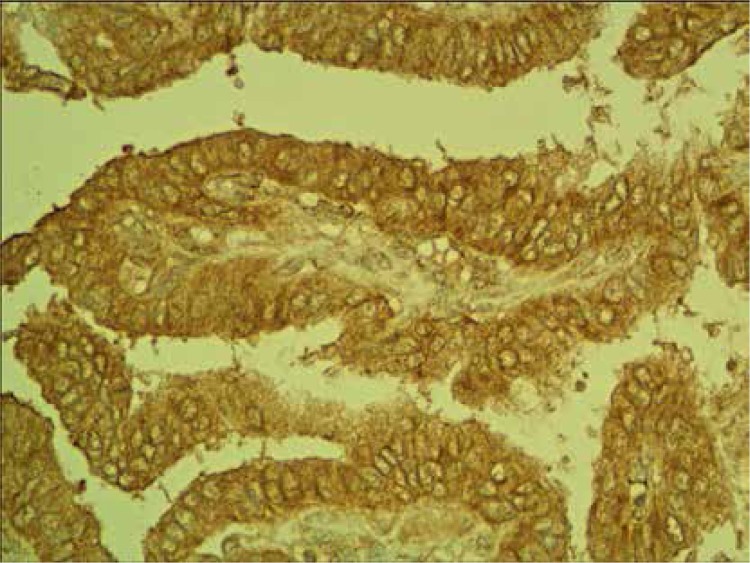
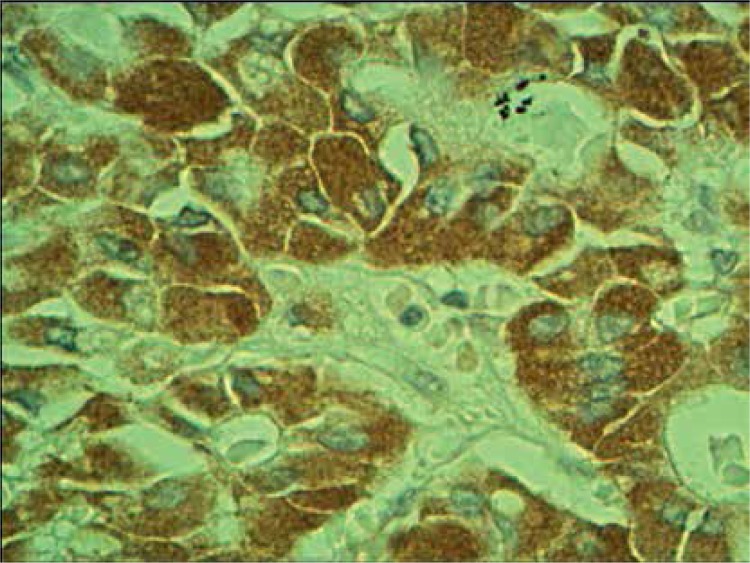
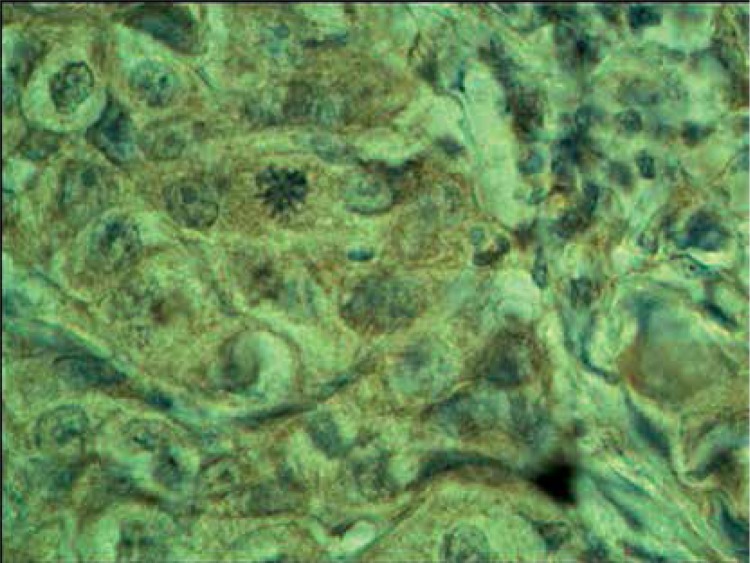
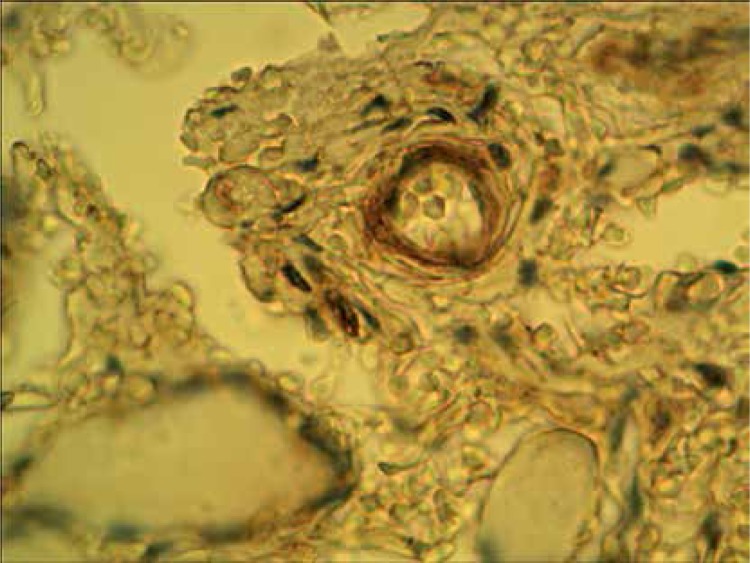
Normal thyroid follicles do not show immunopositivity for FSHR (Figure 1). The same concerns the majority of benign lesions, diagnosed as hyperplasia nodularis or thyroid follicular adenomas. However, FSHR immunoreactivity is positive only in a small proportion of follicles (Figure 2). In all but one papillary cancers and in all follicular cancers 10–100% of tumor cells exhibit positive FSHR immunostaining (Figures 3, 4). In 1 case of anaplastic thyroid cancer all tumor cells are immunopositive with anti-FSHR antibody (Figure 5). In 15/36 (approx. 40%) samples FSHR immunostaining can be observed also in the walls of intrathyroidal blood vessels, particularly in the endothelium (Figure 6).
Figure 1.
Non-neoplastic thyroid follicles. Lack of FSHR immunostaining
Figure 2.
Nodular hyperplasia. Neighboring follicles showing positive and negative FSHR immunostaining
Figure 3.
Positive FSHR immunostaining in papillary cancer
Figure 4.
Positive FSHR immunostaining in follicular cancer
Figure 5.
Positive FSHR immunostaining in anaplastic thyroid cancer
Figure 6.
Peritumoral blood vessel positively immunostained with anti-FSHR antibody in a case of follicular cancer
This positive vascular immunostaining was more frequent in the samples of thyroid cancers (13/27, 48%) than in benign lesions (2/9, 22%).
Discussion
The data presented above show that – like in other endocrine tumors – FSHR immunoreactivity can be detected in tumor cells and in some of the vascular endothelia. The positive reaction with FSHR antibody in intra- and peritumoral blood vessels occurring in thyroid cancers corroborates the earlier observations on other non-endocrine and endocrine cancers [3–6]. However, the expression of FSHR immunopositivity was observed not only in blood vessels, but also in tumor cells of malignant, and, to a lesser degree, benign thyroid tumors. Follicle stimulating hormone receptors expression in tumor cells has also been observed in pituitary adenomas, adrenal tumors [4] and neuroendocrine tumors [5, 7] and sarcomas [8]. In this paper we show that ectopic positive FSHR immunostaining is also present in thyroid cancers, and, to a lesser degree, in benign hyperplastic or adenomatous lesions but not in the normal non-neoplastic thyroid epithelium. However, several questions need to be answered. The first question concerns the specificity of the antibody. The antibody used in this study strongly reacts with Sertoli cells in a positive control sample, and the reaction is totally absent in negative controls. In spite of many similarities between FSHR and thyroid stimulating hormone (TSH) receptors, the cross reaction of anti-FSHR antibody applied in this study with the latter can be rejected since the non-neoplastic thyroid follicles were immunonegative with FSHR antibody, in spite of the known presence of TSH receptors.
Secondly, the question arises whether the immunopositive protein revealed in this study is the active form of FSH receptor. To our knowledge, only Ben-Josef et al. [6] have examined in vitro the response of ectopic FSHR to their ligand (FSH) in prostate cancer cell lines and observed a positive response (increase of cAMP). The next question is, if the ectopic FSHR in tumors are active, what are the consequences of that for the thyroid neoplasia? It is known that in normal ovary, FSH, acting via its receptors, stimulates the proliferation of ovarian granulosa cells and increases the level of vascular endothelial growth factor (VEGF), leading to enhanced angiogenesis [10]. A similar finding was made with ovarian cancer cells SK-OV-3 [11]. The FSH also inhibits ovarian cancer cell apoptosis by up-regulating survivin and down-regulating programmed cell death gene 6 (PDCD6) and death receptor 5 (DR5) [12]. The involvement of FSHR in tumor angiogenesis is also supported by a recent observation that the therapeutic effectiveness of sunitinib in kidney cancer correlates with the density of FSHR-positive blood vessels [13]. Sunitinib is a novel anti-cancer drug acting as a receptor tyrosine kinase inhibitor which inhibits, among others, receptors 1–3 of VEGF [14]. Moreover, ectopic FSHR expression may be considered as a potential marker of malignancy. In thyroid tumors, for instance, FSHR immunostaining may be potentially useful in differential diagnosis between benign follicular adenomas and follicular cancers [15].
In conclusion, all these data, taken together, strongly suggest that ectopic FSHR may constitute a novel biomarker and target in the treatment of cancer, including thyroid cancer.
Acknowledgments
This paper was supported by grant of Medical University of Lodz No. 503/5-020-02/503-51-001.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Simoni M, Gromoll J, Nieschlag E. The follicle stimulating hormone receptor: biochemistry, molecular biology, physiology and pathophysiology. Endocrine Rev. 1997;18:739–73. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 2.Huhtaniemi I. Are gonadotropins tumorigenic – a critical review of clinical and experimental data. Mol Cell Endocrinol. 2010;329:56–61. doi: 10.1016/j.mce.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Radu A, Pichon C, Camparo P, et al. Expression of follicle – stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–30. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 4.Pawlikowski M, Pisarek H, Kubiak R, Jaranowska M, Stępień H. Immunohistochemical detection of FSH receptors in pituitary adenomas and adrenal tumors. Folia Histochem Cytobiol. 2012;50:325–30. doi: 10.5603/17850. [DOI] [PubMed] [Google Scholar]
- 5.Pawlikowski M, Winczyk K, Stępień H. Immunohistochemical detection of follicle stimulating hormone receptor (FSHR) in neuroendocrine tumours. Endokrynol Pol. 2013;64:268–71. doi: 10.5603/ep.2013.0004. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Josef E, Yang SY, Bidart JM, et al. Hormone-refractory prostate cancer cells express functional follicle stimulating hormone receptor (FSHR) J Urol. 1999;161:970–6. [PubMed] [Google Scholar]
- 7.Sardella C, Russo D, Raggi F, et al. Ectopic expression of FSH receptor isoforms in neoplastic but not in endothelial cells from pancreatic neuroendocrine tumours. J Endocrinol Invest. 2013;36:174–9. doi: 10.3275/8472. [DOI] [PubMed] [Google Scholar]
- 8.Renner M, Goeppert B, Siraj MA, et al. Follicle stimulating hormone receptor expression in soft tissue sarcomas. Histopathology. 2013;63:29–35. doi: 10.1111/his.12135. [DOI] [PubMed] [Google Scholar]
- 9.George JW, Dille EA, Heckert LL. Current concepts of follicle stimulating hormone receptor gene regulation. Biol Reprod. 2011;84:7–17. doi: 10.1095/biolreprod.110.085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo SW, Ke FC, Chang GD, Lee MT, Hwang JJ. Potential role of follicle stimulating hormone (FSH) and transforming growth factor (TGF1) in the regulation of ovarian angiogenesis. J Cell Physiol. 2011;226:1608–19. doi: 10.1002/jcp.22491. [DOI] [PubMed] [Google Scholar]
- 11.Park YH, Kim SJ, Jeong BH, et al. Follicular stimulating hormone enhances Notch1 expression in SK-OV-3 ovarian cancer cells. J Gynecol Oncol. 2010;21:119–24. doi: 10.3802/jgo.2010.21.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Jin H, Liou I, et al. FSH inhibits ovarian cancer cell apoptosis by up-regulating survivin and down regulating PDCD6 and DR5. Endocr Relat Cancer. 2010;18:13–26. doi: 10.1677/ERC-09-0308. [DOI] [PubMed] [Google Scholar]
- 13.Siraj MA, Pichon C, Radu A, Ghinea N. Endothelial follicle stimulating hormone receptor in primary kidney cancer correlates with subsequent response to sunitinib. J Cell Mol Med. 2012;16:2010–6. doi: 10.1111/j.1582-4934.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiedmann MW, Mossner J. Safety and efficacy of sunitinib in patients with unresectable pancreatic neuroendocrine tumors. Clin Med Insights Oncol. 2012;6:381–93. doi: 10.4137/CMO.S7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassiliou I, Tympa A, Arkadopoulos N, et al. Total thyroidectomy as the single surgical option for benign and malignant thyroid disease: a surgical challenge. Arch Med Sci. 2013;9:74–78. doi: 10.5114/aoms.2013.33065. [DOI] [PMC free article] [PubMed] [Google Scholar]