Abstract
Background
D2 lymphadenectomy has been increasingly regarded as standard surgical procedure for advanced gastric cancer (GC), while the necessity of No.14v lymph node (14v) dissection for distal GC is still controversial.
Methods
A total of 920 distal GC patients receiving at least a D2 lymph node dissection in Department of Gastric Cancer, Tianjin Medical University Cancer Institute and Hospital were enrolled in this study, of whom, 243 patients also had the 14v dissected. Other 677 patients without 14v dissection were used for comparison.
Results
Forty-five (18.5%) patients had 14v metastasis. There was no significant difference in 3-year overall survival (OS) rate between patients with and without 14v dissection. Following stratified analysis, in TNM stages I, II, IIIa and IV, 14v dissection did not affect 3-year OS; in contrast, patients with 14v dissection had a significant higher 3-year OS than those without in TNM stages IIIb and IIIc. In multivariate analysis, 14v dissection was found to be an independent prognostic factor for GC patients with TNM stage IIIb/IIIc disease [hazard ratio (HR), 1.568; 95% confidence interval (CI): 1.186-2.072; P=0.002]. GC patients with 14v dissection had a significant lower locoregional, especially lymph node, recurrence rate than those without 14v dissection (11.7% vs. 21.1%, P=0.035).
Conclusions
Adding 14v to D2 lymphadenectomy may be associated with improved 3-year OS for distal GC staged TNM IIIb/IIIc.
Keywords: Gastric carcinoma, No.14v lymph node, metastasis, dissection, prognosis
Introduction
Radical gastrectomy with D2 lymph node dissection has become increasingly regarded as the standard surgical procedure for most patients with operable gastric cancer (GC) (1-8). Lymph nodes along the root of superior mesenteric vein are referred to as 14v lymph node. In the second English edition of Japanese Classification of Gastric Carcinoma, 14v lymph node was included in the N2 group for tumors located in the lower third of the stomach, and it should be removed in D2 lymphadenectomy for distal GC (9). However, the 3rd English edition of Japanese gastric cancer treatment guidelines excluded 14v from D2 lymphadenectomy, though it was still defined as regional gastric lymph node (5,10). Previous studies had affirmed the poor survival of 14v positive patients even after curative resection (11,12). An et al. deemed the survival of 14v positive patients was similar to those with M1 disease and 14v should be excluded from regional gastric lymph node (11). However, Masuda et al. confirmed the dissection of 14v may improve the overall survival (OS) for 14v positive patients without No.16 lymph node metastasis (12). Eom et al. even found that 14v dissection was an independent prognostic factor for middle or lower GC of clinical stage III/IV (13).
Until now, the data of 14v lymph node were mainly from Japan and South Korea, and no random prospective trails have been implemented to affirm survival benefit in 14v dissection. The necessity of 14v dissection is still controversial in GC surgery. In China, GC remains diagnosed at advanced stage and early GC accounts for no more than 10 percent (14,15), so the therapeutic strategy should be different from Japan and South Korea where the early GC was very common. The aim of the present study is to elucidate the potential impact of adding 14v to a D2 dissection on the long-term survival for distal GC patients after gastrectomy with curative intent in a single high-volume center in China.
Materials and methods
Patients
A total of 1,598 patients with GC who underwent surgical resection in the Department of Gastric Cancer, Tianjin Medical University Cancer Institute and Hospital between January 2003 and March 2011 were eligible for this study. Eligibility criteria included: (I) adenocarcinoma of the stomach; (II) with primary tumors located at middle or lower of the stomach (including tumors invading lower 1/3, middle 1/3 and both the two areas); (III) no history of gastrectomy or other malignancy; (IV) no history of neoadjuvant chemotherapy; (V) receiving at least D2 lymph node dissection without macroscopically tumor residual; (VI) patients were completely followed-up; and (VII) no patients died during the initial hospital stay or for 1 month after surgery. After excluding 4 patients with gastric neuroendocrine carcinoma, 1 patient with gastric squamous cells carcinoma, 452 patients with tumors located at upper of the stomach, 20 remnant GC patients, 14 patients with other malignant tumors, 38 patients received neoadjuvant chemotherapy, 72 patients underwent less than standard D2 lymph node dissection, 23 patients died within 1 month after surgery and 53 patients lost, ultimately, 920 patients were included in this study.
The 920 patients included 644 males (70%) and 276 females (30%). The age ranges from 20 to 89 years, and the median age was 59 years. All patients were categorized into 2 groups based on status of 14v dissection: dissected group (DG) and un-dissected group (UDG). As a result, 243 patients were assigned to DG, and 677 patients to UDG.
Dissection of No.14v lymph node and evaluation of OS
No.14v lymph nodes are defined as the lymph nodes along the superior mesenteric vein. There are no predefined indications of dissection of the 14v lymph node. The removal of 14v lymph nodes absolutely depended on the surgeons’ willingness and habits. To completely dissect the 14v lymph nodes, the soft tissue around the superior mesenteric vein must be removed along with exposing the superior mesenteric vein.
Potential prognostic factors evaluated included sex, age at surgery, tumor diameter, Borrmann type, histology, curability, TNM stage, postoperative chemotherapy, types of gastrectomy, and status of 14v dissection. The tumors were staged according to the 7th edition Union for International Cancer Control TNM classification system, whereas lymphadenectomy and lymph node stations were defined according to the 3rd English Edition of the Japanese Classification of Gastric Carcinoma (10). Tumors were classified into two groups based on histology: differentiated type, including papillary, well or moderately differentiated adenocarcinoma; and undifferentiated type, including poorly differentiated or undifferentiated adenocarcinoma, signet ring cell carcinoma and mucinous carcinoma.
Follow-up
The patients were followed up every 3 months up to 2 years after surgery, then every 6 months up to 5 years, and then every year or until death. Physical examination, laboratory test, chest X-ray and abdominal ultrasound (US) were performed at each visit, while endoscopy and abdominal computed tomography (CT) were obtained every 6 months or each year. The OS rate was calculated from the day of surgery until time of death or final follow-up. The date of final follow-up was April 30, 2014.
Statistical analysis
For continuous variables, parametric analysis was performing using Student’s t test. Categorical variables were analyzed by means of the Chi-square or Fisher’s exact test. The OS curves were calculated using the Kaplan-Meier method based on the length of time between primary surgical treatment and final follow-up or death. The log-rank test was used to assess statistical differences between curves. Independent prognostic factors were identified by Cox proportional hazards regression model. P<0.05 was considered statistically significant. The statistical analysis was performed using the statistical analysis program package SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Frequency of 14v metastasis and TNM-stratified analysis for OS
Of the 243 GC patients with 14v dissection, 45 (18.5%) had 14v metastasis. The frequency of 14v metastasis according to tumor stages is shown in Table 1. No patients with stage I disease had 14v metastasis. The frequency of 14v involvement was 1.6%, 6.3%, 20.5% and 32.2% respectively in stages II, IIIa, IIIb and IIIc, and it rose to 66.7% in stage IV disease. The co-relationships between metastatic status of 14v and clinicopathological factors including other regional lymph nodes were also analyzed. In univariate logistic regression analysis, factors significantly correlated with 14v metastasis were Borrmann type (III/IV), depth of invasion (T4), N stage (N2-3), distant metastasis (M1) and regional lymph nodes such as 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p and 12a metastasis. Factors including sex, age, tumor diameter, histology and regional lymph nodes 2, 4a, 11d had no significant impact on 14v metastasis. In multivariate analysis, only distant metastasis [relative risk (RR): 5.756, 95% confidence interval (CI), 1.308-25.326, P=0.021], No.4d (RR: 3.782, 95% CI, 1.181-12.112, P=0.025) and No.6 (RR: 4.325, 95% CI, 1.331-14.062, P=0.015) lymph node metastasis were independently associated with 14v metastasis.
Table 1. Frequency of 14v metastasis according to tumor stages.
| Tumor stage | 14v lymph node metastasis |
|
|---|---|---|
| Negative (n) | Positive [n (%)] | |
| I | 23 | 0 (0) |
| II | 63 | 1 (1.6) |
| IIIa | 30 | 2 (6.3) |
| IIIb | 35 | 9 (20.5) |
| IIIc | 40 | 19 (32.2) |
| IV | 7 | 14 (66.7) |
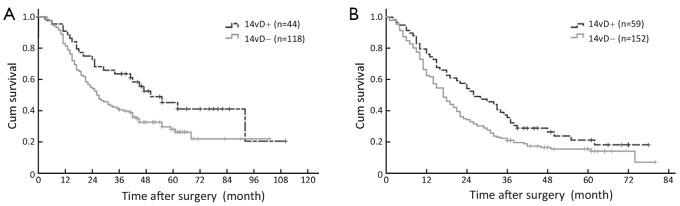
As shown in Table 2, there was no significant difference in 3-year OS between patients with and without 14v dissection. Following stratified analysis, in TNM stages I, II, IIIa and IV, 14v lymph node dissection did not affect 3-year OS; in contrast, patients with 14v lymph node dissection had a significant higher 3-year OS than those without in TNM stage IIIb and IIIc disease (Figure 1).
Table 2. OS differences between gastric cancer patients undergoing gastrectomy with or without 14v dissection stratified by TNM stage.
| TNM | 14vD (−) |
14vD (+) |
χ2 | P | |||
|---|---|---|---|---|---|---|---|
| n | 3-year OS (%) | n | 3-year OS (%) | ||||
| I | 86 | 94.2 | 23 | 100 | 0.081 | 0.776 | |
| II | 182 | 77.5 | 64 | 84.1 | 0.418 | 0.518 | |
| IIIa | 92 | 62.0 | 32 | 77.9 | 2.412 | 0.120 | |
| IIIb | 118 | 41.5 | 44 | 63.6 | 4.400 | 0.033 | |
| IIIc | 152 | 22.4 | 59 | 37.3 | 5.768 | 0.016 | |
| IV | 47 | 4.3 | 21 | 4.8 | 0.011 | 0.917 | |
| Total | 677 | 53.9 | 243 | 63.4 | 2.804 | 0.094 | |
OS, overall survival; 14vD, 14v dissection.
Figure 1.
Survival curves for GC patients with or without 14v dissection (14vD). (A) IIIb stage patients; (B) IIIc stage patients. GC, gastric cancer.
Comparison of clinicopathologic characteristics in TNM stage IIIb/IIIc
As only in stages IIIb and IIIc, 14v lymph node dissection has a significant effect on survival and the metastatic rate of 14v is relatively high. We particularly focus on the GC patients with IIIb/IIIc diseases. No.14v lymph node dissection was performed in 103 patients (DG), other 270 patients without 14v dissection were used for comparison (UDG). Clinicopathologic factors of the two groups are shown in Table 3. There were no significant differences in sex, total number of lymph node metastasis, number of 1-12a lymph node dissected or metastasis, tumor diameter, Borrmann type, histology, curability, TNM stage, postoperative chemotherapy and types of gastrectomy between the two groups, while patients with 14v dissection had a significant larger total number of lymph nodes dissected and a higher ratio of patients aged <65 years old.
Table 3. Comparison of clinicopathological characteristics between TNM IIIb/IIIc GC patients with and without 14v dissection.
| Factors | 14vD (+) | 14vD (−) | t/χ2 | P |
|---|---|---|---|---|
| Total number of metastatic LNs | 9.9 (8.3-11.4) | 10.0 (9.0-11.0) | −0.152 | 0.879 |
| Total number of dissected LNs | 29.7 (26.9-32.4) | 24.2 (23.0-25.4) | 4.194 | <0.001 |
| Number of 1-12a LNs dissected | 26.7 (24.0-29.5) | 24.2 (23.0-25.4) | 1.921 | 0.055 |
| Number of 1-12a LNs metastasis | 9.5 (8.1-11.0) | 10.0 (9.0-11.0) | −0.501 | 0.617 |
| Sex | 2.164 | 0.141 | ||
| Male | 68 (66.0) | 199 (73.7) | ||
| Female | 35 (34.0) | 71 (26.3) | ||
| Age at surgery (year) | 6.985 | 0.008 | ||
| <65 | 79 (76.7) | 168 (62.2) | ||
| ≥65 | 24 (23.3) | 102 (37.8) | ||
| Tumor diameter (cm) | 0.401 | 0.526 | ||
| <5 | 34 (33.0) | 80 (29.6) | ||
| ≥5 | 69 (67.0) | 190 (70.4) | ||
| Borrmann type | 1.189 | 0.756 | ||
| I | 6 (5.8) | 20 (7.4) | ||
| II | 23 (22.3) | 55 (20.4) | ||
| III | 62 (60.2) | 154 (57.0) | ||
| IV | 12 (11.7) | 41 (15.2) | ||
| Histology | 0.500 | 0.480 | ||
| Differentiated | 18 (17.5) | 56 (20.7) | ||
| Undifferentiated | 85 (82.5) | 214 (79.3) | ||
| Curability | 2.165 | 0.141 | ||
| R0 | 94 (91.3) | 231 (85.6) | ||
| R1 | 9 (8.7) | 39 (14.4) | ||
| TNM stage | 0.029 | 0.864 | ||
| IIIb | 44 (42.7) | 118 (43.7) | ||
| IIIc | 59 (57.3) | 152 (56.3) | ||
| Postoperative chemotherapy | 0.351 | 0.553 | ||
| Yes | 66 (64.1) | 164 (60.7) | ||
| No | 37 (35.9) | 106 (39.3) | ||
| Type of gastrectomy | 0.917 | 0.338 | ||
| Subtotal | 64 (62.1) | 153 (56.7) | ||
| Total | 39 (37.9) | 117 (43.3) |
GC, gastric cancer; 14vD, 14v dissection; LN, lymph node.
Univariate and multivariate survival analysis of patients of stage IIIb/IIIc
A total of 8 factors evaluated in univariate analysis had a significant effect on survival: age at surgery, tumor diameter, Borrmann type, histology, curability, TNM stage, postoperative chemotherapy and status of 14v dissection. Patients with 14v dissection had a significant higher OS rate than those without 14v dissection (3-year OS: 48.5% vs. 30.7%, P=0.003), while the survival of R0 resection patients without 14v dissection was similar to those who got a R1 resection regardless of status of 14v dissection (3-year OS: 32% vs. 29.2%, P=0.084) (Figure 2). In multivariate analysis, Borrmann type (III/IV), TNM stage, postoperative chemotherapy and status of 14v dissection still had a significant effect on 3-year OS. Dissection of 14v was an independent prognostic factor in GC patients with TNM stage IIIb/IIIc disease (Table 4).
Figure 2.

Survival curves for GC patients categorized by curability and 14v dissection (14vD) status. The 3-year OS was 32.0% for 14v un-dissected patients with R0 resection and 29.2% for R1 resection patients (regardless of 14v dissection), respectively (P=0.084, R0 resection without 14v dissection vs. R1 resection regardless of the status of 14v dissection, log-rank test). GC, gastric cancer; OS, overall survival.
Table 4. Univariate and multivariate survival analysis of TNM IIIb/IIIc GC patients by Cox proportional hazards model.
| Characteristics | n | 3-year OS (%) | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| Sex | |||||||
| Male | 267 | 37.1 | 1 (ref) | ||||
| Female | 106 | 32.1 | 1.015 (0.783-1.315) | 0.911 | |||
| Age at surgery (year) | |||||||
| <65 | 247 | 40.1 | 1 (ref) | 1 (ref) | |||
| ≥65 | 126 | 27.0 | 1.473 (1.157-1.876) | 0.002 | 1.238 (0.962-1.593) | 0.097 | |
| Tumor diameter (cm) | |||||||
| <5 | 114 | 44.7 | 1 (ref) | 1 (ref) | |||
| ≥5 | 259 | 31.7 | 1.413 (1.089-1.834) | 0.009 | 1.262 (0.967-1.646) | 0.086 | |
| Borrmann type | |||||||
| I | 26 | 69.2 | 1 (ref) | 1 (ref) | |||
| II | 78 | 38.5 | 1.490 (0.840-2.641) | 0.172 | 1.506 (0.843-2.690) | 0.167 | |
| III | 216 | 33.8 | 1.875 (1.105-3.181) | 0.020 | 1.743 (1.016-2.990) | 0.044 | |
| IV | 53 | 22.6 | 2.770 (2.541-4.976) | 0.001 | 2.346 (1.285-4.280) | 0.005 | |
| Histology | |||||||
| Differentiated | 74 | 47.3 | 1 (ref) | 1 (ref) | |||
| Undifferentiated | 299 | 32.8 | 1.430 (1.052-1.942) | 0.022 | 1.305 (0.954-1.785) | 0.095 | |
| Curability | |||||||
| R0 | 325 | 36.6 | 1 (ref) | 1 (ref) | |||
| R1 | 48 | 29.2 | 1.511 (1.080-2.115) | 0.016 | 1.208 (0.847-1.724) | 0.297 | |
| TNM stage | |||||||
| IIIb | 162 | 47.5 | 1 (ref) | 1 (ref) | |||
| IIIc | 211 | 26.6 | 1.742 (1.367-2.220) | <0.001 | 1.769 (1.385-2.261) | <0.001 | |
| Postoperative chemotherapy | |||||||
| Yes | 230 | 42.2 | 1 (ref) | 1 (ref) | |||
| No | 143 | 25.2 | 1.485 (1.171-1.881) | 0.001 | 1.488 (1.161-1.906) | 0.022 | |
| Type of gastrectomy | |||||||
| Subtotal | 217 | 37.3 | 1 (ref) | ||||
| Total | 156 | 33.3 | 1.168 (0.922-1.479) | 0.197 | |||
| Status of 14v dissection | |||||||
| 14vD (+) | 103 | 48.5 | 1 (ref) | 1 (ref) | |||
| 14vD (−) | 270 | 30.7 | 1.500 (1.143-1.968) | 0.003 | 1.568 (1.186-2.072) | 0.002 | |
GC, gastric cancer; OS, overall survival; HR, hazard ratio; CI, confidence interval; 14vD, 14v dissection.
Recurrence data of IIIb/IIIc stage disease
The patterns and incidence of recurrence in patients with IIIb/IIIc disease are shown in Table 5. Although there were no significant differences in peritoneal, hematogenous and combined recurrence between the two groups, patients with 14v dissection had a significantly lower overall recurrence rate than those without 14v dissection (45.6% vs. 63.3%, P=0.003). The proportion of locorregional recurrence was greater in patients without 14v dissection (21.1% vs. 11.7%, P=0.035). For patients with locoregional recurrence, lymph node recurrence was greater in patients without 14v dissection (15.9% vs. 6.8%, P=0.021), while other recurrence types, including gastric stump, anastomosis and gastric bed recurrence, showed no significant differences between the two groups.
Table 5. Types of initial recurrence of GC patients staged TNM IIIb/IIIc.
| Types of recurrence | n (%) |
P | |
|---|---|---|---|
| 14vD (+) (N=103) | 14vD (−) (N=270) | ||
| Locoregional | 12 (11.7) | 57 (21.1) | 0.035 |
| Lymph node | 7 (6.8) | 43 (15.9) | 0.021 |
| Gastric stump | 2 (1.9) | 3 (1.1) | 0.533 |
| Anastomosis | 0 (0) | 3 (1.1) | 0.283 |
| Gastric bed | 3 (2.9) | 8 (3.0) | 0.980 |
| Peritoneal | 15 (14.6) | 44 (16.3) | 0.682 |
| Hematogenous | 11 (10.7) | 32 (11.9) | 0.751 |
| Combined | 9 (8.7) | 38 (14.1) | 0.165 |
| Overall recurrence | 47 (45.6) | 171 (63.3) | 0.003 |
GC, gastric cancer; 14vD, 14v dissection.
Discussion
No.14v lymph node is one of regional gastric lymph nodes. The dissection of 14v had been a hot spot in GC surgery. Some researches are in favor of the removal of 14v in D2 gastrectomy for distal GC (13,16-21), while others are not (11,12). In the present study, we found that GC patients with 14v dissection had a significant higher 3-year OS, but lower locorregional recurrence rate than those without in TNM stages IIIb and IIIc after a D2 resection, and the status of 14v dissection was an independent prognostic factor in patients with TNM stage IIIb/IIIc disease [hazard ratio (HR), 1.568; 95% CI, 1.186-2.072; P=0.002].
The necessity of lymph node dissection was based on metastatic pathway, frequency and its impact on survival. The 14v lymph node is anatomically downstream of No.6 in the lymphatic flow for distal GC, and it receives the lymphatic flow from No.6, and then flows to No.16 lymph node station. Theoretically, once the No.6 lymph node is invaded, the 14v is at high risk of metastasis. Actually, it is reported that No.6 was a useful predictive factor for 14v metastasis with high accuracy (99.0%) and low false negative rate (1.9%) (11). In the 3rd edition of Japanese gastric cancer treatment guideline, 14v was excluded from D2 lymphadenectomy in distal GC, meanwhile the “guideline” pointed out that 14v dissection may be beneficial for patients with No.6 lymph node apparently involved (5). In the present study, the frequency of 14v metastasis was relatively as high as 18.5 percent, and No.4d and No.6 lymph node metastases were independently associated with 14v metastasis. We think the status of No.4d and No.6 was a useful indicator for 14v metastasis. If the No.4d and No.6 lymph nodes are negative, there is no need to remove 14v.
As we all know, if the dissected lymph node is rarely invaded, there is no need to remove it. The frequency of 14v metastasis was reported to be correlated with tumor stage. In the study by Masuda et al, the incidence of 14v metastasis was merely 1.3 percent in early GC, and it raised 19.7 percent in advanced GC (12). An et al. reported the metastatic rate of 14v was 11.0% in N2 stage and 40.5% in N3 stage, and in N1 stage, only 1.2 percent patients had positive 14v (11). In the present study, no patients with stage I disease had 14v metastasis; the frequency of 14v involvement was 1.7%, 6.3%, 20.5% and 32.2% respectively in stages II, IIIa, IIIb and IIIc, and it rose to 66.7% in stage IV disease. Considering the low incidence of 14v metastasis in stage I and II GC, 14v dissection was not recommended in these patients. Our results also affirmed that dissection of 14v could not bring any survival benefits to such patients, which was in accordance with a previous study (9). Though stage IV patients had a high incidence of 14v metastasis, they also benefited little from 14v dissection according to our data. We believe that stage IV GC is a systemic disease beyond surgical cure, even after curative resection, these patients may suffer from recurrence immediately which may obscure the effect of 14v dissection. In stages IIIb and IIIc, 14v dissection did significantly improve the 3-year OS in the present study, and even after R0 dissection, the survival of patients without 14v dissection was similar to those with R1 resection regardless of status of 14v dissection, which may indicate that if the 14v lymph node is not removed, some tumor cells are more or less left behind. Because of high incidence of 14v metastasis in IIIb/IIIc disease, adding 14v to a D2 lymphadenectomy decreases the possibility of locoregional tumor residual and increases the chance of curable resection, which may account for the improved OS.
To verify the tumor residual of IIIb/IIIc stage GC patients without 14v lymph node dissection, the recurrence data were also analyzed. We found that patients with 14v dissection had a significant lower lymph node recurrence rate than those without 14v dissection (6.8% vs. 15.9%, P=0.021). This was in accordance with a previous study which had proved that the proportion of locoregional recurrence was greater in patients without 14v dissection (13). From this point of view, 14v dissection did reduce the residual of tumors microscopically or macroscopically along the superior mesenteric vein.
Conclusions
Considering the low incidence of 14v metastasis in stage I, II and IIIa disease, the correlated bleeding and the incurability of the M1 disease, we concluded 14v dissection did not affect OS in these patients and it should be avoided. In patients with stage IIIb and IIIc disease, the dissection of 14v is suggested for these apparent metastases to No.4d or No.6 lymph nodes, as such patients were at high risk of 14v metastasis. Adding 14v lymph node to a D2 lymphadenectomy may be associated with improved OS and lower lymph node recurrence rate for distal GC patients with TNM IIIb/IIIc disease.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [DOI] [PubMed] [Google Scholar]
- 2.Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:584-91. [DOI] [PubMed] [Google Scholar]
- 3.NCCN clinical practice guidelines in oncology (gastric cancer), version 3, 2015. Available online: http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- 4.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer 2011;14:97-100. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric Cancer 2011;14:113-23. [DOI] [PubMed] [Google Scholar]
- 6.Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [DOI] [PubMed] [Google Scholar]
- 7.Hu JK, Yang K, Zhang B, et al. D2 plus para-aortic lymphadenectomy versus standardized D2 lymphadenectomy in gastric cancer surgery. Surg Today 2009;39:207-13. [DOI] [PubMed] [Google Scholar]
- 8.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association . Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer 1998;1:10-24. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [DOI] [PubMed] [Google Scholar]
- 11.An JY, Pak KH, Inaba K, et al. Relevance of lymph node metastasis along the superior mesenteric vein in gastric cancer. Br J Surg 2011;98: 667-72. [DOI] [PubMed] [Google Scholar]
- 12.Masuda TA, Sakaguchi Y, Toh Y, et al. Clinical characteristics of gastric cancer with metastasis to the lymph node along the superior mesenteric vein (14v). Dig Surg 2008;25:351-8. [DOI] [PubMed] [Google Scholar]
- 13.Eom BW, Joo J, Kim YW, et al. Improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer. Surgery 2014;155:408-16. [DOI] [PubMed] [Google Scholar]
- 14.Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921-30. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014;26:48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokunaga M, Ohyama S, Hiki N, et al. Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion: is the posterior pancreatic head lymph node dissection beneficial? Ann Surg Oncol 2009;16:1241-6. [DOI] [PubMed] [Google Scholar]
- 17.Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995;82:346-51. [DOI] [PubMed] [Google Scholar]
- 18.Xu KF, Zhou YB, Li Y, et al. Study on metastasis and micrometastasis in No.14v lymph nodes of patients with lower third gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2011;14:125-7. (in Chinese) [PubMed] [Google Scholar]
- 19.Liang YX, Liang H, Ding XW, et al. Significance of No.14v lymph node dissection for advanced gastric cancer undergoing D2 lymphadenectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2013;16:632-6. (in Chinese) [PubMed] [Google Scholar]
- 20.Watanabe T, Nakayama H, Tomibayashi A, et al. A case of long-term survival after resection of metastatic lymph nodes along the superior mesenteric vein from early gastric cancer. Gan To Kagaku Ryoho 2013;40:515-7. (in Japanese) [PubMed] [Google Scholar]
- 21.Blouhos K, Boulas KA, Tsalis K, et al. Right-sided bursectomy as an access plane for aesthetic resection of the posterior leaf of the lesser sac from the head of the pancreas en block with the No. 6 and 14v lymph nodes in advanced lower third gastric cancer. Surgery 2015;158:1742. [DOI] [PubMed] [Google Scholar]