Abstract
Background
Fluorouracil-based preoperative chemoradiotherapy has become the standard treatment for stage II/III rectal cancer. In order to improve the overall survival (OS) and disease-free survival (DFS), we added oxaliplatin to the standard treatment, and compared the effectiveness of these two treatment patterns.
Methods
A total of 206 patients enrolled in the prospective study had histologically confirmed rectal cancer of clinical stage II/III during July 2007 to July 2010. They were randomized into the experimental group received oxaliplatin and capecitabine in combination with radiotherapy, and the control group received capecitabine in combination with radiotherapy. All patients received surgery in 6−10 weeks after chemoradiotherapy and adjuvant chemotherapy with mFOLFOX6. The primary endpoints were DFS and OS, and the secondary endpoints included toxicity, compliance, and histopathological response.
Results
The 3-year OS in the experimental group and the control group was 90.29% vs. 86.41% (P>0.05), and the 3-year DFS was 80.58% vs. 69.90% (P>0.05). The pathological complete remission (pCR) rates were 23.30% and 19.42%, respectively (P=0.497). The 3-year local recurrence rates were 4.85% vs. 5.83% (P=0.694), and the 3-year distant metastasis rates were 16.50% and 28.16%, respectively (P=0.045). There were no significant differences in most grade 3−4 toxicities between two groups, however, grade 3−4 diarrhea occurred in 16.50% (17/103) of the experimental group, compared with 6.80% (7/103) of the control group (P=0.030). Also, the total grade 3−4 acute toxicity showed a significant difference (10.68% vs. 21.36%, P=0.037).
Conclusions
The experimental treatment did not lead significantly improved OS and DFS, and thus longer follow-up is warranted for our patient cohort. Adding oxaliplatin to capecitabine-based preoperative chemoradiotherapy can significantly reduce metastasis, but has only minimal impact on local recurrence. Although grade 3−4 toxicity rate increased (primarily gastrointestinal toxicity), patients can stand to be followed up with allopathic treatment.
Keywords: Oxaliplatin, capecitabine, preoperative chemoradiotherapy, rectal adenocarcinoma
Introduction
Colorectal cancer is a common malignant tumor, and the mortality rate of rectal cancer is 7.7% (1) worldwide, after lung cancer (39.1%), liver cancer (34.6%), stomach cancer (30.8%) and esophageal cancer (19.7%). Compared with the US and Europe, colorectal cancer in China has three characteristics: (I) rectal cancer is more common than colon cancer; (II) the proportion of lower rectal cancer is higher; and (III) patients are much younger. Patients younger than 30 years of age accounted for 10% to 30%. Surgery combined with radiotherapy and chemotherapy has been the standard treatment since 1990s. Several large randomized studies have shown that preoperative fluorouracil-based chemoradiotherapy can significantly reduce the local recurrence rate and improve sphincter preservation and the quality of life.
With the preoperative fluorouracil-based chemoradiotherapy being accepted by oncologists, it has become T3, T4 and/or lymph node-positive patients’ primary therapy. However CAO/ARo/AIO-94 (1,2) showed that compared with neoadjuvant chemotherapy, preoperative fluorouracil-based chemoradiotherapy doesn’t improve overall survival (OS) and progression-free survival (PFS). Therefore, oncologists have started to search for more effective preoperative chemoradiotherpy regimen. In this study, we combined oxaliplatin with the standard treatment, and compared and analyzed the effectiveness of these two treatment patterns.
Materials and methods
Patient population
A total of 206 patients treated in Liaoning Cancer Hospital & Institute and enrolled in the prospective study had histologically confirmed rectal cancer of clinical stage II/III during July 2007 to July 2010. They were randomized (1:1 ratio) into the experimental group (oxaliplatin + capecitabine + radiotherapy) and the control group (capecitabine + radiotherapy). Informed consent was obtained from all the patients.
Inclusion criteria included histologically confirmed rectal adenocarcinoma of clinical stage II/III. Rectal adenocarcinoma should be accessible to digital rectal examination as pretreatment assessment including chest, abdominal and pelvis CT/ultrasound/X-ray scan in case of any suspicious shadows which insured M0 (no distant metastasis). Baseline assessment also included transrectal ultrasonography and a rigid rectoscopy inspection to ensure the inferior margin located no more than 12 cm above the anal verge. For local staging, magnetic resonance imaging (MRI) was recommended but was not mandatory. These options were also used to confirm T3/T4 or lymph node-positive. If the findings of endorectal ultrasound and MRI differed, the highest stage was recorded. Lymph nodes measuring more than 5 mm were considered positive. With no previous pelvic treatment, patients were eligible for preoperative chemoradiotherapy if evidence of perirectal fat infiltration (any cT3) or resectable (potentially to achieve a R0 or R1 resection with no evidence of infiltration of the pelvic wall, prostate, or base of the urinary bladder) infiltration of adjacent tissues or organs (cT4) or regional lymph-node metastases (cN1-2) were shown. In addition, patients with T2 Nx tumors located in the distal anterior or lower rectum were also eligiblely included, regardless of biopsy or radiologic evidence. The hematological parameters reflect bone marrow function, and liver and renal function should be adequate. Bone marrow: white blood cell (WBC) >4.0×109/L, hemoglobin >10 g/dL, neutrophils >1,500 cells/µL, platelets >100,000 cells/µL. Liver: direct bilirubin <1.5× the upper limit of normal; aspartate aminotransferase, alanineamino transferase <3× the upper limit of normal. Kidney: creatinine <1.5× the upper limit of normal. Other inclusion criteria were Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, aged 18 years or older, but less than 80 years with no heart failure, diabetes and other serious complicated diseases.
Exclusion criteria were patients who had history of any other malignancies no matter previous or concurrent with the exception of adequately treated basal cell carcinoma (BCC) of the skin or in situ carcinoma of the uterine cervix, any serious diseases such as clinically significant cardiac disease (unstablecardiac angina, myocardial infarction and heart failure) within the past 6 months, serious liver disease, or kidney failure. Further exclusion criteria were fluorouracil and oxaliplatin drug allergies such as peripheral neuropathy more than grade 2 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 3.0), treatment related metabolic disorders such as chronic diarrhoea more than grade 1, as well as women in pregnancy, lactation or lack of proper contraception.
Device and treatment agent
The major radiotherapy devices included Varian 2300CD Linac, Pinnacle 3D plan system, thermoplastic mouldings, and CT simulated positioner. Capecitabine (Xeloda, 0.5 g/tablet, 12 tablets/box) is produced by Roche (Shanghai, China), which is one of oral chemotherapy drugs, and oxaliplatin (AiHeng, 100 mg/bottle) is produced by HengRui Company (Jiangsu, China).
Treatment plan
Radiotherapy
All patients received immobilization posture and 3D radiotherapy plan on CT image before radiotherapy. Patients needed to empty their bladder before CT locating their radiotherapy center, and then drank 1,000 mL of water, which was mixed with 20 mL 20% meglumine diatrizoate. The radiotherapy areas included the tumor, the surrounding tissue extending 1 cm around the tumor, the mesentery of rectum, presacral region, pelvic sidewall, internal iliac lymph region and external iliac lymph region, the upper bound among 5th lumbar vertebrae and 1st sacral vertebrae, the bilateral small pelvis, the anterior limiting lamina including 1/3 or 1/4 of the anterior wall of the filled bladder, and the posterior parietal including half of the cortical sacrum (upper 3rd sacrum) or behind the cortical sacrum (below 3rd sacrum). Varian 2300 CD was used with 10 M. Radiotherapy was delivered to three or four fields to the tumor and lymph node regions, and perirectal soft tissue structures. All patients also received 50 Gy radiation in 25 fractions over 5 weeks (200 cGy per fraction per day).
Chemotherapy
During radiotherapy, the control group also received oral capecitabine (800 mg/m2 b.i.d., d 1−14 and d 22−25), while the experimental group received capecitabine (800 mg/m2 b.i.d., d 1−14 and d 22−25) plus oxaliplatin (60 mg/m2, i.v. over 2 h, on d 1, 8, 22 and 29). After surgery, all patients also received 6−8 cycles of FOLFOX (5-FU 400 mg/m2 i.v. bolus on d 1 then 2,400 mg/m2 over 46−48 h, oxaliplatin 85 mg/m2 i.v. over 2 h on d 1, and leucovorin 400 mg/m2 i.v. over 2 h on d 1) after surgery.
Surgery and histopathology
Surgery was performed 6−10 weeks (3,4) after completion of chemoradiotherapy. If possible total mesorectal excision (TME) with sphincter preservation is preferred. Histopathological examination of resected specimen was performed according to Quirke’s method (5). A positive circumferential resection margin (CRM) was defined as tumor ≤1 mm from the margin (National Comprehensive Cancer Network, NCCN). This assessment included both tumor within a lymph node and direct tumor extension.
Assessments during and after treatment
All patients were evaluated before treatment by collecting health history and results from physical examination, pelvic CT/MRI, abdominal and neck lymph node ultrasound, chest CT, electrocardiogram (ECG), liver and kidney function, tumor markers, and complete blood test. After surgery, the residual tumors were re-staged according to the American Joint Committee on Cancer/International Union against Caner (AJCC/UICC) TNM system. During therapy, adverse events (AEs) were evaluated according to National Cancer Institute Common Terminology Criteria (CTC version 3.0). All patients were scheduled to be followed up for at least 3 years to assess OS, disease-free survival (DFS), local recurrence, metastasis and AEs.
Statistical analysis
The primary endpoints were OS and DFS. OS is defined as the time interval between diagnosis and death or the last follow-up, whichever occurred first. DFS is defined as the time interval between diagnosis and the first occurrence of any of the following events, including non-radical surgery of the primary tumor (R2 resection), local recurrence after R0/1 resection, distant metastasis, progression or death from any cause. Patients who were alive and did not have any disease recurrence were censored for the analysis of DFS. Secondary endpoints included compliance, pathological complete remission (pCR) and toxicities. Data were expressed as x±s for continuous variables and frequency (percentage) for categorical variables. Chi-squared test was used to assess the difference between the two treatment groups in terms of categorical variables and two-sample t-test was used to compare the distribution of continuous variables. The probabilities of OS and DFS were estimated using the Kaplan-Meier method. Log-rank test was performed to compare OS and PFS between the two treatment groups. K-S test was used to evaluate the distribution. P<0.05 was considered statistically significant. All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA). All eligible patients were included in the analysis according to the intention-to-treat principle except for compliance and safety related endpoints which included all of the patients as treated. Our hypothesis was to increase OS from 84% in the control group to 96% in the experimental group. In order to detect such a difference and achieve a power of 80%, with α=0.025 (two-tailed), 206 randomly assigned patients were required.
The patients were enrolled by the investigators of this study. The computer-generated randomization codes (sequential permuted blocks) were stratified by clinical T category (cT1−3 vs. cT4) and clinical N category (cN0 vs. cN1−2). Because the patients were involved in different treatment schedules, they were not masked throughout the study.
Results
Follow up
Patients’ characteristics are summarized in Table 1. The median follow-up time was 48.7 months in this study, and the longest follow-up time was 79 months. In the experimental group, 10 patients died within 3 years, of whom 9 died of local recurrence and distance metastasis, and 1 died of non-rectal cancer. There were 2 cases lost to follow-up. In the control group, 14 cases died within 3 years. Among them, 12 cases died of local recurrence and/or distance metastasis, and 2 cases died of non-rectal reasons. There was 1 case lost to follow-up. The detailed follow-up data of patients are shown in Table 2.
Table 1. Patients’ baseline characteristics.
| Variable | Experimental group (N=103) | Control group (N=103) | P |
|---|---|---|---|
| Age (year) | 0.082 | ||
| <60 | 63 | 63 | |
| 60−70 | 36 | 28 | |
| >70 | 4 | 12 | |
| Median age | 56 | 58 | |
| Mean age () | 55.81±2.45 | 55.95±2.33 | |
| Sex | 0.197 | ||
| Male | 59 | 68 | |
| Female | 44 | 35 | |
| T stage | 0.736 | ||
| T1−2 | 2 | 3 | |
| T3 | 66 | 61 | |
| T4 | 35 | 39 | |
| N stage | 0.541 | ||
| N0 | 22 | 23 | |
| N1 | 54 | 56 | |
| N2 | 27 | 24 | |
| Distance to anus (cm) | 0.986 | ||
| <4 | 24 | 25 | |
| 4−8 | 58 | 57 | |
| >8 | 21 | 21 | |
| ECOG status | 0.924 | ||
| 0 | 83 | 82 | |
| 1 | 15 | 16 | |
| 2 | 5 | 5 |
ECOG, Eastern Cooperative Oncology Group.
Table 2. Patients’ follow-up data.
| Data | Experimental group | Control group | P |
|---|---|---|---|
| Lost to follow-up | 2 | 1 | |
| Eligible number | 101 | 102 | |
| Total number of recurrence and metastasis | 20 | 31 | 0.106 |
| Distant metastasis only | 15 | 25 | |
| Local recurrence only | 3 | 2 | |
| Recurrence with metastasis | 2 | 4 | |
| 3-year local recurrence rate | 4.85% (5/103) | 5.83% (6/103) | 0.694 |
| 3-year distant metastasis rate | 16.50% (17/103) | 28.16% (29/103) | 0.045 |
| 3-year progression rate | 19.42% (20/103) | 30.10% (31/103) | 0.106 |
| Total number of death | 10 | 14 | 0.515 |
| Died from local recurrence and/or distance metastasis | 9 | 12 | |
| Died from non-rectal reasons | 1 | 2 | |
| 3-year DFS rate | 80.58% (83/103) | 69.90% (72/103) | 0.076 |
| 3-year OS rate | 90.29% (93/103) | 86.41% (89/103) | 0.515 |
DFS, disease-free survival; OS, overall survival.
The primary endpoints of this study were DFS and OS. The 3-year OS in the experimental group and the control group were 90.29% and 86.41%, respectively (P>0.05) (Figure 1), with a power of 18.42%. The 3-year DFS for the experimental group and the control group were 80.58% and 69.90%, respectively (P>0.05) (Figure 2), with a power of 22.06%. Although no significant differences were found in the primary endpoints, both OS and DFS increased by about 5−10% in the experimental group. From the survival curve, the experimental group tends to increase the survival compared with the control group with the time prolonging. The 3-year distant metastasis rate in the experimental group and the control group was 16.50% and 28.16%, respectively (P=0.045) (Figure 3).
Figure 1.
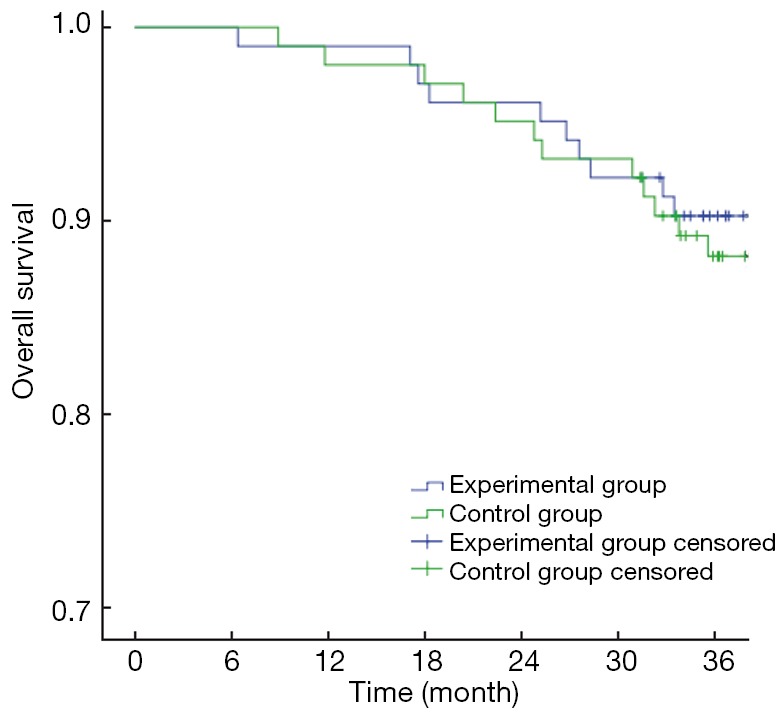
Curves of overall survival.
Figure 2.
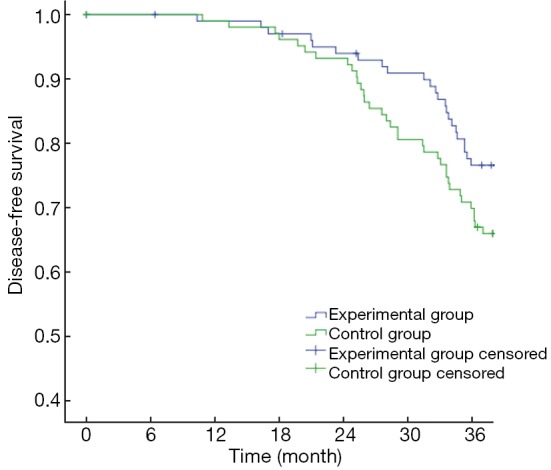
Curves of disease-free survival.
Figure 3.
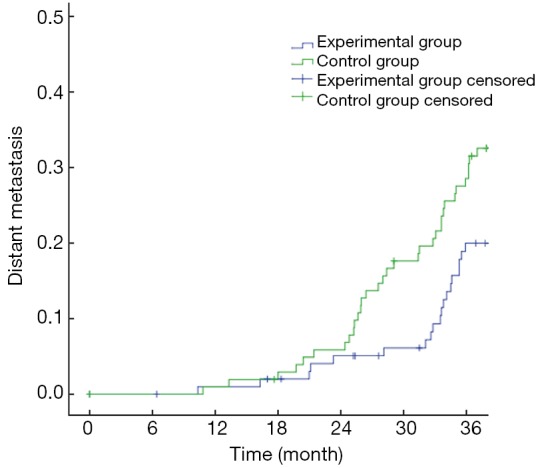
Curves of distant metastasis rate.
Compliance and acute toxicity
During chemoradiotherapy, 90 patients (87.38%) in the control group received full-dose chemoradiotherapy, while only 81/103 (78.64%) received full-dose in the experimental group. In the experimental group, 6 patients received only 46 Gy radiation and 1 patient received only 44 Gy and full dose chemotherapy because of unbearable local anal pain; while 5 patients in the control group received only 46 Gy. Three patients received 60% to 80% of the full chemotherapy dose due to grade 3/4 diarrhea, and 2 patients received only 50% of the total dose because of hematologic toxicity. In the experimental group, 1 patient received 75% of the oxaliplatin dose because of neurotoxicity, 1 patient received 75% of the total chemotherapy dose because of local pain, 8 patients received 50−75% of the total chemotherapy dose due to grade 3/4 diarrhea, 3 patients received 25−75% of the total chemotherapy dose due to hematologic toxicity, and 4 patients received 46−48 Gy radiation.
Due to grade 3/4 toxicities such as hematologic toxicity, abdominal pain and diarrhea, only 88 patients (85.44%) in the experimental group received 100% of the capecitabine dose, 84 patients (81.53%) received 100% of the oxaliplatin dose. In the control group, 90 patients received full dose of chemoradiotherapy. Table 3 summarizes patient compliance of the two groups.
Table 3. Patients’ compliance.
| Treatment | Experimental group (N=103) |
Control group (N=103) |
P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Radiotherapy | ||||||
| Full dose (50 Gy) | 96 | 93.20 | 98 | 95.15 | 0.768 | |
| ≥90% planed dose | 102 | 99.03 | 103 | 100 | ||
| Chemotherapy | ||||||
| Capecitabine | ||||||
| Full dose | 88 | 85.44 | 90 | 87.38 | 0.555 | |
| ≥90% planed dose | 97 | 94.17 | 98 | 95.15 | ||
| ≥80% planed dose | 100 | 97.09 | 100 | 97.09 | ||
| Oxaliplatin | ||||||
| Full dose | 84 | 81.55 | – | – | ||
| ≥75% planed dose | 91 | 88.35 | – | – | ||
| ≥50% planed dose | 102 | 99.03 | – | – | ||
The most common grade 1−2 toxicities were gastrointestinal reactions and hematologic toxicities, however, most of these acute toxicities were well tolerated. There were no significant differences between the two treatment arms in most grade 3/4 toxicities except grade 3−4 diarrhea and the total grade 3/4 acute toxicities. Table 4 describes the incidence of acute toxicities during the course of treatment.
Table 4. Common acute toxicities during chemoradiotherapy (N=103).
| Toxicity | Grade 1−2 [n (%)] |
Grade 3−4 [n (%)] |
P | |||
|---|---|---|---|---|---|---|
| Control group | Experimental group | Control group | Experimental group | |||
| Diarrhea | 44 (42.72) | 50 (48.54) | 7 (6.80) | 17 (16.50) | 0.030 | |
| Nausea | 20 (19.42) | 42 (40.78) | 0 (0) | 3 (2.91) | >0.05 | |
| Abdominal pain | 5 (4.85) | 20 (19.42) | 0 (0) | 2 (1.94) | >0.05 | |
| Anemia | 17 (16.50) | 19 (18.45) | 3 (2.91) | 5 (4.85) | >0.05 | |
| Neurotoxicity | 0 (0) | 2 (1.94) | 0 (0) | 1 (0.97) | >0.05 | |
| Fever | 5 (4.85) | 6 (5.83) | 1 (0.97) | 1 (0.97) | >0.05 | |
| Fatigue | 20 (19.42) | 23 (22.33) | 0 (0) | 3 (2.91) | >0.05 | |
| Dysuria | 25 (24.27) | 28 (27.18) | 1 (0.97) | 2 (1.94) | >0.05 | |
| Total | 90 (87.38) | 93 (90.29) | 11 (10.68) | 22 (21.36) | 0.037 | |
Surgery and pathology
All patients received surgical treatment, and the median time between surgery and chemoradiotherapy was 52 d (range, 46−80 d) for the control group, and 56 d (range, 40−85 d) for the experimental group. A total of 87 patients underwent Dixon operation, 15 patients received Miles operation, and 1 patient received Hartmann’s operation in the experimental group. In the control group, 80 patients underwent Dixon, 22 patients underwent Miles and 1 received Hartmann. All patients were TNM restaged based on pathology after surgery, and the results are summarized in Table 5. No patient achieved R2 resection in the experimental group, while 1 patient achieved R2 resections in the control group.
Table 5. Surgical procedures and grading of patients after surgery.
| Grade | Experimental group (N=103) |
Control group (N=103) |
P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Surgery type | 0.495 | |||||
| Dixon | 87 | 84.47 | 80 | 77.67 | ||
| Miles | 15 | 14.56 | 22 | 21.36 | ||
| Hartmann | 1 | 0.97 | 1 | 0.97 | ||
| T stage | 0.962 | |||||
| 0 | 24 | 23.30 | 20 | 19.42 | ||
| 1 | 20 | 19.42 | 21 | 20.39 | ||
| 2 | 21 | 20.39 | 20 | 19.42 | ||
| 3 | 23 | 22.33 | 26 | 25.24 | ||
| 4 | 15 | 14.56 | 16 | 15.53 | ||
| N stage | 0.313 | |||||
| 0 | 74 | 71.84 | 65 | 63.11 | ||
| 1 | 23 | 22.33 | 27 | 26.21 | ||
| 2 | 6 | 5.83 | 11 | 10.68 | ||
| M stage | – | |||||
| 0 | 103 | 100 | 103 | 100 | ||
| 1 | 0 | 0 | 0 | 0 | ||
| Phase | 0.455 | |||||
| 0 | 24 | 23.30 | 20 | 19.42 | ||
| I | 50 | 48.54 | 45 | 43.69 | ||
| II | 20 | 19.42 | 22 | 21.36 | ||
| III | 9 | 8.74 | 16 | 15.53 | ||
| IV | 0 | 0 | 0 | 0 | ||
| CRM | 0.761 | |||||
| R0 | 100 | 97.09 | 98 | 95.15 | ||
| R1 | 2 | 1.94 | 3 | 2.91 | ||
| R2 | 1 | 0.97 | 2 | 1.94 | ||
| pCR | 24 | 23.30 | 20 | 19.42 | 0.497 | |
TME, total mesorectal excision; CRM, circumferential resection margin; pCR, pathological complete remission.
The number of patients who achieved R1 resection was 2 and 3, respectively, for the experimental group and the control group. The remaining patients achieved R0 resections. The pCR rate in the experimental group was 23.30%, and was 19.42% in the control group. There was no significant difference in the pCR rate between the two groups (P=0.497).
Discussion
The fluorouracil-based chemoradiotherapy gradually become the standard treatment for stage II/III rectal cancers since 1990s. But it cannot reduce local recurrence and distant metastasis (6). How to prolong survival, how to reduce local recurrence and distant metastasis, and how to reduce the side effects of the treatment of rectal cancer have become a key problem. INT-0114 study (7) has shown that radiotherapy combined with continuous infusion of 5-FU compared with radiotherapy combined with bolus 5-FU can significantly reduce the local recurrence rate and toxicity. A recently research (8) found that capecitabine combined with radiotherapy is not inferior to continuous infusion of 5-FU combined with radiotherapy. Also, hematological toxicity was less severe than that of continuous infusion of 5-FU, however, the hand-foot syndrome was more severe. Multivariate analyses showed that the 5-year OS and 3-year DFS were significantly improved in patients with hand-foot syndrome.
Treatment-related toxicity is an important factor affecting patients’ compliance, although there are several classic studies showing that capecitabine and oxaliplatin combined with radiotherapy significantly increased treatment-related toxicity rate, but did not improve patients’ OS, nor did it reduce local recurrence and distant metastasis compared with capecitabine in combination with radiotherapy. The STAR-01 (9) study randomly assigned 747 cases into two groups, and it found that grade 3/4 toxicities increased from 8% to 24% when treated with fluorouracil-based chemoradiotherapy plus oxaliplatin. The most common grade 3/4 toxicity is diarrhea, and it increased to 15% compared with 4% in the control group. The study also showed that capecitabine plus oxaliplatin significantly increased the rates of other grade 3−4 toxicities, such as vomiting, hematologic toxicity, neuro-toxicitiy and fatigue. The CAO/AIO/ARO-04 (10,11) study showed that the grade 3−4 gastrointestinal toxicities in oxaliplatin group and the control group were 20% vs. 15%. The most common toxicity is still diarrhea, nausea and vomiting, but the differences are not significant. The study concluded that fluorouracil-based chemotherapy plus oxaliplatin added no additional toxicities. Our study also found that gastrointestinal toxicities such as diarrhea, nausea and vomiting are the most common toxicities, and the rates of these toxicities significantly increased in the experimental group.
In our study, grade 3−4 diarrhea occurred in 16.5% and 6.8%, respectively, in the experimental group and the control group (P<0.05). These gastrointestinal toxicities were partly alleviated after being treated, and chemoradiotherapy was resumed in those patients. Also, other grade 3−4 toxicities did not increase with the experimental treatment. In regard to treatment compliance, 78.64% of patients in the experimental group received full dose of treatment, compared to 87.38% in the control group. Therefore, the authors believe that capecitabine and oxaliplatin combined with radiotherapy did increase the rate of grade 3−4 diarrhea, but the toxicity was generally well tolerated.
In our study, although the sphincter preservation rate increased by nearly 10% in the experimental group, the difference is not statistically significant, which may be due to the fact that the sample size is not large enough. pCR is an important indicator to evaluate short-term treatment effect. The rate of pCR for neoadjuvant capecitabine and oxaliplatin in combination with radiotherapy in rectal cancer patients varies between 0% and 26% in the literature (12-15). In this study, the pCR rates in the experimental group and the control group were 23.40% and 19.42%, respectively (P=0.497). ACCORD 12/0405-prodige 2 studies (16) randomly divided 598 patients into two groups with or without oxaliplatin, and the pCR rates were 19% and 15%, respectively. However, the difference was not statistically significant. STAR-01 (9) has a similar result, however, the CAO/ARO/AIO-04 study (6,12-14) showed that oxaliplatin and fluorouracil in combination with radiotherapy improved the pCR rate, with no additional emerging toxicities compared with fluorouracil combined with radiotherapy. That study also showed that fluorouracil plus oxaliplatin did not significantly reduce local recurrence rate. However, the schedule of fluorouracil was not the same in that study, which may contribute to the positive result.
The treatment failure for rectal cancer is often due to local recurrence and metastasis. Though fluorouracil-based chemoradiotherapy as the standard treatment significantly reduces the local recurrence rate, clinical studies have showed that compared with preoperative radiotherapy, preoperative chemoradiotherapy does not reduce the distant metastasis. In this study, we have found that adding oxaliplatin to capecitabine-based chemotherapy can significantly reduce the rate of distant metastasis compared to capecitabine-based chemotherapy alone (3-year distant metastasis rate of 16.50% vs. 28.16%, P=0.045).
This trial had some limitations. First, due to the hypothesis of OS of 84% in the control group was too low, the study is underpowered to reach statistical significance for the main endpoint. To detect a difference of 12%, the number of patients (N=206) was small. Second, a follow-up duration of 3 years was too short to capture a full clinical endpoint event such as OS, rate of local recurrence, and late toxicity occurring in the patients both in the experimental and control groups. Analysis of these endpoints will be provided in the near future. Third, a large proportion of our patients are stage III younger patients, which may have contributed to our findings. Other studies (17-19) have also showed that adding oxaliplatin to fluorouracil-based chemotherapy failed to gain a survival benefit in elderly stage II patients, yet oxaliplatin combined with fluorouracil can significantly improve OS in stage III rectal cancer. The NSABP c-07 (20,21) study has shown that there was no significant difference in OS between patients treated with oxaliplatin combined with capecitabine and those treated with oxaliplatin in combination with 5-FU. Another study (22) also showed that elderly patients of stage III can benefit from XELOX, but the benefit is more significant in younger patients (23). Aschele also has similar findings that oxaliplatin added to fluorouracil-based chemoradiotherapy could reduce the rate of distant metastasis (9).
As a result, even though the power is low, we still can see adding oxaliplatin to fluorouracil-based chemoradiotherapy does not tend to increase recent effects, but tend to reduce the distant metastasis rate, thus long-term effect also needs longer time to observe.
Conclusions
The study finds that adding oxaliplatin to capecitabine-based preoperative chemoradiotherapy can significantly reduce distant metastasis, but it did not reduce the rate of local recurrence. Although the rate of grade 3−4 toxicities is higher in the experimental group (primarily gastrointestinal toxicities), patients can stand to be followed up with allopathic treatment. OS was not improved with the experimental treatment, and longer follow-up time may be required to confirm the findings.
Acknowledgements
None.
Footnotes
Conflicts of Interests: The authors have no conflicts of interest to declare.
References
- 1.Sauer R, Fietkau R, Wittekind C, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 2003;5:406-15. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [DOI] [PubMed] [Google Scholar]
- 3.Glehen O, Chapet O, Adham M, et al. Long-term results of the Lyons R90-01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter-saving surgery in rectal cancer. Br J Surg 2003;90:996-8. [DOI] [PubMed] [Google Scholar]
- 4.Foster JD, Jones EL, Falk S, et al. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum 2013;56:921-30. [DOI] [PubMed] [Google Scholar]
- 5.Quirke P, Palmer T, Hutchins GG, et al. Histopathological work-up of resection specimens, local excisions and biopsies in colorectal cancer. Dig Dis 2012;30 Suppl 2:2-8. [DOI] [PubMed] [Google Scholar]
- 6.Schmoll HJ, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: Disease-free survival results at interim analysis. J Clin Oncol 2014;32:abstr 3501.
- 7.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol 2014;32:1927-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [DOI] [PubMed] [Google Scholar]
- 10.Reibetanz J, Germer CT. Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin in rectal cancer: initial results of the CAO/ARO/AIO-04 study. Chirurg 2012;83:995. [DOI] [PubMed] [Google Scholar]
- 11.Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [DOI] [PubMed] [Google Scholar]
- 12.Lefevre JH, Rousseau A, Svrcek M, et al. A multicentric randomized controlled trial on the impact of lengthening the interval between neoadjuvant radiochemotherapy and surgery on complete pathological response in rectal cancer (GRECCAR-6 trial): rationale and design. BMC Cancer 2013;13:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Aguilar J, Shi Q, Thomas CR, Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu SK, Bhangu A, Tait DM, et al. Chemoradiotherapy response in recurrent rectal cancer. Cancer Med 2014;3:111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012;30:4558-65. [DOI] [PubMed] [Google Scholar]
- 17.Miyake Y, Ikeda K, Osawa H, et al. Fluoropyrimidines with oxaliplatin (L-OHP) as an adjuvant chemotherapy for Stage III colon cancer. Gan To Kagaku Ryoho 2012;39:2161-3. [PubMed] [Google Scholar]
- 18.Shiroiwa T, Takeuchi T, Fukuda T, et al. Cost-effectiveness of adjuvant FOLFOX therapy for stage III colon cancer in Japan based on the MOSAIC trial. Value Health 2012;15:255-60. [DOI] [PubMed] [Google Scholar]
- 19.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Jiang H, Niu J, et al. Correlation of ADC value with pathologic indexes in colorectal tumor homografts in Balb/c mouse. Chin J Cancer Res 2014;26:444-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamboni BA, Yothers G, Choi M, et al. Conditional survival and the choice of conditioning set for patients with colon cancer: an analysis of NSABP trials C-03 through C-07. J Clin Oncol 2010;28:2544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353-60. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Peng S. Identification of metastasis-associated genes in colorectal cancer through an integrated genomic and transcriptomic analysis. Chin J Cancer Res 2013;25:623-36. [DOI] [PMC free article] [PubMed] [Google Scholar]


