Abstract
FGF1 is a nonclassically released growth factor that regulates carcinogenesis, angiogenesis and inflammation. In vitro and in vivo, FGF1 export is stimulated by cell stress. Upon stress, FGF1 is transported to the plasma membrane where it localizes prior to transmembrane translocation. To determine which proteins participate in the submembrane localization of FGF1 and its export, we used immunoprecipitation mass spectrometry to identify novel proteins that associate with FGF1 during heat shock. The heat shock-dependent association of FGF1 with the large protein AHNAK2 was observed. Heat shock induced the translocation of FGF1 and AHNAK2 to the cytoskeletal fraction. In heat-shocked cells, FGF1 and the C-terminal fragment of AHNAK2 colocalized with F-actin in the vicinity of the cell membrane. Depletion of AHNAK2 resulted in a drastic decrease of stress-induced FGF1 export but did not affect spontaneous FGF2 export and FGF1 release induced by the inhibition of Notch signaling. Thus, AHNAK2 is an important element of the FGF1 nonclassical export pathway.
Keywords: AHNAK2, FGF1, nonclassical secretion, actin cytoskeleton, heat shock
A large group of secreted proteins lack signal peptides, and are thus unable to use the classical secretion pathway that involves the endoplasmic reticulum and Golgi. Instead, these molecules use various mechanisms of nonclassical export (Prudovsky et al., 2013; Rabouille et al., 2012). The two most ubiquitously expressed members of the fibroblast growth factor (FGF) family, FGF1 and FGF2, lost their signal peptides during evolution (Coulier et al., 1997). They are released as a result of direct translocation through the cell membrane (Prudovsky et al., 2013; Rabouille et al., 2012). An interesting and heretofore unexplained feature of the release of FGF1 and 2 is that FGF2 export is constitutive (Engling et al., 2002), whereas FGF1 release is stimulated by various types of cell stress, including heat shock, hypoxia and growth factor starvation (Ananyeva et al., 1997; Jackson et al., 1992; Mouta Carreira et al., 2001; Shin et al., 1996). FGF1 release is preceded by stress-induced accumulation of FGF1 in the vicinity of the cell membrane (Prudovsky et al., 2002).
Stress-dependent FGF1 secretion requires copper ions (Landriscina et al., 2001a) and depends on several signal peptide-less nonclassically exported proteins: the small calcium-binding protein S100A13 (Landriscina et al., 2001b); 40 kDa synaptotagmin 1 (Bagala et al., 2003; Tarantini et al., 1998), which is the product of alternative initiation of synaptotagmin 1 (Syt1) mRNA translation, and the enzyme sphingosine kinase 1 (Soldi et al., 2007). FGF1 binds acidic phospholipids, particularly phosphatidylserine (PS), with high affinity (Tarantini et al., 1995). In the plasma membrane of nonstressed cells, PS is localized exclusively in the inner leaflet of the plasma membrane (Schroeder, 1984). Under certain conditions, including different forms of stress, PS translocates to the outer membrane leaflet (Kirov et al., 2012; Shvedova et al., 2002). We have recently found that stress-induced FGF1 export proceeds through discretely localized domains of the cell surface, which are characterized by externalization of PS (Kirov et al., 2012).
In the present study, we used immunoprecipitation mass spectrometry to identify previously unrecognized proteins that associate with FGF1 during the process of stress-mediated secretion. We found that in stressed cells, FGF1 functionally associates with the protein AHNAK2, which is required for nonclassical FGF1 secretion. Interestingly, stress induces association of both FGF1 and AHNAK2 with the cytoskeleton and their co-localization in the vicinity of the cell membrane. Thus, these studies identify a new mechanistic component of stress-mediated FGF1 secretion.
Experimental Procedures
Cell culture
Murine NIH 3T3 cells (ATCC, Manassas, VA) were grown in DMEM (HyClone, Logan, UT) supplemented with 10% bovine calf serum (HyClone).
Genetic constructs
Human FGF1 cloned in the pMEXneo expression vector (Jackson et al., 1992) was recloned into the SalI and EcoRI restriction sites of the pcDNA3/HA vector (Clontech, Mountain View, CA) thus forming FGF1:HA. Next, FGF1:HA was cloned into the multiple cloning site of the shuttle vector pAdlox (a generous gift of Lisa Phipps, Somatix Therapy Corporation, CA). Human FGF2:HA, a gift of A. Baird (UC San Diego), was cloned in pAdlox. dnCBF1 (gift of T. Honjo, University of Kyoto) was Myc tagged and cloned in pAdlox as described (Kacer et al., 2011).
Genetic constructs pGFP-V-RS expressing shRNAs that suppress the expression of mouse AHNAK2 were obtained from Origene (Rockville, MD). The following AHNAK2-specific sequences were used: 1. 5’-TGCTGAGATGGAGGCTAGAACAGGAAGCT-3’; 2. CCACATCTGGAGGTGATTTGGTAGTCGTA-3’; 3. 5’-GCAATGGTGGCATCCTCTGCGAGAACAGA-3’.
The construct MHSS1010-9205042, which comprises the plasmid pCMV-SPORT6 and the human AHNAK2 cDNA coding for the C-terminal 483 amino acid fragment of this protein (ctAHNAK2) between NotI and SalI sites was obtained from Open Biosystems. It was originally produced by Strausberg et al. (Strausberg et al., 2002). The ctAHNAK2 was C-terminally tagged with the V5 tag and cloned between the BamHI and SmaI sites of the pAdlox construct.
Production of adenoviruses and cell transduction
Recombinant FGF1:HA, FGF2:HA, dnCBF1 and ctAHNAK2:V5 adenoviruses were produced, purified, and titered as described (Duarte et al., 2008). Briefly, CRE8 cells were transfected with SfiI-digested pAdlox-derived constructs, and infected with the ψ5 virus. The lysates were prepared 2 days after infection. The virus was passaged twice through CRE8 cells, and purified from the second passage via cesium density gradient centrifugation. The virus preparations were quantified by optical density at 260 nm, and the bioactivity was determined by a plaque-forming unit assay. Adenoviral transduction was performed in serum-free DMEM with approximately 103 viral particles/cell in the presence of poly-D-lysine hydrobromide (Sigma) (5×103 molecules/viral particle) for 2 h at 37°C. Next, the adenovirus-containing medium was removed and replaced with serum-containing medium. The cells were plated for experiments 24-48 h after transduction.
Immunoprecipitation mass spectrometry
NIH 3T3 cells transduced with the FGF1:HA adenovirus were subjected to 90 min incubation at 37°C, or 42°C heat stress, in serum-free DMEM. Following incubation, the cells were scraped into ice-cold PBS and then lysed in the buffer of the following composition: 20 mM Tris HCl pH 7.5, containing 300 mM sucrose, 60 mM KCl, 150 mM NaCl, 5% glycerol, 2 mM EDTA, 1% Triton X-100, 0.2% deoxycholate, and Protease Inhibitor Cocktail (Sigma) in the dilution recommended by the manufacturer. The cell lysates were centrifuged at 10,000 rpm, and supernatants were pre-cleared by overnight rotation with mouse IgG and protein A-G-Sepharose beads (Santa Cruz Biochemicals), followed by centrifugation. The pre-cleared supernatants were divided into equal aliquots and rotated overnight with mouse IgG or mouse anti-HA monoclonal antibodies (Covance) in presence of Protein A-G Sepharose. After rotation, the Sepharose beads were precipitated by centrifugation at 5000 g for 5 min, washed with lysis buffers, and used for mass spectrometric analysis.
Candidate protein-derived tryptic peptides were identified by liquid nanoscale chromatography-mass spectrometry (LC-MS) as described previously (Prudovsky et al., 2012; Romero et al., 2011; Young et al., 2012). Briefly, purified proteins (approximately 30 μg) were digested with sequencing grade trypsin (Sigma) and the resulting tryptic peptides were isolated by utilizing reverse phase C18 spin columns (PepClean C18 column, Pierce). The extracted peptides were analyzed by LC-MS via quadrupole-time-of-flight and linear ion trap mass spectrometry (QSTAR, 4000QTRAP, respectively, ABSciex) mass spectrometers. For mass spectrometry, peptides were separated by using a linear 0-60% water/acetonitrile gradient (0.1% formic acid) on a U-3000 RSLC nanoscale capillary liquid chromatograph (Thermofisher-Dionex) fitted with an Acclaim PepMap reversed-phase capillary column (3 μm, C18, 100 A° pore size, 75 μm ID × 15 cm, 15 μm tip; LC Packings), and an inline PepMap 100 precolumn (C18, 300 μm ID × 5 mm; LC Packings) that served as a loading column.
Peak list generation, peptide mass fingerprint peak picking, and relative quantification and protein identification were performed with the ProteinPilot™ software (ABSciex). Default parameters were used for all analyses. Protein database searches were conducted using the UniProtKB/Swiss-Prot protein knowledge base released not later than January 2014. MS/MS spectra were searched against a database of mouse and human protein sequences. Threshold score for accepting individual MS/MS spectra was 95% confidence. Trypsin autolysis peaks and keratin tryptic peptides were known contaminants that were excluded from database searching.
shRNA suppression of AHNAK2 expression
NIH 3T3 cells were transfected with the constructs coding for shRNAs against AHNAK2 or with control scrambled shRNA using the Fugene 6 transfection reagent (Promega, Madison, WI) according to the manufacturer’s protocol. The transfected clones were selected using medium with 2 μg/ml puromycin (Sigma). RT-PCR analysis of AHNAK2 expression was performed with total RNA isolated from transfectant cells by utlizing the RNeasy kit (Qiagen, Valencia, CA). Total RNA (1 μg) was used as a template for the PCR reaction performed with the Platinum Tap One Step RT-PCR Kit (Invitrogen). The following mouse AHNAK2 PCR primers were utilized: forward 5'-CCTGTGCAGGCCTGTGTGTATAC-3' and reverse 5'-GTATCCATGCCAGAGACCTTAAC-3'. β-actin expression served as a control for RNA levels. The amplification products were visualized by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Confocal fluorescence microscopy
To simultaneously assess the stress-induced translocation of AHNAK2 and FGF1, NIH 3T3 cells were co-transduced with FGF1:HA and ctAHNAK2:V5 adenoviruses and plated on coverslips for 24 h, incubated for 90 min at 37°C or 42°C, formalin fixed, and co-stained with FITC-conjugated anti-HA monoclonal antibodies (Sigma) and CY3-conjugated anti-V5 antibody (Sigma). Fluorescent preparations were embedded in Vectashield (Vector Laboratories, Burlingame, CA), and images were taken by utilizing a Leica SP1 or SP8 confocal microscope. Both mass spectrometry and confocal microscopy were conducted using the MMCRI COBRE in Vascular Biology DNA and Protein Analysis and Cell Imaging Core facility.
Analysis of FGF1 release
The NIH 3T3 cells stably transfected with AHNAK2 shRNAs or control scrambled shRNAs were used to study FGF1 release 48 h after adenoviral transduction with FGF1. The heat shock-induced FGF1 release assay was performed by incubation of the cells at 42°C or 37°C (control) for 90 min in serum-free DMEM containing 5 U/ml of heparin (Sigma), as previously described (Jackson et al., 1992). Conditioned media were collected, briefly centrifuged at 1,000g to remove detached cells, and FGF1 was isolated for immunoblot analysis via heparin-Sepharose chromatography as described (Jackson et al., 1992). Cell viability was assessed by measuring lactate dehydrogenase (LDH) activity in the medium by utilizing the Promega CytoTox kit (Promega).
Results
1. Cell stress results in the association of FGF1 with AHNAK2
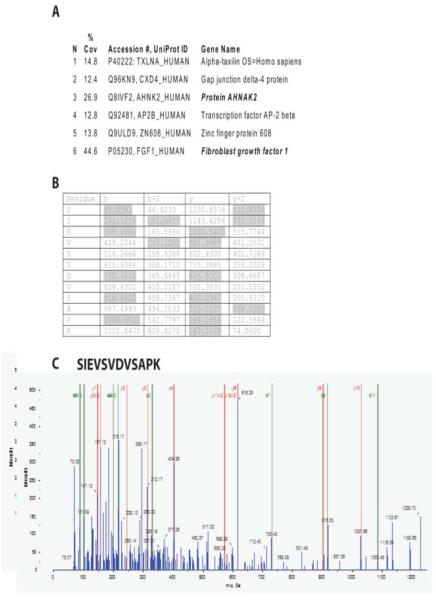
Heat shock stimulates translocation of FGF1 to the cell periphery and their release to the extracellular compartment (Prudovsky et al., 2002). We hypothesized that these processes depend on the stress-induced association of FGF1 with protein(s) exhibiting submembrane localization. To identify these proteins, we applied the method of immunoprecipitation LC-MS. NIH 3T3 cells were adenovirally transduced with FGF1 with a carboxy terminal HA epitope tag. FGF1:HA-transduced cells were subjected to a 90 min incubation at 37°C (control) or at 42°C (heat shock). The cells were lysed in a buffer containing Triton X100, and supernatants of cell lysates were precleared and immunoprecipitated using anti-HA antibodies or control IgG. A minor portion of the immunoprecipitates served to verify the specific immunoprecipitation of FGF1 and the remainder was used for mass spectrometry. Following tryptic digestion, mass spectrometry of the anti-HA precipitates confirmed the presence of FGF1 both at 37°C and 42°C (Figure 1), but not in the control IgG precipitates. In addition, preparations from heat shocked cells but not those maintained at 37°C included the large submembrane protein AHNAK2. Peptides across the entire length of this 5795 amino acid polypeptide were identified by mass spectrometry (Figure 1). The association of FGF1 and AHNAK2 in heat shocked cells was confirmed in a repeated immunoprecipitation/mass spectrometry experiment.
Figure 1. Mass spectrometric analysis of FGF1:HA immunoprecipitated protein suggests AHNAK2 as a candidate binding protein at 42°C.
(A) Partial sequence from human AHNAK2 showing high (<95% confidence, bold face font) and medium confidence (>90% confidence) peptides that were consistent with MSMS-TOF-derived protein sequence. (B) MSMS-TOF collision-induced decay protein sequence data supporting identification of the high confidence AHNAK2 peptide. (C) MSMS-TOF spectrum corresponding to the data in (B).
2. Cell stress enhances the association of FGF1 with the C-terminal domain of AHNAK2
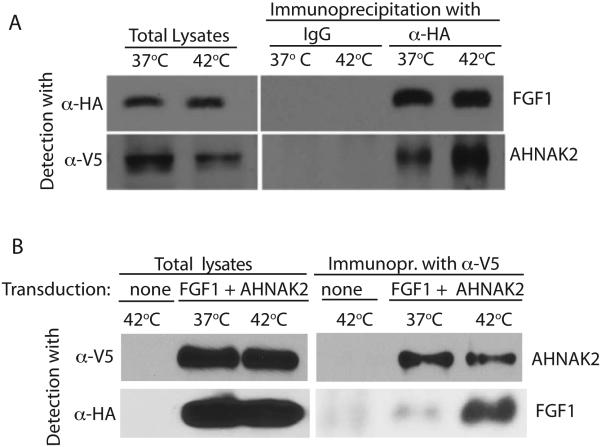
AHNAK2 is an understudied protein, and the reagents for its exploration are scarce. Although we repeatedly confirmed the expression of AHNAK2 in NIH3T3 cells by mass-spectrometry and RT PCR, commercially available anti-AHNAK2 antibodies failed to reliably detect AHNAK2 both in immunoblotting and immunofluorescence. A close relative of AHNAK2, the 5890 amino acid AHNAK1 protein is much better characterized. In particular, it has been shown that the C-terminal portion of AHNAK1 binds several membrane-associated proteins (De Seranno et al., 2006; Haase et al., 2004; Hohaus et al., 2002). We assessed the binding of FGF1 to the C-terminus of AHNAK2 under normal conditions and heat shock. To accomplish this, we tagged with the V5 sequence the AHNAK2 cDNA available from Open Biosystems and coding for 483 C-terminal amino acids of human AHNAK2. The V5-tagged C-terminal fragment of AHNAK2 (ctAHNAK2) was then subcloned into the adenovirus expression vector. NIH 3T3 cells were co-transduced with ctAHNAK2:V5 and FGF1:HA. The cells were subjected to 90 min incubation at 37°C or 42°C, and cell lysates were prepared and immunoprecipitated using anti-V5 or anti-HA antibodies. Immunoprecipitation experiments confirmed the association of ctAHNAK2 and FGF1, which was strongly enhanced by heat shock (Figure 2).
Figure 2. Heat shock enhances the association between FGF1 and C-terminus (ct) of AHNAK2.
NIH 3T3 cells were adenovirally co-transduced with FGF1:HA and ctAHNAK2:V5 and incubated in serum-free medium at 37°C or 42°C for 90 min. Cell lysates were prepared. FGF1 was precipitated from cell lysates by using anti-HA antibodies (A) and ctAHNAK2 by using anti-V5 antibodies (B). Immunoprecipitates were resolved by SDS PAGE and immunoblotted by using anti-HA and anti-V5 antibodies.
3. Heat shock results in submembrane co-localization of FGF1 and ctAHNAK2, and their association with the cytoskeleton fraction
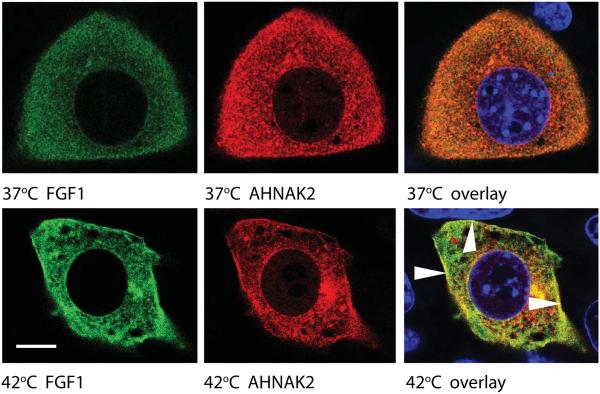
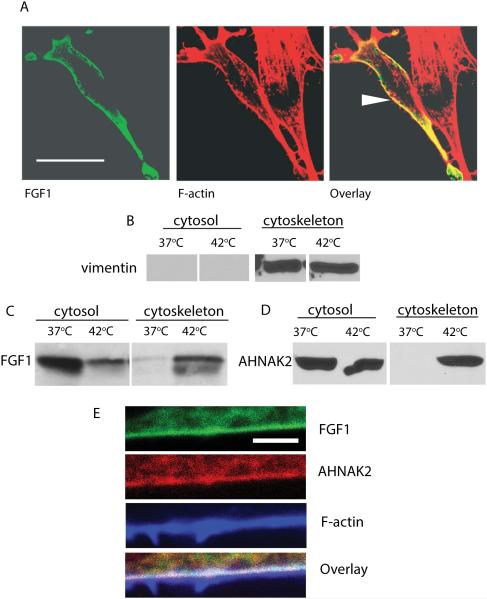
Heat shock induces the translocation of FGF1 to the vicinity of the cell membrane (Prudovsky et al., 2002). Several studies demonstrate the submembrane localization of AHNAK1 (De Seranno et al., 2006; Hashimoto et al., 1995; Marg et al., 2010). We co-transduced NIH 3T3 cells with FGF1:HA and ctAHNAK2:V5, and used confocal microscopy to study their localization under normal conditions and after heat shock. While at normal temperature both proteins exhibited a diffuse cytoplasmic distribution with a considerable co-localization, heat shock resulted in their partial concentration in the submembrane areas, where they were co-localized (Figure 3). Confocal microscopy also showed that in heat-shocked cells, the peripherally distributed FGF1 was colocalized with the submembrane F-actin (Figure 4A). Interestingly, the C-terminal domain of AHNAK1 binds both G- and F–actin (Haase et al., 2004). Taking these data into consideration, we determined the association of FGF1 and ctAHNAK2 with the cytoskeleton, under normal conditions and after heat shock, by utilizing subcellular fractionation (Qproteome, Qiagen). In this way, we found that heat shock resulted in the appearance of FGF1:HA in the cytoskeletal fraction of adenovirally transduced NIH 3T3 cells (Figure 4B,C). Similar behavior was demonstrated for ctAHNAK:V5 (Figure 4D). Confocal immunofluorescence microscopy of NIH 3T3 cells co-transduced with FGF1:HA and ctAHNAK2:V5 demonstrated their co-localization with submembrane F actin after heat shock (Figure 4E).
Figure 3. Heat shock induces a peripheral localization of FGF1 and ctAHNAK2.
NIH 3T3 were adenovirally transduced with FGF1:HA and ctAHNAK2:V5 and plated on glass coverslips. Adherent co-transduced cells were incubated in serum-free medium at 37°C or stressed at 42°C for 90 min and then formalin fixed. Cells were co-stained with FITC-labeled anti-HA antibodies (green), CY3-labeled anti-V5 antibodies (red), DAPI (blue), and studied via a confocal microscope. Scale bar indicates 8 μm. Arrows indicate AHNAK2 and FGF1 colocalization at the periphery of a heat shocked cell.
Figure 4. Heat shock induces the association of FGF1 and ctAHNAK2 with the cytoskeleton.
(A) NIH 3T3 cells transfected with FGF1:HA were subjected to a 90 min incubation at 42°C. FGF1 was detected by using FITC-labeled anti-HA antibodies (green) and F actin by using CY3-labeled phalloidin (red). Cells were studied via a confocal microscope. Arrow indicates FGF1 and F-actin colocalization at the cell periphery. Scale bar – 32 μm. (B,C) NIH 3T3 cells transfected with FGF1:HA were incubated for 90 min at 37°C or 42°C. Cytosolic and cytoskeletal fractions were prepared by utilizing the Qiagen kit, resolved by SDS PAGE and immunoblotted with vimentin antibodies (cytoskeleton marker) (B) or FGF1 antibodies (C). (D) NIH 3T3 cells adenovirally transduced with ctAHNAK2:V5 were treated and fractionated similar to (B), ctAHNAK2 was detected by using anti-V5 antibodies. E. Co-localization of FGF1, AHNAK2 and F-actin at the periphery of a stressed cell. NIH 3T3 cells were co-transduced with FGF1:HA and ctAHNAK2:V5, and heat shocked. Fixed cells were immunofluorescently stained with FITC-labeled anti-HA antibodies (green), CY3-labeled anti-V5 antibodies (red) and Alexa 633-labeled phalloidin (blue), and studied via a confocal microscope. Scale bar – 2 μm.
4. AHNAK2 is critical for stress-induced FGF1 export
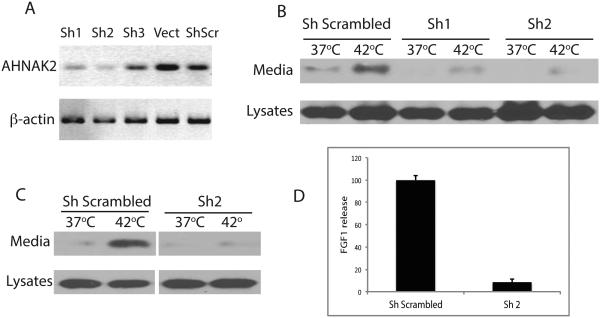
The heat shock-induced association of FGF1 and AHNAK2 and their submembrane colocalization indicated that AHNAK2 could play a role in FGF1 export. To test this hypothesis, we assessed the effect of shRNA knockdown of endogenous AHNAK2 on FGF1 secretion. AHNAK2 shRNA expression constructs were stably transfected to NIH 3T3 cells. RT-PCR analysis demonstrated that two out of three tested shRNAs decreased the expression of AHNAK2 (5A). Cells transfected with AHNAK2 shRNAs 1 and 2, and scrambled shRNA were adenovirus-transduced with FGF1 and tested for their ability to release FGF1 under heat shock conditions. We found that cells transfected during thermal stress-induced FGF1 export were drastically suppressed by AHNAK2 shRNAs (Figure 5B). These data demonstrate that AHNAK2 is required for the stress-induced export of FGF1. In all the following experiments, we used cells expressing AHNAK2 shRNA 2, which exhibited a more than ten-fold decrease of FGF1 release during the heat shock (Figure 5C,D).
Figure 5. shRNA knockdown of AHNAK2 expression inhibits the stress-induced FGF1 export.
(A) NIH 3T3 cells were stably transfected with AHNAK2 shRNAs, scrambled shRNA or empty vector. AHNAK2 mRNA levels in transfected cells were assessed by RT PCR. Two of three shRNA constructs suppressed AHNAK2 expression. (B) AHNAK2 sRNA 1 and 2, and scrambled shRNA transfectant cells were adenovirally transduced with FGF1:HA and incubated for 90 min at 37°C or 42°C. FGF1 was isolated from the cell culture media by heparin chromatography. The levels of FGF1 in cell lysates and media were determined by SDS PAGE and immunoblotting using anti-FGF1 antibodies. (C,D) Quantification of the effect of shRNA 2 transfection on FGF1 release in a separate experiment. Immunoblotting (C) and photometric quantification (D) results are presented. FGF1 release from scrambled shRNA and AHNAK2 shRNA transfectants was normalized to FGF1 content in cell lysates at 37°C. The normalized heat shock-induced release from scrambled shRNA transfectants is presented as 100%. The data are representative of three independent shRNA experiments, which gave consistent results.
5. FGF1 export induced by the inhibition of Notch signaling is refractory to AHNAK2 knockdown
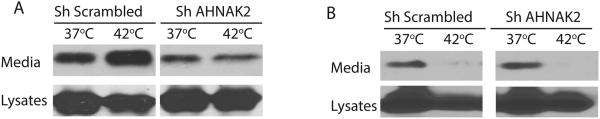
FGF1 release is induced not only by stress but also by inhibition of Notch signaling (Small et al., 2003), particularly through the expression of a dominant negative (dn) mutant of CBF1, the key transcription factor of the Notch signaling cascade (Kacer et al., 2011). Therefore, we assessed the importance of AHNAK2 for this type of FGF1 export. NIH 3T3 cells transfected with AHNAK2 shRNA or scrambled shRNA were adenovirally co-transduced with FGF1 and dnCBF1. We found that at 37°C, AHNAK2 knockdown did not noticeably change the intensive export of FGF1 from dnCBF1-expressing cells; however, it prevented its further increase at 42°C (Figure 6A).
Figure 6. (A) Stress-independent FGF1 export induced by the inhibition of Notch signaling does not depend on AHNAK2.
NIH 3T3 cells stably transfected with scrambled shRNA or AHNAK2 shRNA (construct 2) were adenovirally co-transduced with FGF1:HA and dnCBF1. Cells were incubated for 90 min at 37°C or 42°C. FGF1 was isolated from the cell culture media by heparin chromatography. The levels of FGF1 in cell lysates and media were determined by SDS PAGE and immunoblotting using anti-FGF1 antibodies. dnCBF1 transduced cells released FGF1 at 37°C. AHNAK2 knockdown in dnCBF1 transduced cells did not affect the release of FGF1 at 37°C but prevented its enhancement by heat shock. (B) Spontaneous FGF2 export is insensitive to AHNAK2 depletion. NIH 3T3 cells stably transfected with scrambled shRNA or AHNAK2 shRNA (construct 2) were co-transduced with FGF2:HA and incubated for 90 min at 37°C or 42°C. FGF2:HA was isolated from the cell culture media by heparin chromatography. The levels of FGF2:HA in cell lysates and media were determined by SDS PAGE and immunoblotting using anti-HA antibodies. The data presented in A and B are each representative of three independent shRNA experiments, which gave consistent results.
6. Constitutive nonclassical FGF2 export does not require AHNAK2
Unlike FGF1, nonclassical export of FGF2 does not depend on stress conditions (Engling et al., 2002). On the contrary, we found that heat shock decreases FGF2 export (Lopez-Castejon et al., 2013). We used the cells transfected with AHNAK2 shRNA 2 to elucidate the importance of AHNAK2 for FGF2 export. The knockdown of AHNAK2 expression did not interfere with the spontaneous release of FGF2 (Figure 6B).
Discussion
We found that AHNAK2 is a stress-dependent association partner of FGF1 that is required for stress-induced FGF1 export. Little is known about AHNAK2 functions, while, the closely related protein AHNAK1 is much better understood (for review see (Davis et al., 2014)). It participates in various processes localized at the cell membrane. For example, AHNAK1 regulates the L type calcium membrane channels in multiple cell types (Haase et al., 2005; Matza et al., 2009) and activates PKCα through the dissociation of the PKC-PP2A complex (Lee et al., 2008). Interestingly, AHNAK1 serves as a scaffold for the formation of submembrane protein complexes. Indeed, it binds Erk, PAK, and p21-activated kinase-interacting exchange factor β (Lim et al., 2013), PLCγ-1 and PKCα (Lee et al., 2004), Annexin2/S100A10 heterotetramer (De Seranno et al., 2006), natriuretic peptide receptor (Alli and Gower, 2009), dysferlin (Cacciottolo et al., 2011), calpain 3 (Huang et al., 2008), and S100B (Tsoporis et al., 2009). We found that the C-terminus of AHNAK2 is sufficient for association with FGF1. While nothing is known about the functional domains of AHNAK2, it is noteworthy that the C-terminal portion of AHNAK1 is critical for most of its interactions with other proteins (Alli and Gower, 2009; De Seranno et al., 2006; Haase et al., 2004; Hohaus et al., 2002; Ozorowski et al., 2013; Pankonien et al., 2012). Submembrane translocation of AHNAK1 is stimulated by stress, which is mediated by its C-terminus and calcium ions (De Seranno et al., 2006). AHNAK1 expressed in endothelial cells apparently participates in brain-blood barrier formation and neoangiogenesis (Gentil et al., 2005; von Boxberg et al., 2006). AHNAK1 expression is important for smooth muscle cell migration (Lim et al., 2013). However, targeting of AHNAK1 in mice does not result in significant phenotypic change (Kouno et al., 2004). It is possible that AHNAK1 deficiency could be accentuated by AHNAK2 knockout. Interestingly, the localization of both proteins in muscle cells is identical (Marg et al., 2010).
Here we present the first data on a cellular function of AHNAK2: its participation in FGF1 export. Based on the stress-induced association of both AHNAK2 and FGF1 with the cytoskeleton and their stress-induced submembrane co-localization, we hypothesize that AHNAK2, through its C-terminus, serves for localizing FGF1 in the vicinity of cell membrane, in association with actin cytoskeleton. It is noteworthy that AHNAK1 through its C-terminus binds actin (Haase et al., 2004) and that agrees with the observed stress-induced localization of AHNAK2 in the cytoskeleton. Interestingly, while intracellular translocation of AHNAK1 is regulated by PKC (Hashimoto et al., 1995) and calcium ions (Nie et al., 2000), our studies on promonocytic cells show that PMA, the stimulator of PKC, induces calcium-dependent export of FGF1 (Kirov et al., 2012). Another indication that AHNAK proteins could participate in the nonclassical protein export is that synchronous high expression of AHNAK1 and MIF, a cytokine, which is similar to FGF1 released through a nonclassical pathway, predicts poor survival in laryngeal carcinoma (Dumitru et al., 2013).
The results of this study identify AHNAK2 as a novel component of stress-induced FGF1 secretion pathway. FGF1 is an important regulator of tissue repair and angiogenesis, which is involved in many cardiovascular disorders and also tumorigenesis. Thus, our data warrant further investigations of the specific interactions between AHNAK2, FGF1, actin cytoskeleton and cell membrane. In addition, they demonstrate a mechanistic difference between FGF1 secretion pathways induced by stress and by inhibition of Notch signaling, of which the latter does not require AHNAK2. Similarly, spontaneous secretion of FGF2 does not depend on AHNAK2. These differences are not surprising considering that the export of FGF1 stimulated in the cells with inhibited Notch signaling (Kacer et al., 2011) and spontaneous release of FGF2 (Engling et al., 2002) are not accompanied by massive redistribution of these proteins to cell periphery, which is observed in stress conditions (Prudovsky et al., 2002). Such results demonstrate the existence of mechanistically distinct nonclassical export pathways (Nickel and Rabouille, 2009; Prudovsky et al., 2007) not only for different signal peptide-less proteins, but also for the same protein, such as FGF1.
Acknowledgments
The study has been supported by a Maine Cancer Foundation grant to IP, NIH grant HL35627 to IP and MMCRI institutional support to IP. In this work, we used the facilities of the Protein, Nucleic Acid and Cell Imaging Core and of the Viral Core, supported by NIH grant P30 GM103392 to Robert Friesel. We are grateful to Norma Albrecht for editorial assistance, and to Open Biosystems for the construct coding for the C-terminal fragment of AHNAK2. The authors have no conflict of interest to declare.
Contract grant numbers: NIH HL35627, NIH P30 GM103392.
Contract grant sponsors: NIH, Maine Cancer Foundation
Abbreviations
- FGF1
fibroblast growth factor 1
- PS
phosphatidylserine
References
- Alli AA, Gower WR., Jr The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol. 2009;297:C1157–67. doi: 10.1152/ajpcell.00219.2009. [DOI] [PubMed] [Google Scholar]
- Ananyeva NM, Tijurmin AV, Berliner JA, Chisolm GM, Liau G, Winkles JA, Haudenschild CC. Oxidized LDL mediates the release of fibroblast growth factor-1. Arterioscler.Thromb.Vasc.Biol. 1997;17:445–453. doi: 10.1161/01.atv.17.3.445. [DOI] [PubMed] [Google Scholar]
- Bagala C, Kolev V, Mandinova A, Soldi R, Mouta C, Graziani I, Prudovsky I, Maciag T. The alternative translation of synaptotagmin 1 mediates the non-classical release of FGF1. Biochem Biophys Res Commun. 2003;310:1041–7. doi: 10.1016/j.bbrc.2003.09.119. [DOI] [PubMed] [Google Scholar]
- Cacciottolo M, Belcastro V, Laval S, Bushby K, di Bernardo D, Nigro V. Reverse engineering gene network identifies new dysferlin-interacting proteins. J Biol Chem. 2011;286:5404–13. doi: 10.1074/jbc.M110.173559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulier F, Pontarotti P, Roubin R, Hartung H, Goldfarb M, Birnbaum D. Of worms and men: an evolutionary perspective on the fibroblast growth factor (FGF) and FGF receptor families. J Mol Evol. 1997;44:43–56. doi: 10.1007/pl00006120. [DOI] [PubMed] [Google Scholar]
- Davis TA, Loos B, Engelbrecht AM. AHNAK: The giant jack of all trades. Cell Signal. 2014;26:2683–2693. doi: 10.1016/j.cellsig.2014.08.017. [DOI] [PubMed] [Google Scholar]
- De Seranno S, Benaud C, Assard N, Khediri S, Gerke V, Baudier J, Delphin C. Identification of an AHNAK binding motif specific for the Annexin2/S100A10 tetramer. J Biol Chem. 2006;281:35030–8. doi: 10.1074/jbc.M606545200. [DOI] [PubMed] [Google Scholar]
- Duarte M, Kolev V, Kacer D, Mouta-Bellum C, Soldi R, Graziani I, Kirov A, Friesel R, Liaw L, Small D, Verdi J, Maciag T, Prudovsky I. Novel cross-talk between three cardiovascular regulators: thrombin cleavage fragment of Jagged1 induces fibroblast growth factor 1 expression and release. Mol Biol Cell. 2008;19:4863–74. doi: 10.1091/mbc.E07-12-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru CA, Bankfalvi A, Gu X, Zeidler R, Brandau S, Lang S. AHNAK and inflammatory markers predict poor survival in laryngeal carcinoma. PLoS One. 2013;8:e56420. doi: 10.1371/journal.pone.0056420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engling A, Backhaus R, Stegmayer C, Zehe C, Seelenmeyer C, Kehlenbach A, Schwappach B, Wegehingel S, Nickel W. Biosynthetic FGF-2 is targeted to non-lipid raft microdomains following translocation to the extracellular surface of CHO cells. J Cell Sci. 2002;115:3619–31. doi: 10.1242/jcs.00036. [DOI] [PubMed] [Google Scholar]
- Gentil BJ, Benaud C, Delphin C, Remy C, Berezowski V, Cecchelli R, Feraud O, Vittet D, Baudier J. Specific AHNAK expression in brain endothelial cells with barrier properties. J Cell Physiol. 2005;203:362–71. doi: 10.1002/jcp.20232. [DOI] [PubMed] [Google Scholar]
- Haase H, Alvarez J, Petzhold D, Doller A, Behlke J, Erdmann J, Hetzer R, Regitz-Zagrosek V, Vassort G, Morano I. Ahnak is critical for cardiac Ca(V)1.2 calcium channel function and its beta-adrenergic regulation. FASEB J. 2005;19:1969–77. doi: 10.1096/fj.05-3997com. [DOI] [PubMed] [Google Scholar]
- Haase H, Pagel I, Khalina Y, Zacharzowsky U, Person V, Lutsch G, Petzhold D, Kott M, Schaper J, Morano I. The carboxyl-terminal ahnak domain induces actin bundling and stabilizes muscle contraction. FASEB J. 2004;18:839–41. doi: 10.1096/fj.03-0446fje. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Gamou S, Shimizu N, Kitajima Y, Nishikawa T. Regulation of translocation of the desmoyokin/AHNAK protein to the plasma membrane in keratinocytes by protein kinase C. Exp Cell Res. 1995;217:258–66. doi: 10.1006/excr.1995.1085. [DOI] [PubMed] [Google Scholar]
- Hohaus A, Person V, Behlke J, Schaper J, Morano I, Haase H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002;16:1205–16. doi: 10.1096/fj.01-0855com. [DOI] [PubMed] [Google Scholar]
- Huang Y, de Morree A, van Remoortere A, Bushby K, Frants RR, den Dunnen JT, van der Maarel SM. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum Mol Genet. 2008;17:1855–66. doi: 10.1093/hmg/ddn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci U S A. 1992;89:10691–5. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacer D, McIntire C, Kirov A, Kany E, Roth J, Liaw L, Small D, Friesel R, Basilico C, Tarantini F, Verdi J, Prudovsky I. Regulation of non-classical FGF1 release and FGF-dependent cell transformation by CBF1-mediated notch signaling. J Cell Physiol. 2011;226:3064–75. doi: 10.1002/jcp.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov A, Al-Hashimi H, Solomon P, Mazur C, Thorpe PE, Sims PJ, Tarantini F, Kumar TK, Prudovsky I. Phosphatidylserine externalization and membrane blebbing are involved in the nonclassical export of FGF1. J Cell Biochem. 2012;113:956–66. doi: 10.1002/jcb.23425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouno M, Kondoh G, Horie K, Komazawa N, Ishii N, Takahashi Y, Takeda J, Hashimoto T. Ahnak/Desmoyokin is dispensable for proliferation, differentiation, and maintenance of integrity in mouse epidermis. J Invest Dermatol. 2004;123:700–7. doi: 10.1111/j.0022-202X.2004.23412.x. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Bagala C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem. 2001a;276:25549–57. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Soldi R, Bagala C, Micucci I, Bellum S, Tarantini F, Prudovsky I, Maciag T. S100A13 participates in the release of fibroblast growth factor 1 in response to heat shock in vitro. J Biol Chem. 2001b;276:22544–52. doi: 10.1074/jbc.M100546200. [DOI] [PubMed] [Google Scholar]
- Lee IH, Lim HJ, Yoon S, Seong JK, Bae DS, Rhee SG, Bae YS. Ahnak protein activates protein kinase C (PKC) through dissociation of the PKC-protein phosphatase 2A complex. J Biol Chem. 2008;283:6312–20. doi: 10.1074/jbc.M706878200. [DOI] [PubMed] [Google Scholar]
- Lee IH, You JO, Ha KS, Bae DS, Suh PG, Rhee SG, Bae YS. AHNAK-mediated activation of phospholipase C-gamma1 through protein kinase C. J Biol Chem. 2004;279:26645–53. doi: 10.1074/jbc.M311525200. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Kang DH, Lim JM, Kang DM, Seong JK, Kang SW, Bae YS. Function of Ahnak protein in aortic smooth muscle cell migration through Rac activation. Cardiovasc Res. 2013;97:302–10. doi: 10.1093/cvr/cvs311. [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J Biol Chem. 2013;288:2721–33. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marg A, Haase H, Neumann T, Kouno M, Morano I. AHNAK1 and AHNAK2 are costameric proteins: AHNAK1 affects transverse skeletal muscle fiber stiffness. Biochem Biophys Res Commun. 2010;401:143–8. doi: 10.1016/j.bbrc.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Matza D, Badou A, Jha MK, Willinger T, Antov A, Sanjabi S, Kobayashi KS, Marchesi VT, Flavell RA. Requirement for AHNAK1-mediated calcium signaling during T lymphocyte cytolysis. Proc Natl Acad Sci U S A. 2009;106:9785–90. doi: 10.1073/pnas.0902844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouta Carreira C, Landriscina M, Bellum S, Prudovsky I, Maciag T. The comparative release of FGF1 by hypoxia and temperature stress. Growth Factors. 2001;18:277–85. doi: 10.3109/08977190109029116. [DOI] [PubMed] [Google Scholar]
- Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- Nie Z, Ning W, Amagai M, Hashimoto T. C-Terminus of desmoyokin/AHNAK protein is responsible for its translocation between the nucleus and cytoplasm. J Invest Dermatol. 2000;114:1044–9. doi: 10.1046/j.1523-1747.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Ozorowski G, Milton S, Luecke H. Structure of a C-terminal AHNAK peptide in a 1:2:2 complex with S100A10 and an acetylated N-terminal peptide of annexin A2. Acta Crystallogr D Biol Crystallogr. 2013;69:92–104. doi: 10.1107/S0907444912043429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankonien I, Otto A, Dascal N, Morano I, Haase H. Ahnak1 interaction is affected by phosphorylation of Ser-296 on Cavbeta(2) Biochem Biophys Res Commun. 2012;421:184–9. doi: 10.1016/j.bbrc.2012.03.132. [DOI] [PubMed] [Google Scholar]
- Prudovsky I, Bagala C, Tarantini F, Mandinova A, Soldi R, Bellum S, Maciag T. The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export. J Cell Biol. 2002;158:201–8. doi: 10.1083/jcb.200203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Kumar TK, Sterling S, Neivandt D. Protein-phospholipid interactions in nonclassical protein secretion: problem and methods of study. Int J Mol Sci. 2013;14:3734–72. doi: 10.3390/ijms14023734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Tarantini F, Landriscina M, Neivandt D, Soldi R, Kirov A, Small D, Kathir KM, Rajalingam D, Kumar TK. Secretion without Golgi. J Cell Biochem. 2007 doi: 10.1002/jcb.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Vary CP, Markaki Y, Olins AL, Olins DE. Phosphatidylserine colocalizes with epichromatin in interphase nuclei and mitotic chromosomes. Nucleus. 2012;3:200–10. doi: 10.4161/nucl.19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Malhotra V, Nickel W. Diversity in unconventional protein secretion. J Cell Sci. 2012;125:5251–5. doi: 10.1242/jcs.103630. [DOI] [PubMed] [Google Scholar]
- Romero D, O'Neill C, Terzic A, Contois L, Young K, Conley BA, Bergan RC, Brooks PC, Vary CP. Endoglin regulates cancer-stromal cell interactions in prostate tumors. Cancer Res. 2011;71:3482–93. doi: 10.1158/0008-5472.CAN-10-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F. Role of membrane lipid asymmetry in aging. Neurobiol Aging. 1984;5:323–33. doi: 10.1016/0197-4580(84)90010-1. [DOI] [PubMed] [Google Scholar]
- Shin JT, Opalenik SR, Wehby JN, Mahesh VK, Jackson A, Tarantini F, Maciag T, Thompson JA. Serum-starvation induces the extracellular appearance of FGF-1. Biochim Biophys Acta. 1996;1312:27–38. doi: 10.1016/0167-4889(96)00013-4. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Tyurina JY, Kawai K, Tyurin VA, Kommineni C, Castranova V, Fabisiak JP, Kagan VE. Selective peroxidation and externalization of phosphatidylserine in normal human epidermal keratinocytes during oxidative stress induced by cumene hydroperoxide. J Invest Dermatol. 2002;118:1008–18. doi: 10.1046/j.1523-1747.2002.01759.x. [DOI] [PubMed] [Google Scholar]
- Small D, Kovalenko D, Soldi R, Mandinova A, Kolev V, Trifonova R, Bagala C, Kacer D, Battelli C, Liaw L, Prudovsky I, Maciag T. Notch activation suppresses fibroblast growth factor-dependent cellular transformation. J Biol Chem. 2003;278:16405–13. doi: 10.1074/jbc.M300464200. [DOI] [PubMed] [Google Scholar]
- Soldi R, Mandinova A, Venkataraman K, Hla T, Vadas M, Pitson S, Duarte M, Graziani I, Kolev V, Kacer D, Kirov A, Maciag T, Prudovsky I. Sphingosine kinase 1 is a critical component of the copper-dependent FGF1 export pathway. Exp Cell Res. 2007;313:3308–18. doi: 10.1016/j.yexcr.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA, Mammalian Gene Collection Program T Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini F, Gamble S, Jackson A, Maciag T. The cysteine residue responsible for the release of fibroblast growth factor-1 residues in a domain independent of the domain for phosphatidylserine binding. J Biol Chem. 1995;270:29039–42. doi: 10.1074/jbc.270.49.29039. [DOI] [PubMed] [Google Scholar]
- Tarantini F, LaVallee T, Jackson A, Gamble S, Mouta Carreira C, Garfinkel S, Burgess WH, Maciag T. The extravesicular domain of synaptotagmin-1 is released with the latent fibroblast growth factor-1 homodimer in response to heat shock. J Biol Chem. 1998;273:22209–16. doi: 10.1074/jbc.273.35.22209. [DOI] [PubMed] [Google Scholar]
- Tsoporis JN, Overgaard CB, Izhar S, Parker TG. S100B modulates the hemodynamic response to norepinephrine stimulation. Am J Hypertens. 2009;22:1048–53. doi: 10.1038/ajh.2009.145. [DOI] [PubMed] [Google Scholar]
- von Boxberg Y, Salim C, Soares S, Baloui H, Alterio J, Ravaille-Veron M, Nothias F. Spinal cord injury-induced up-regulation of AHNAK, expressed in cells delineating cystic cavities, and associated with neoangiogenesis. Eur J Neurosci. 2006;24:1031–41. doi: 10.1111/j.1460-9568.2006.04994.x. [DOI] [PubMed] [Google Scholar]
- Young K, Conley B, Romero D, Tweedie E, O'Neill C, Pinz I, Brogan L, Lindner V, Liaw L, Vary CP. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120:4263–4273. doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]