Abstract
For now, magnetic resonance (MR) is the best noninvasive imaging modality to evaluate vertebral bone marrow thanks to its inherent soft-tissue contrast and non-ionizing nature. A daily challenging scenario for every radiologist interpreting MR of the vertebral column is discerning the diseased from normal marrow. This requires the radiologist to be acquainted with the used MR techniques to judge the spinal marrow as well as its normal MR variants. Conventional sequences used basically to image marrow include T1W, fat-suppressed T2W and short tau inversion recovery (STIR) imaging provides gross morphological data. Interestingly, using non-routine MR sequences; such as opposed phase, diffusion weighted, MR spectroscopy and contrasted-enhanced imaging; may elucidate the nature of bone marrow heterogeneities; by inferring cellular and chemical composition; and adding new functional prospects. Recalling the normal composition of bone marrow elements and the physiologic processes of spinal marrow conversion and reconversion eases basic understanding of spinal marrow imaging. Additionally, orientation with some common variants seen during spinal marrow MR imaging as hemangiomas and bone islands is a must. Moreover, awareness of the age-associated bone marrow changes as well as changes accompanying different variations of the subject’s health state is essential for radiologists to avoid overrating normal MR marrow patterns as pathologic states and metigate unnecessary further work-up.
Keywords: Magnetic resonance imaging, Normal, Spinal, Marrow, Variants
Core tip: Magnetic resonance (MR) remains the ideal noninvasive imaging modality to evaluate vertebral bone marrow. Radiologists have to be aware by age-associated bone marrow changes as well as changes accompanying different variations of the subject’s health state. Moreover, acquaintation with the used MR techniques, their privileges and limitations, in evaluation of spinal marrow is a prime requirement for radiologist to discern the normal spinal marrow as well as its variants from diseased one.
INTRODUCTION
The spine is the largest store of bone marrow in the body[1,2]. Addressing bone marrow signal pattern is an integral part of the spinal magnetic resonance (MR) imaging evaluation. By far, magnetic resonance imaging (MRI) is the best imaging modality to depict bone marrow thanks to its inherent soft-tissue contrast and non-ionizing nature[3-5].
Bone marrow is a dynamic organ with continued changes occurring with increased age and increased hematopoietic needs in different environmental and health states[4,6]. Similarly, it is the target of a lot of pathologic processes that results in altered signal intensity or heterogenous signal pattern on MR imaging. A daily challenging scenario for every radiologist interpreting MR of the spine is to discern the diseased from normal marrow. This requires the radiologist to be acquainted with normal MR patterns of the spinal bone marrow, its chronological conversion, and its different common variants. This review starts with a brief discussion of the composition and physiology of the spinal marrow, followed by a concise discussion on the common MR sequences used to evaluate the spinal marrow. In the latter section the normal spinal marrow MR patterns and common variants are displayed.
Spinal bone marrow buildup and physiology
Imaging-wise, the spinal bone marrow is a mix of cellular elements enclosed within a cortical bone shell; the vertebral body. These cellular elements are enmeshed within the medullary bony trabeculae; predominantly vertically oriented; that provide both structural support and storage of minerals as calcium and phosphate; thicker in the lumbar region[2,5,6]. There are two types of bone marrow in the spine: the red marrow, named after its richness in hemoglobin in erythrocytes lineage and is richly vascular; and the yellow marrow, named after abundant carotenoid bodies in its fat cells and is scarily vascular[7]. Either marrow type, whether red or yellow, is composed of a blend of fat, water and proteins in different proportions (Table 1). The 3 components vary in volume with normal growth and in response to different stimuli. Hence, their proportions are the main determinants of spinal marrow MR signal characteristics[8-10]. The nutrition of spinal marrow is derived from ambient sinusoids branching from nutrient vessels piercing the vertebral cortices and drained via the Batson’s venous plexus emerging from the posterior vertebral bodies’ cortices. Nerves accompany this vascular network and few lymph nodes can be identified within the vertebral marrow[7]. The function of bone marrow is to provide different blood cell lineages involved in tissue nutrition, oxygenation and body’s immune reactions[7].
Table 1.
Vertebral bone marrow chemical composition and cellular buildup
| Marrow phenotype | Chemical composition | Cellular components |
| Red marrow | 40%-60% lipids | 60% hematopoietic cells |
| 30%-40% water | 40% fat cells | |
| 10%-20% proteins | ||
| Yellow marrow | 80% lipids | 95% fat cells |
| 15% water | 5% hematopoietic cells | |
| 5% proteins |
Spinal marrow conversion
At birth, the whole spinal marrow is metabolically active (hematopoietic/red marrow). This pattern gradually, and in orderly fashion, turns into a less metabolically active (fat/yellow) marrow with growing up. This temporal physiologic phenomenon is known as normal marrow conversion and concludes around age of 25-30 years[2,6,11,12]. As in other skeletal region, the pattern of spinal marrow conversion is centripetal starting in the subcortical and subendplates regions and going to the center of vertebral body[8]. At all times, both red and yellow marrow are spinal marrow cohabitant yet the prevalent type is used to address the type of marrow in focus[2,4,12]. Moreover, a peculiar character of the spinal marrow is the persistence of red marrow over all ages, especially in the lumbar region[1]. Consequently, heterogeneity of the spinal marrow is a normal phenomenon, especially in adolescence and middle age. Focal areas of red marrow may be a challenge to disclose its nature in some clinical scenarios and mandates making use of different MR pulse sequences to disclose its nature.
Spinal marrow reconversion
During lifetime, various physiologic and pathologic states require increased tissue demands for more oxygen and hemoglobin. The metabolically inactive fat marrow dynamically repopulates into the metabolically active red type, capable of responding to tissues’ needs of oxygen in a process named marrow reconversion[5,6,13,14].
It could be in response to physiologic stimuli as in obesity, cigarette smokers and heavy training athletes; or pathologic conditions as chronic hemolytic anemias and marrow replacing disorders[2,13,14].
In contrast to the orderly fashion of normal marrow conversion, reconversion is a patchy and an asymmetrical process where areas of red marrow are embedded within the surrounding yellow marrow[15]. That is why recognition of this physiologic phenomenon is mandatory to rule out underlying myeloproliferative disorder on MR imaging.
MRI technique for the spinal marrow
Routine evaluation of spinal marrow will include spin echo T1 and T2W pulse sequences in the sagittal plane. Some institutes add short tau inversion recovery (STIR) sequences in the sagittal plane as a routine. The author’s prefer to use the STIR in the coronal plane to discourse the neutral axis and its meningeal sleeves, especially in cervical and lumbar regions, abnormalities of the facets and sacroiliac joints and exploration of accidental extra-spinal pathologies not apparent on routine sagittal and axial planes. Any suspicious bone marrow lesion on the routine planes could be ascertained on this additional coronal STIR image. Axial planes will be advantageous in labeling presence of extra-medullary extensions and neural axis involvement by any marrow pathology.
Routine MR sequences for spinal bone marrow imaging
T1-weighted imaging: Both red and fat marrows contain lipid and water with various proportions. The red marrow appears as low signal due to its higher water content on T1W images yet it has to be higher than that of intervertebral discs and paraspinal muscles[16]. On the contrary, a high lipid content of yellow marrow returns high signal intensity comparable to that of subcutaneous fat on T1W images[16]. This makes T1W the money’s worth sequence of MR screening of bone marrow[3,17,18].
T2-weighted imaging: The signal returning from both water and fat are high yet signal returning from red marrow is slightly lower than that of yellow marrow[19]. So, the ability of T2-sequence to differentiate marrow hyperplasia from marrow lesions is limited without the use of fat suppression especially on the fast spin echo (FSE) acquisitions[10,20]. This mandates the use of fat-suppression for better utility of T2 FSE used in clinical imaging of the spines. On fat suppression T2W sequences the red marrow will be of slightly higher signal than muscle while the yellow marrow has signal lower than it[6,10].
STIR sequence: It enhances the difference in longitudinal relaxation of fat and water on T1W imaging. A non-selective 180° inversion pulse applied at specified inversion time followed by refocusing 90 pulse can cancel any signal from fat and the returning signal will be of the non-fatty components, e.g., water[10,21]. This enhances contrast of bone marrow lesions within the suppressed background. The main drawback of STIR imaging, that it suppresses any signal other than fat as hematomas and gadolinium enhancement[22].
Problem-solving MR sequences for spinal bone marrow imaging
Chemical-shift imaging: Chemical-shift or opposed phase imaging relies on the fact that water and fat have different resonance frequencies so that when they are resonating aligned their signal is summed (in-phase imaging) while when they are opposed (out-phase imaging) their signals are subtracted with subsequent signal drop[23]. As fat and water intermix in both types of marrow, the signal of red marrow will not significantly drop in out phase while that of yellow marrow will[23]. However, this is not absolute and a cut off value of 20% signal drop has postulated[24].
The main value of opposed phase imaging is to rule out neoplastic replacement of the marrow. Metastatic and infiltrative marrow neoplasia will destroy normal marrow and retain high water content with resultant high-signal on out-phase imaging[23]. This has proved beneficiary in differentiation neoplastic and osteoporotic fractures[25,26].
However, false negative results can rise from fat-containing metastasis (e.g., from renal cell carcinoma) and false positive results can results from marrow fibrosis as well as susceptibility artifacts accompanying marrow hematomas and sclerotic metastasis[25,27]. This may necessitate marrow biopsy for histopathologic confirmation.
Diffusion-weighted imaging
Diffusion imaging addresses the free mobility of protons in a specific tissue[28]. This could be qualitative[29], i.e., bull eying or quantitive[30] using the apparent diffusion coefficient (ADC). It is a sound fast sequence that can comprehend functional aspects of the examined tissues in addition to the available routine morphologic sequences. Normal marrow that is rich in protons will show free diffusion and high ADC values (i.e., high signal intensity on both the diffusion image and ADC map)[31,32]. Contrarily, in metastatic lesions with densely packed cells and in cytotoxic edematous cells following trauma lower ADC values are seen (i.e., high signal intensity on the diffusion image and low signal on ADC map)[31,32]. However, studies on the use of diffusion weighted imaging of the marrow are controversial and it should be interpreted in line with the routine marrow sequences[31,33].
Currently, common clinical musculoskeletal applications of diffusion weighted imaging of the spine are differencing osteoporotic fractures and neoplastic vertebral body collapse[34], differentiation of infective and degenerative sub-endplates changes[35] and follow-up treatment response of neoplastic marrow lesions[36].
Contrast-enhanced marrow imaging
Contrast enhancement is used to depict marrow lesions. The normal spinal marrow may show mild homogenous contrast enhancement in neonates and pediatrics due to abundant blood flow, prominent extravascular space and rich diverse cellularity[17,37]. This progressively become imperceptible as a function of age and increased fatty marrow content[38]. In normal adults spinal marrow doesn’t show perceptible enhancement following administration of gadolinium based T1W agents[17,37,38].
Dynamic contrast studies of the spinal marrow had been used to diagnose and follow-up myelo-proliferative disorders[39]. Following rapid IV gadolinium-based contrast agent administration, the changes in longitudinal relaxation of vertebral marrow are measured and signal time intensity curve is reproduced. Various parameters have been used like maximum intensity, slope of the curve and contrast washout[40]. Normal vertebral marrow shows decreased maximal enhancement, slope of enhancement and washout indices with increased age and fat marrow content[38,41]. However, dynamic contrast-enhanced studies have not been widely used in clinical practices.
Another less commonly used class of MR contrast agents affect the T2- or T2* imaging characteristics. Theses contrast agents, e.g., ultra small particles iron oxides are engulfed by the hematopoietic cells of the normal bone marrow, produce local field inhomogeneities with resultant suppression of normal bone marrow[42]. This results in increased conspicuity of marrow lesion that will not take these agents. They are used to differentiate infiltrative marrow lesions from reactive marrow hyperplasia[43]. Also they can differentiate bone metastasis from infection[44].
Proton MR spectroscopy
MR spectroscopy is a non-invasive method of quantification of fat content of the marrow and evaluation of its chemical composition[45]. A single or multi-voxel method can used to asses one or more vertebral bodies and the fat content is expressed as a percentage (due to multiple lipid peaks) not an absolute value[46,47]. Previous reports emphasized age and sex related physiologic changes of the fat content of the spinal bone marrow[48,49].
However, it is not widely used clinically as same information could be achieved by the above used tools.
MRI appearance of the normal spinal bone marrow
As mentioned earlier, the bone marrow is a mix of red and yellow marrow supported by a trabecular marrow network. The trabecular marrow appears as a mesh of linear intermingled low signal intensities within both red and yellow marrow on all pulse sequences, especially prominent on the gradient recalled one. The trabecular marrow changes have little effects on the spinal marrow MR signal, if present.
Actually, the relative ratio of fat and water is the main determinant for the MR signal of spinal bone marrow as well as the used MR pulse sequence[1,4,5,9,13,50,51].
On T1W images, the vertebral fat marrow is high-signal intensity similar to subcutaneous fat in adults[2,5,6]. However, at birth all spinal marrow is of the red type with high water content resulting in low signal intensity of the vertebral bodies even relative to the intervertebral discs and muscles on T1W images (Figure 1)[14,52]. After that, gradual increased amount of fat cells, especially at the sub-endplates region and anterior part of the vertebral body (Figure 2), in the marrow results into the adulthood heterogenous vertebral marrow pattern (Figure 3)[52].
Figure 1.
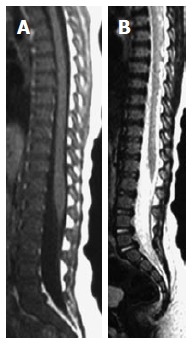
Sagittal T1W (A) and T2W (B) images of 2-year-old boy showing low-signal of the spinal marrow just barely brighter than intervertebral discs on T1W images due to richness in red marrow.
Figure 2.
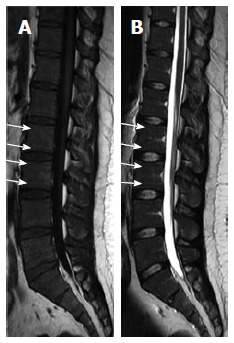
Sagittal T1W (A) and T2W (B) images of 25-year-old male showing linear high-signal of the normal fat marrow at the sub-endplate zones (white arrows) at LV2 through LV5 levels. Note also, linear focal fat depositions along the basi-vertebral veins posteriorly.
Figure 3.
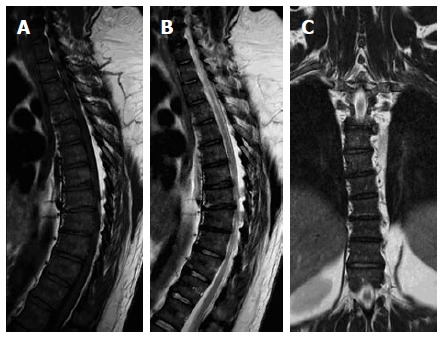
Sagittal T1W (A), T2W (B) and coronal STIR (C) images of the dorsal spines of a 60-year-old male with mild scoliotic deformity of the mid dorsal region. The figure shows heterogeneous vertebral marrow with predominantly T1W high-signal and T2W intermediate signal meanwhile, the whole marrow did not exhibit abnormal signal on STIR images. STIR: Short tau inversion recovery.
On T2W images, fatty marrow exhibits signal intensity near to that of the subcutaneous fat[2,6,10,17,18,53]. So use of fat suppression on fast/turbo spin echo T2 imaging is a must for better clinical utility of T2W sequence.
On fat-suppressed T2W and STIR images (Figure 3), the red marrow emits an intermediate signal slightly higher than adjacent paravertebral muscles against the black background of suppressed fatty marrow. However, it is far less intense compared to pathologic lesions with high cellular and water contents[10].
Following intravenous gadolinium-based contrast media administration, the red marrow; predominantly in children and young adolescents; shows appreciable visual enhancement and increased quantitive parameters on MR dynamic contrast studies inferring its abundant vascularity, well perfusion and increased metabolic activity. However, this SI increased has to be less than 35% by the age of 35 years[54]. On the other hand, this enhancement pattern is hardly perceptible in the fat marrow, in adults[37,38,55,56].
On chemical-shift imaging, the red marrow shows no remarkable signal drop on the out-phase image thanks to its near equal contents of both water and fat protons[57]. However, some signal drop may be seen in the yellow marrow (less than 20%) yet it is far less than malignant destructive processes[25].
On DWI, the normal red marrow shows intermediate signal that does not show lost signal on the corresponding ADC map.
Common variant of the normal spinal marrow on MR imaging
As bone marrow is a dynamic organ with the normal processeses of conversion and reconversion in response to various environmental and health stresses, and spines are the largest marrow reservoir of our body, heterogeneity of vertebral marrow MR signal is a common finding in daily clinical MR examinations. Additionally, this is more complicated by age- and sex-related variations as fat marrow is higher in men than women[49,58] and water content is higher in females child-bearing age[59].
In the same vertebral body of an adult, bone marrow is homogenously distributed with more abundance of the cellular red-marrow (50% of the spinal marrow by age of 70 years) near the endplates and anterior portion of the vertebral body while fat marrow is abundant around the basi-vertebral vein[2,60].
These spatial and sex-related changes are common between individuals of the same age group. However, it is important to recognize that these variations have to be homogenous between vertebral bodies of the same subject[54].
Spinal marrow heterogeneities’ may be seen in all spinal regions but it is more common in the lumbar spines[1,6,9]. These changes could be in a focal or diffuse pattern, produced by either yellow or red marrow variant distributions[6,54,61,62].
LOCALIZED NORMAL VARIANTS
Focal T1W hyper-intensities
Basi-vertebral vein fat: On T1W and T2W imaging, areas of focal fat deposition are commonly seen in the posterior elements of the vertebrae as well as areas of high vascularity with active processes of conversion and reconversion. This will include the sub-endplates and subcortical zones and around the basivertebral vein (Figure 4)[5,32,60].
Figure 4.
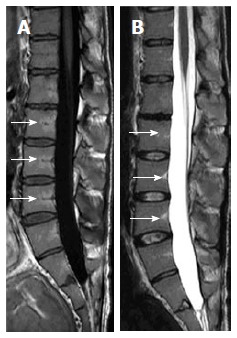
Sagittal T1W (A) and T2W (B) images of 24-year-old male showing linear high-signal intensities along the course of basi-vertebral veins with near ending of normal marrow conversion into the mature/fat type.
Vertebral-end plate degenerative changes: Progressive degenerative changes of the vertebral endplates are not uncommon findings on spinal MR (Figure 5)[63]. Modic and colleagues described band-like sub-end plate marrow changes that exhibit water-like (low T1W and high T2W) MR signal for type-I, fat-like (high T1W and T2W signals) for type-II, and calcium-like (low T1W and T2W signals) for type-III Modic changes[64]. Recognition of associated disc dehydration and presence of intra-discal gas precludes underling pathologies, e.g., discitis.
Figure 5.
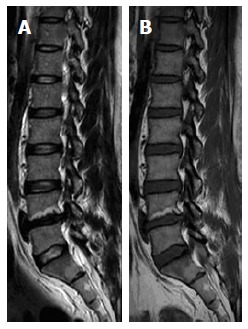
Sagittal T1W (A) and T2W (B) images of 53-year-old male showing LV4 lower end plate irregularities with subjacent Type-II Modic changes with high-T1W and T2W signal. Note adjacent LV4-5 disc desiccations.
Focal fatty marrow islands (Focal fatty metaplasia): A developmental variation of the bone marrow conversion process is the localized aggregates of areas of fat marrow. It can occur in any vertebral level yet it is a common variant in the lumbar spines (Figure 6) and lateral sacral ala of males than females; an area where sex-related marrow changes are important as age-related changes as proved by chemical-shift imaging and spectroscopic data[49,65]. It can take the eye of an inexperienced interpreter if seen in the turbo-spin echo T2W images. However, recognizing its high signal on T1W images and vanishing on fat-suppressed images will disclose its nature. Their corresponding radiographs and CT examinations will show preserved trabecular and cortical bone. They are not uncommon finding on daily spinal MR evaluations and should not raise clinical awkward.
Figure 6.
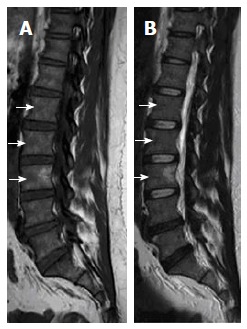
Sagittal T1W (A) and T2W (B) images of 41-year-old female showing LV3 patch of high-signal intensity (unchanged on serial magnetic resonance follow-up; not shown) on both T1W and T2W with fuzzy margins, proved to focal fatty metaplasia. Note areas of low signal intensity under the anterior cortex of multiple adjacent vertebral bodies (short white arrows) corresponding to focal nodules of red marrow.
HAEMANGIOMA
Histologically, hemangiomas are developmental vascular malformations consist of endothelial lined, thin-walled, blood-filled vessels and sinuses, containing and supported by fat and interspersed among the longitudinally oriented trabeculae of bones[66]. They are common in vertebral bodies than posterior elements. Hemangiomas are not exceedingly uncommon finding in MR studies of the spines. They are commonly asymptomatic and multiple. On T2W images as well as STIR images, typical hemangiomas have high-signal intensity due to slow flow in vascular channels[67]. Its benign nature is ascertained by corresponding high-signal intensity on T1W images due to its abundant fat content (Figure 7)[67]. Some atypical patterns of hemangioma may mimic more worrisome neoplastic lesions on MR imaging (Figure 8). Commonly, the intervening thickened trabeculae exhibits linear low-signal intensity on all pulse sequences[68]. Spinal hemangiomas shows variable patterns of enhancement and can be confused for serious bony lesions[69]. Radiography (Figure 8) and CT can help to solve such confusing situations by showing prominent trabeculae with the pathognomonic polka-dot sign (Figure 8) on axial images[70,71].
Figure 7.
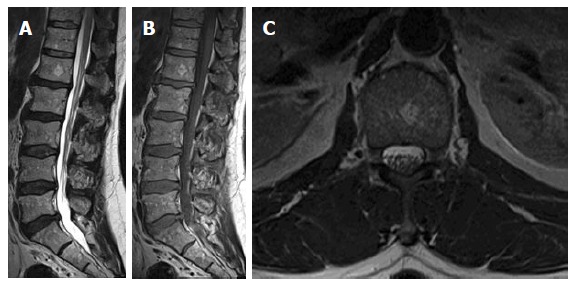
Sagittal T1W (A), T2W (B) and axial T2W images of 63-year-old osteoporotic female showing heterogeneous lumbar vertebral marrow signal with diffuse increased high-signal intensities due to higher fat content. There is a focal round patch of increased signal on both T1 and T2 weighting in LV1 body with small punctuate areas of low signal intensities; seen unchanged from previous 2 magnetic resonance examinations (not shown here) confirmed to be a small typical vertebral hemangioma.
Figure 8.
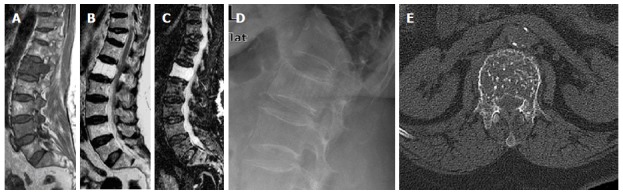
Sagittal T1W (A), T2W (B) and STIR (C) images of 65-year-old female showing L1 vertebral body atypical hemangioma with diffuse low signal intensity on T1W image and high-signal intensity on T2W and STIR images presented on a background of lumbar spondylotic changes. Note also, DV11 old porotic wedging. Companion imaging showed prominent trabecular pattern on focused radiography (D) and CT (E) of the LV1 with characteristic polka-dot sign. STIR: Short tau inversion recovery.
Focal T1W hypo-intensities
Vertebral enostosis: Enostosis (or bone island) is a common imaging finding on all imaging modalities assessing skeletal parts, especially the spine with an incidence of about 14%. It is thought as a benign osseous hamartoma of developmental origin[72]. It is composed of cortical bone layers embedded within the surrounding vertebral marrow cavity and it is usually endosteal surface based. It is common in the mid-dorsal and lumbar regions, although it can occur anywhere. It has low signal on all MR pulse sequences[72]. However, a previous report described a rare pattern of peripheral rim of high-signal intensity on STIR images making it difficult to differentiate from sclerotic metastasis[73]. However, correlation with radiography and CT will help to disclose the lesion’s nature.
Focal nodular hyperplasia of the red marrow
Bone marrow hyperplasia is an abberance of normal marrow conversion-reconversion process with abundance of red marrow[74,75]. Mild regional forms can be seen in endurance athletes, obese subjects and heavy smokers[15,76]. A more pronounced form can show up in some hematologic disorders (e.g., Hemolytic anemias) and malignancies as well as patients treated with granulocyte colony stimulating factors (GCSF) used to relive marrow suppression associated with chemotherapeutic regimens[77,78].
Rarely a localized focal form can be seen in the spinal (Figure 6) and pelvic marrow. On MR imaging these areas follow the signal criteria of normal red marrow, i.e., low signal intensity on T1W images, intermediate or no signal increase on T2W, Fat-suppressed and STIR imaging. On gadolinium administration faint or no enhancement is observed. Sometimes, these focal lesions can show increased signal intensity on T2 FSE sequences. This is contradictory to high T2W signal and contrast enhancement seen in neoplastic cases[74,75,79].
Presences within areas rich in red marrow (sub-cortical and around basi-vertebral vein), elongated shape of the lesions, presence of central high-spot on T1W images (Figure 9), fuzzy margins are predictors of their benignity[1,3]. On corresponding radiographs and CT studies, radiolucency, geographic nature and absence of cortical disruption will ascertain their benign nature.
Figure 9.
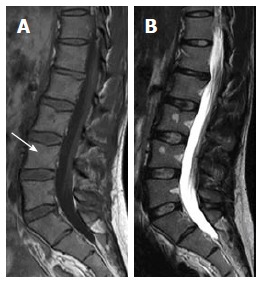
Sagittal T1W (A) and T2W (B) images of 33-year-old male showing focal geographic low signal intensity patches targeting LV3 and LV4 bodies centers as well as around basi-vertebral veins. These patches still of high-signal intensity on T2W image. Note, the central fat spot (white arrow) and fuzzy margins of LV4 lesion inferring benignity features are consistent with focal nodular marrow reconversion.
Benign notochordal cell tumors
Benign notochordal cell tumors are increasingly recognized intraosseous; presumably; benign lesions of notochordal remnants[80]. Its reported incidence in autopsies reaches 20% of clivus and vertebral bodies[81]. They are incidental finding on radiologic and histologic examinations and have to be distinguished from chordomas to save inadvertent extensive surgeries[82]. These lesions were found to emit homogenous low- to iso-signal intensity on T1W images and high-signal on T2W images with no enhancement on MR contrast studies[82,83]. Lesions that are sizable enough to be picked on CT and radiographs are sclerotic in nature. However, topographic features of the lesion, i.e., intravertebral, preserved trabecular pattern and non-enhancement following MR contrast administration, are equally important to rule the possibility of chordoma; the extremely malignant spectrum of notchordal cell lineage[82,83]. A recent report described malignant, transformation into chordoma of L1 vertebral body supporting the postulation of a relation of the two entities[84]. MR imaging is the best modality to address and followup these lesions.
Diffuse normal variants
Diffuse hematopoietic marrow hyperplasia: Diffuse hematopoietic marrow hyperplasia is an exaggeration of the normal marrow reconversion discussed in an earlier section. It can occur in response to different physiologic stimuli as discussed before. Moreover, it is increasingly recognized in patients under chemo- and radio-therapeutic regimens whom are treated with GCSF to lessen the associated bone marrow suppression[85,86].
It can be confused with diffuse marrow infiltrative processes in the vertebral marrow thanks to both red and fat marrow cohabitation. As a rule of thumb, marrow hyperplasia exhibits a signal similar to that of red marrow. The vertebral hyperplastic marrow shows low signal on T1W images that may be even lower than adjacent intervertebral discs[1,6,13,14,87,88]. it may show mild to moderate enhancement following IV gadolinium administration[86]. However, the signal is relatively higher than paravertebral muscles on STIR and fat-saturated T2 imaging[1,6,13,14,87,88]. In chronic hemoglobinopathies, low signal may be seen on T2W images due to chronic hemosidine deposition[5]. Problem-solving MR sequences may be utilized in some difficult cases.
Fat conversion of the marrow
Under certain conditions, there may be premature conversion of red marrow into the fat type with increased MR signal compared to the age and sex matched subjects. This can be seen in subjects with hypercortisolism (whether endogenous or exogenous) and in some feeding disorders as anorexia nervosa[89]. This supposed to be mediated via hormonal effects on the preferential differentiation of bone marrow progenitor cells[90]. It has to be considered as a normal variation of the bone marrow for the health status of those subjects and not a pathologic marrow disease.
Serous conversion of the marrow
In conditions of severe systemic illness associated with loss body fat stores, e.g., malignant cachexia, AIDS, anorexia nervosa or even following severe infections in pediatrics, a rare phenomenon of serous or gelatinous transformation of the bone marrow may commence in either diffuse or focal forms[91]. It starts in peripheral skelton yet it eventually reaches the axial skeleton. Pathologically; it is characterized by paucicellular marrow including both fat and hematopoietic cells which become embedded in hyaluronic acid-rich extracellular gelatinous substances[91,92]. These pathologic changes are recognized on MR as fat-poor marrow, which emits water-like signal on all pulse sequences, i.e., low on T1W and high on T2W and STIR sequences[3,93]. Visual loss of normal fat stores of the subcutis and inter-tissues fascial spaces will raise this suspicion[93,94]. On 18FDG PET/CT it may show increased tracer uptake[95].
CONCLUSION
MR is the gold standard noninvasive imaging modality to evaluate vertebral bone marrow. Conventional sequences used basically to image marrow include T1W, fat-suppressed T2W and STIR imaging provides gross morphological data. Moreover, non-routine MR sequences may elucidate the nature of bone marrow heterogeneities; by inferring cellular and chemical composition; and adding new functional prospects. Awareness of the age-related bone marrow changes as well as changes accompanying different variations of the subject’s health state is essential for radiologists. This will avoid overrating normal MR marrow patterns as pathologic states and avoid unnecessary further work-up.
ACKNOWLEDGMENTS
The authors would thank to Dr. Osamah Al Atyah, anesthesia and ICU Consultant, Hussein Al Ali Hospital, Al Ehsa, Saudi Arabia, for his critical linguistic revision of the final manuscript.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 5, 2015
First decision: August 8, 2015
Article in press: October 27, 2015
P- Reviewer: Anil G, Gao BL, Shen J, Sureka B S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Alyas F, Saifuddin A, Connell D. MR imaging evaluation of the bone marrow and marrow infiltrative disorders of the lumbar spine. Magn Reson Imaging Clin N Am. 2007;15:199–219, vi. doi: 10.1016/j.mric.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DT, Moynagh MR, Eustace SJ, Kavanagh EC. Bone marrow. Magn Reson Imaging Clin N Am. 2010;18:727–735. doi: 10.1016/j.mric.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Vande Berg BC, Lecouvet FE, Michaux L, Ferrant A, Maldague B, Malghem J. Magnetic resonance imaging of the bone marrow in hematological malignancies. Eur Radiol. 1998;8:1335–1344. doi: 10.1007/s003300050548. [DOI] [PubMed] [Google Scholar]
- 4.Strong PN, Goerke J, Oberg SG, Kelly RB. beta-Bungarotoxin, a pre-synaptic toxin with enzymatic activity. Proc Natl Acad Sci USA. 1976;73:178–182. doi: 10.1073/pnas.73.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tall MA, Thompson AK, Vertinsky T, Palka PS. MR imaging of the spinal bone marrow. Magn Reson Imaging Clin N Am. 2007;15:175–198, vi. doi: 10.1016/j.mric.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Vande Berg BC, Malghem J, Lecouvet FE, Maldague B. Magnetic resonance imaging of the normal bone marrow. Skeletal Radiol. 1998;27:471–483. doi: 10.1007/s002560050423. [DOI] [PubMed] [Google Scholar]
- 7.Riley RS, Williams D, Ross M, Zhao S, Chesney A, Clark BD, Ben-Ezra JM. Bone marrow aspirate and biopsy: a pathologist’s perspective. II. interpretation of the bone marrow aspirate and biopsy. J Clin Lab Anal. 2009;23:259–307. doi: 10.1002/jcla.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vande Berg BC, Malghem J, Lecouvet FE, Maldague B. Normal bone marrow: dynamic aspects in magnetic resonance imaging. J Radiol. 2001;82:127–135. [PubMed] [Google Scholar]
- 9.Vande Berg BC, Lecouvet FE, Galant C, Simoni P, Malghem J. Normal variants of the bone marrow at MR imaging of the spine. Semin Musculoskelet Radiol. 2009;13:87–96. doi: 10.1055/s-0029-1220879. [DOI] [PubMed] [Google Scholar]
- 10.Shah LM, Hanrahan CJ. MRI of spinal bone marrow: part I, techniques and normal age-related appearances. AJR Am J Roentgenol. 2011;197:1298–1308. doi: 10.2214/AJR.11.7005. [DOI] [PubMed] [Google Scholar]
- 11.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:108–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Małkiewicz A, Dziedzic M. Bone marrow reconversion - imaging of physiological changes in bone marrow. Pol J Radiol. 2012;77:45–50. doi: 10.12659/pjr.883628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillerman RP. Marrow: red, yellow and bad. Pediatr Radiol. 2013;43 Suppl 1:S181–S192. doi: 10.1007/s00247-012-2582-0. [DOI] [PubMed] [Google Scholar]
- 15.Poulton TB, Murphy WD, Duerk JL, Chapek CC, Feiglin DH. Bone marrow reconversion in adults who are smokers: MR Imaging findings. AJR Am J Roentgenol. 1993;161:1217–1221. doi: 10.2214/ajr.161.6.8249729. [DOI] [PubMed] [Google Scholar]
- 16.Carroll KW, Feller JF, Tirman PF. Useful internal standards for distinguishing infiltrative marrow pathology from hematopoietic marrow at MRI. J Magn Reson Imaging. 1997;7:394–398. doi: 10.1002/jmri.1880070224. [DOI] [PubMed] [Google Scholar]
- 17.Vogler JB, Murphy WA. Bone marrow imaging. Radiology. 1988;168:679–693. doi: 10.1148/radiology.168.3.3043546. [DOI] [PubMed] [Google Scholar]
- 18.Long SS, Yablon CM, Eisenberg RL. Bone marrow signal alteration in the spine and sacrum. AJR Am J Roentgenol. 2010;195:W178–W200. doi: 10.2214/ajr.09.4134. [DOI] [PubMed] [Google Scholar]
- 19.Levine CD, Schweitzer ME, Ehrlich SM. Pelvic marrow in adults. Skeletal Radiol. 1994;23:343–347. doi: 10.1007/BF02416990. [DOI] [PubMed] [Google Scholar]
- 20.Silva JR, Hayashi D, Yonenaga T, Fukuda K, Genant HK, Lin C. MRI of bone marrow abnormalities in hematological malignancies. Diagn Interv Radiol Ank Turk. 2013;19:393–3999. doi: 10.5152/dir.2013.067. [DOI] [PubMed] [Google Scholar]
- 21.Mirowitz SA, Apicella P, Reinus WR, Hammerman AM. MR imaging of bone marrow lesions: relative conspicuousness on T1-weighted, fat-suppressed T2-weighted, and STIR images. AJR Am J Roentgenol. 1994;162:215–221. doi: 10.2214/ajr.162.1.8273669. [DOI] [PubMed] [Google Scholar]
- 22.Bydder GM, Steiner RE, Blumgart LH, Khenia S, Young IR. MR imaging of the liver using short TI inversion recovery sequences. J Comput Assist Tomogr. 1985;9:1084–1089. doi: 10.1097/00004728-198511000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Disler DG, McCauley TR, Ratner LM, Kesack CD, Cooper JA. In-phase and out-of-phase MR imaging of bone marrow: prediction of neoplasia based on the detection of coexistent fat and water. AJR Am J Roentgenol. 1997;169:1439–1447. doi: 10.2214/ajr.169.5.9353477. [DOI] [PubMed] [Google Scholar]
- 24.Zajick DC, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA. Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology. 2005;237:590–596. doi: 10.1148/radiol.2372040990. [DOI] [PubMed] [Google Scholar]
- 25.Erly WK, Oh ES, Outwater EK. The utility of in-phase/opposed-phase imaging in differentiating malignancy from acute benign compression fractures of the spine. AJNR Am J Neuroradiol. 2006;27:1183–1188. [PMC free article] [PubMed] [Google Scholar]
- 26.Ragab Y, Emad Y, Gheita T, Mansour M, Abou-Zeid A, Ferrari S, Rasker JJ. Differentiation of osteoporotic and neoplastic vertebral fractures by chemical shift {in-phase and out-of phase} MR imaging. Eur J Radiol. 2009;72:125–133. doi: 10.1016/j.ejrad.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Swartz PG, Roberts CC. Radiological reasoning: bone marrow changes on MRI. AJR Am J Roentgenol. 2009;193:S1–4, Quiz S5-9. doi: 10.2214/AJR.09.7069. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich O, Biffar A, Reiser MF, Baur-Melnyk A. Diffusion-weighted imaging of bone marrow. Semin Musculoskelet Radiol. 2009;13:134–144. doi: 10.1055/s-0029-1220884. [DOI] [PubMed] [Google Scholar]
- 29.Lichy MP, Aschoff P, Plathow C, Stemmer A, Horger W, Mueller-Horvat C, Steidle G, Horger M, Schafer J, Eschmann SM, et al. Tumor detection by diffusion-weighted MRI and ADC-mapping--initial clinical experiences in comparison to PET-CT. Invest Radiol. 2007;42:605–613. doi: 10.1097/RLI.0b013e31804ffd49. [DOI] [PubMed] [Google Scholar]
- 30.Costa FM, Ferreira EC, Vianna EM. Diffusion-weighted magnetic resonance imaging for the evaluation of musculoskeletal tumors. Magn Reson Imaging Clin N Am. 2011;19:159–180. doi: 10.1016/j.mric.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Baur A, Huber A, Dürr HR, Nikolaou K, Stäbler A, Deimling M, Reiser M. Differentiation of benign osteoporotic and neoplastic vertebral compression fractures with a diffusion-weighted, steady-state free precession sequence. Rofo. 2002;174:70–75. doi: 10.1055/s-2002-19534. [DOI] [PubMed] [Google Scholar]
- 32.Biffar A, Baur-Melnyk A, Schmidt GP, Reiser MF, Dietrich O. Multiparameter MRI assessment of normal-appearing and diseased vertebral bone marrow. Eur Radiol. 2010;20:2679–2689. doi: 10.1007/s00330-010-1833-4. [DOI] [PubMed] [Google Scholar]
- 33.Castillo M, Arbelaez A, Smith JK, Fisher LL. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol. 2000;21:948–953. [PMC free article] [PubMed] [Google Scholar]
- 34.Karchevsky M, Babb JS, Schweitzer ME. Can diffusion-weighted imaging be used to differentiate benign from pathologic fractures? A meta-analysis. Skeletal Radiol. 2008;37:791–795. doi: 10.1007/s00256-008-0503-y. [DOI] [PubMed] [Google Scholar]
- 35.Eguchi Y, Ohtori S, Yamashita M, Yamauchi K, Suzuki M, Orita S, Kamoda H, Arai G, Ishikawa T, Miyagi M, et al. Diffusion magnetic resonance imaging to differentiate degenerative from infectious endplate abnormalities in the lumbar spine. Spine (Phila Pa 1976) 2011;36:E198–E202. doi: 10.1097/BRS.0b013e3181d5ff05. [DOI] [PubMed] [Google Scholar]
- 36.Padhani AR, Koh DM, Collins DJ. Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology. 2011;261:700–718. doi: 10.1148/radiol.11110474. [DOI] [PubMed] [Google Scholar]
- 37.Baur A, Stäbler A, Bartl R, Lamerz R, Scheidler J, Reiser M. MRI gadolinium enhancement of bone marrow: age-related changes in normals and in diffuse neoplastic infiltration. Skeletal Radiol. 1997;26:414–418. doi: 10.1007/s002560050257. [DOI] [PubMed] [Google Scholar]
- 38.Montazel JL, Divine M, Lepage E, Kobeiter H, Breil S, Rahmouni A. Normal Spinal Bone Marrow in Adults: Dynamic Gadolinium-enhanced MR Imaging1. Radiol. 2003;229:703–709. doi: 10.1148/radiol.2293020747. [DOI] [PubMed] [Google Scholar]
- 39.Rahmouni A, Montazel JL, Divine M, Lepage E, Belhadj K, Gaulard P, Bouanane M, Golli M, Kobeiter H. Bone marrow with diffuse tumor infiltration in patients with lymphoproliferative diseases: dynamic gadolinium-enhanced MR imaging. Radiology. 2003;229:710–717. doi: 10.1148/radiol.2293020748. [DOI] [PubMed] [Google Scholar]
- 40.Erlemann R, Reiser M, Peters PE, Wuisman P, Niendorf HP, Kunze V. Time-dependent changes in signal intensity in neoplastic and inflammatory lesions of the musculoskeletal system following intravenous administration of Gd-DTPA. Radiologe. 1988;28:269–276. [PubMed] [Google Scholar]
- 41.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–951. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 42.Simon GH, Raatschen HJ, Wendland MF, von Vopelius-Feldt J, Fu Y, Chen MH, Daldrup-Link HE. Ultrasmall superparamagnetic iron-oxide-enhanced MR imaging of normal bone marrow in rodents: original research original research. Acad Radiol. 2005;12:1190–1197. doi: 10.1016/j.acra.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Daldrup-Link HE, Rummeny EJ, Ihssen B, Kienast J, Link TM. Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin’s lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. Eur Radiol. 2002;12:1557–1566. doi: 10.1007/s00330-001-1270-5. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda Y, Ando K, Ishikura R, Kotoura N, Tsuda N, Kato N, Yoshiya S, Nakao N. Superparamagnetic iron oxide (SPIO) MRI contrast agent for bone marrow imaging: differentiating bone metastasis and osteomyelitis. Magn Reson Med Sci. 2006;5:191–196. doi: 10.2463/mrms.5.191. [DOI] [PubMed] [Google Scholar]
- 45.Hu HH, Kan HE. Quantitative proton MR techniques for measuring fat. NMR Biomed. 2013;26:1609–1629. doi: 10.1002/nbm.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Kuo D, Schafer AL, Porzig A, Link TM, Black D, Schwartz AV. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging. 2011;33:974–979. doi: 10.1002/jmri.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98:2294–2300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung CM, Kugel H, Schulte O, Heindel W. [Proton-MR spectroscopy of the spinal bone marrow. An analysis of physiological signal behavior] Radiologe. 2000;40:694–699. doi: 10.1007/s001170050798. [DOI] [PubMed] [Google Scholar]
- 49.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13:263–268. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 50.Panicek DM, Schwartz LH. MR Imaging of Bone Marrow in Patients with Musculoskeletal Tumors. Sarcoma. 1999;3:37–41. doi: 10.1080/13577149977857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanel D, Dromain C, Tardivon A. MRI of bone marrow disorders. Eur Radiol. 2000;10:224–229. doi: 10.1007/s003300050038. [DOI] [PubMed] [Google Scholar]
- 52.Foster K, Chapman S, Johnson K. MRI of the marrow in the paediatric skeleton. Clin Radiol. 2004;59:651–673. doi: 10.1016/j.crad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Hilfiker P, Zanetti M, Debatin JF, McKinnon G, Hodler J. Fast spin-echo inversion-recovery imaging versus fast T2-weighted spin-echo imaging in bone marrow abnormalities. Invest Radiol. 1995;30:110–114. doi: 10.1097/00004424-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Vande Berg BC, Lecouvet FE, Galant C, Maldague BE, Malghem J. Normal variants and frequent marrow alterations that simulate bone marrow lesions at MR imaging. Radiol Clin North Am. 2005;43:761–770, ix. doi: 10.1016/j.rcl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Bollow M, Knauf W, Korfel A, Taupitz M, Schilling A, Wolf KJ, Hamm B. Initial experience with dynamic MR imaging in evaluation of normal bone marrow versus malignant bone marrow infiltrations in humans. J Magn Reson Imaging. 1997;7:241–250. doi: 10.1002/jmri.1880070138. [DOI] [PubMed] [Google Scholar]
- 56.Biffar A, Dietrich O, Sourbron S, Duerr HR, Reiser MF, Baur-Melnyk A. Diffusion and perfusion imaging of bone marrow. Eur J Radiol. 2010;76:323–328. doi: 10.1016/j.ejrad.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Blebea JS, Houseni M, Torigian DA, Fan C, Mavi A, Zhuge Y, Iwanaga T, Mishra S, Udupa J, Zhuang J, et al. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med. 2007;37:185–194. doi: 10.1053/j.semnuclmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell DG, Rao VM, Dalinka M, Spritzer CE, Axel L, Gefter W, Kricun M, Steinberg ME, Kressel HY. Hematopoietic and fatty bone marrow distribution in the normal and ischemic hip: new observations with 1.5-T MR imaging. Radiology. 1986;161:199–202. doi: 10.1148/radiology.161.1.3763867. [DOI] [PubMed] [Google Scholar]
- 59.Ishijima H, Ishizaka H, Horikoshi H, Sakurai M. Water fraction of lumbar vertebral bone marrow estimated from chemical shift misregistration on MR imaging: normal variations with age and sex. AJR Am J Roentgenol. 1996;167:355–358. doi: 10.2214/ajr.167.2.8686603. [DOI] [PubMed] [Google Scholar]
- 60.Ricci C, Cova M, Kang YS, Yang A, Rahmouni A, Scott WW, Zerhouni EA. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology. 1990;177:83–88. doi: 10.1148/radiology.177.1.2399343. [DOI] [PubMed] [Google Scholar]
- 61.Hajek PC, Baker LL, Goobar JE, Sartoris DJ, Hesselink JR, Haghighi P, Resnick D. Focal fat deposition in axial bone marrow: MR characteristics. Radiology. 1987;162:245–249. doi: 10.1148/radiology.162.1.3786770. [DOI] [PubMed] [Google Scholar]
- 62.Baur A, Dietrich O, Reiser M. Diffusion-weighted imaging of bone marrow: current status. Eur Radiol. 2003;13:1699–1708. doi: 10.1007/s00330-003-1873-0. [DOI] [PubMed] [Google Scholar]
- 63.Kuisma M, Karppinen J, Niinimäki J, Kurunlahti M, Haapea M, Vanharanta H, Tervonen O. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine (Phila Pa 1976) 2006;31:1714–1718. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 64.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 65.Duda SH, Laniado M, Schick F, Strayle M, Claussen CD. Normal bone marrow in the sacrum of young adults: differences between the sexes seen on chemical-shift MR imaging. AJR Am J Roentgenol. 1995;164:935–940. doi: 10.2214/ajr.164.4.7726052. [DOI] [PubMed] [Google Scholar]
- 66.Hart JL, Edgar MA, Gardner JM. Vascular tumors of bone. Semin Diagn Pathol. 2014;31:30–38. doi: 10.1053/j.semdp.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Baudrez V, Galant C, Vande Berg BC. Benign vertebral hemangioma: MR-histological correlation. Skeletal Radiol. 2001;30:442–446. doi: 10.1007/s002560100390. [DOI] [PubMed] [Google Scholar]
- 68.Ross JS, Masaryk TJ, Modic MT, Carter JR, Mapstone T, Dengel FH. Vertebral hemangiomas: MR imaging. Radiology. 1987;165:165–169. doi: 10.1148/radiology.165.1.3628764. [DOI] [PubMed] [Google Scholar]
- 69.Rodallec MH, Feydy A, Larousserie F, Anract P, Campagna R, Babinet A, Zins M, Drapé JL. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics. 2008;28:1019–1041. doi: 10.1148/rg.284075156. [DOI] [PubMed] [Google Scholar]
- 70.Laredo JD, Reizine D, Bard M, Merland JJ. Vertebral hemangiomas: radiologic evaluation. Radiology. 1986;161:183–189. doi: 10.1148/radiology.161.1.3763864. [DOI] [PubMed] [Google Scholar]
- 71.Persaud T. The polka-dot sign. Radiology. 2008;246:980–981. doi: 10.1148/radiol.2463050903. [DOI] [PubMed] [Google Scholar]
- 72.Murphey MD, Andrews CL, Flemming DJ, Temple HT, Smith WS, Smirniotopoulos JG. From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996;16:1131–1158. doi: 10.1148/radiographics.16.5.8888395. [DOI] [PubMed] [Google Scholar]
- 73.Proceedings of UK Radiological Congress 1997. The British Institute of Radiology, 1997; 150. Available from: http://www.birpublications.org/doi/abs/10.1259/conf-pukrc.1997.
- 74.Bordalo-Rodrigues M, Galant C, Lonneux M, Clause D, Vande Berg BC. Focal nodular hyperplasia of the hematopoietic marrow simulating vertebral metastasis on FDG positron emission tomography. AJR Am J Roentgenol. 2003;180:669–671. doi: 10.2214/ajr.180.3.1800669. [DOI] [PubMed] [Google Scholar]
- 75.Detti B. A Case of Focal Haematopoietic Hyperplasia of a Vertebral Body and Review of the Modern Literature. J Nucl Med Radiat Ther. 2013;4:In press. [Google Scholar]
- 76.Deutsch AL, Mink JH, Rosenfelt FP, Waxman AD. Incidental detection of hematopoietic hyperplasia on routine knee MR imaging. AJR Am J Roentgenol. 1989;152:333–336. doi: 10.2214/ajr.152.2.333. [DOI] [PubMed] [Google Scholar]
- 77.Hollinger EF, Alibazoglu H, Ali A, Green A, Lamonica G. Hematopoietic cytokine-mediated FDG uptake simulates the appearance of diffuse metastatic disease on whole-body PET imaging. Clin Nucl Med. 1998;23:93–98. doi: 10.1097/00003072-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Stäbler A, Doma AB, Baur A, Krüger A, Reiser MF. Reactive bone marrow changes in infectious spondylitis: quantitative assessment with MR imaging. Radiology. 2000;217:863–868. doi: 10.1148/radiology.217.3.r00dc23863. [DOI] [PubMed] [Google Scholar]
- 79.Steiner RM, Mitchell DG, Rao VM, Schweitzer ME. Magnetic resonance imaging of diffuse bone marrow disease. Radiol Clin North Am. 1993;31:383–409. [PubMed] [Google Scholar]
- 80.Amer HZ, Hameed M. Intraosseous benign notochordal cell tumor. Arch Pathol Lab Med. 2010;134:283–288. doi: 10.5858/134.2.283. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi T, Suzuki S, Ishiiwa H, Shimizu K, Ueda Y. Benign notochordal cell tumors: A comparative histological study of benign notochordal cell tumors, classic chordomas, and notochordal vestiges of fetal intervertebral discs. Am J Surg Pathol. 2004;28:756–761. doi: 10.1097/01.pas.0000126058.18669.5d. [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi T, Iwata J, Sugihara S, McCarthy EF, Karita M, Murakami H, Kawahara N, Tsuchiya H, Tomita K. Distinguishing benign notochordal cell tumors from vertebral chordoma. Skeletal Radiol. 2008;37:291–299. doi: 10.1007/s00256-007-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishiguchi T, Mochizuki K, Ohsawa M, Inoue T, Kageyama K, Suzuki A, Takami T, Miki Y. Differentiating benign notochordal cell tumors from chordomas: radiographic features on MRI, CT, and tomography. AJR Am J Roentgenol. 2011;196:644–650. doi: 10.2214/AJR.10.4460. [DOI] [PubMed] [Google Scholar]
- 84.Nishiguchi T, Mochizuki K, Tsujio T, Nishita T, Inoue Y. Lumbar vertebral chordoma arising from an intraosseous benign notochordal cell tumour: radiological findings and histopathological description with a good clinical outcome. Br J Radiol. 2010;83:e49–e53. doi: 10.1259/bjr/63846600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altehoefer C, Bertz H, Ghanem NA, Langer M. Extent and time course of morphological changes of bone marrow induced by granulocyte-colony stimulating factor as assessed by magnetic resonance imaging of healthy blood stem cell donors. J Magn Reson Imaging. 2001;14:141–146. doi: 10.1002/jmri.1164. [DOI] [PubMed] [Google Scholar]
- 86.Ciray I, Lindman H, Aström GK, Wanders A, Bergh J, Ahlström HK. Effect of granulocyte colony-stimulating factor (G-CSF)-supported chemotherapy on MR imaging of normal red bone marrow in breast cancer patients with focal bone metastases. Acta Radiol. 2003;44:472–484. doi: 10.1080/j.1600-0455.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 87.Caldemeyer KS, Smith RR, Harris A, Williams T, Huang Y, Eckert GJ, Slemenda CW. Hematopoietic bone marrow hyperplasia: correlation of spinal MR findings, hematologic parameters, and bone mineral density in endurance athletes. Radiology. 1996;198:503–508. doi: 10.1148/radiology.198.2.8596857. [DOI] [PubMed] [Google Scholar]
- 88.Hanrahan CJ, Shah LM. MRI of spinal bone marrow: part 2, T1-weighted imaging-based differential diagnosis. AJR Am J Roentgenol. 2011;197:1309–1321. doi: 10.2214/AJR.11.7420. [DOI] [PubMed] [Google Scholar]
- 89.Geiser F, Mürtz P, Lutterbey G, Träber F, Block W, Imbierowicz K, Schilling G, Schild H, Liedtke R. Magnetic resonance spectroscopic and relaxometric determination of bone marrow changes in anorexia nervosa. Psychosom Med. 2001;63:631–637. doi: 10.1097/00006842-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 90.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 91.Jain R, Singh ZN, Khurana N, Singh T. Gelatinous transformation of bone marrow: a study of 43 cases. Indian J Pathol Microbiol. 2005;48:1–3. [PubMed] [Google Scholar]
- 92.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.García AI, Milinkovic A, Tomás X, Rios J, Pérez I, Vidal-Sicart S, Pomés J, Del Amo M, Mallolas J. MRI signal changes of the bone marrow in HIV-infected patients with lipodystrophy: correlation with clinical parameters. Skeletal Radiol. 2011;40:1295–1301. doi: 10.1007/s00256-011-1147-x. [DOI] [PubMed] [Google Scholar]
- 94.Tins B, Cassar-Pullicino V. Marrow changes in anorexia nervosa masking the presence of stress fractures on MR imaging. Skeletal Radiol. 2006;35:857–860. doi: 10.1007/s00256-005-0053-5. [DOI] [PubMed] [Google Scholar]
- 95.Chong A, Song HC, Oh JR, Ha JM, Min JJ, Bom HS, Choi YD, Lee JS. Gelatinous degeneration of the bone marrow mimicking osseous metastasis on 18F-FDG PET/CT. Clin Nucl Med. 2012;37:798–800. doi: 10.1097/RLU.0b013e31825ae455. [DOI] [PubMed] [Google Scholar]


