Abstract
OBJECTIVES:
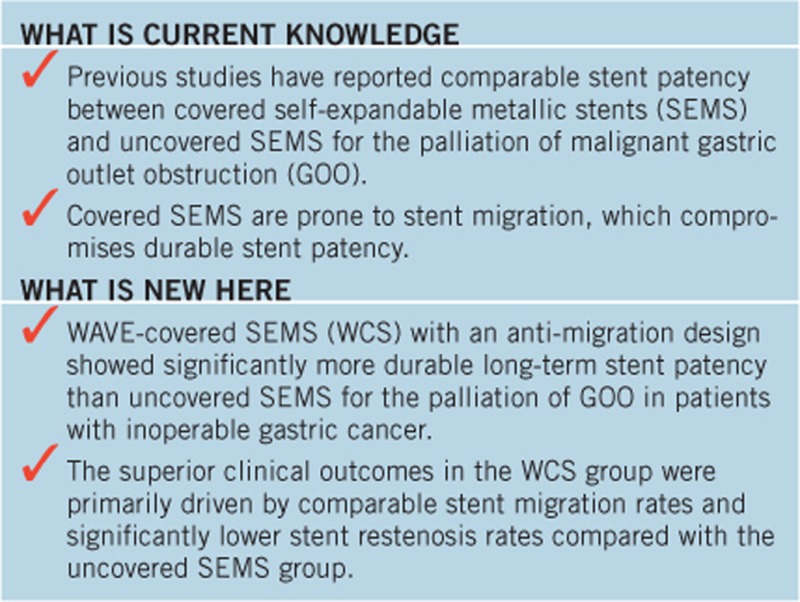
Previous studies reported comparable stent patency between covered self-expandable metallic stents (SEMS) and uncovered SEMS (UCS) for palliation of malignant gastric outlet obstruction (GOO). The aim of this study was to evaluate the efficacy and safety of the newly developed WAVE-covered SEMS (WCS), which has an anti-migration design, compared with UCS in gastric cancer patients with symptomatic GOO.
METHODS:
A total of 102 inoperable gastric cancer patients with symptomatic GOO were prospectively enrolled from five referral centers and randomized to undergo UCS or WCS placement. Stent patency and recurrence of obstructive symptoms were assessed at 8 weeks and 16 weeks after stent placement.
RESULTS:
At the 8-week follow-up, both stent patency rates (72.5% vs. 62.7%) and re-intervention rates (19.6% vs. 19.6%) were comparable between the WCS and the UCS groups. Both stent stenosis (2.4% vs. 8.1%) and migration rates (9.5% vs. 5.4%) were comparable between WCS and UCS groups. At the 16-week follow-up, however, the WCS group had a significantly higher stent patency rate than the UCS group (68.6% vs. 41.2%). Re-intervention rates in the WCS and UCS groups were 23.5% and 39.2%, respectively. Compared with the UCS group, the WCS group had a significantly lower stent restenosis rate (7.1% vs. 37.8%) and a comparable migration rate (9.5% vs. 5.4%). Overall stent patency was significantly longer in the WCS group than in the UCS group. No stent-associated significant adverse events occurred in either the WCS or UCS groups. In the multivariate analysis, WCS placement and chemotherapy were identified as independent predictors of 16-week stent patency.
CONCLUSIONS:
WCS group showed comparable migration rate and significantly more durable long-term stent patency compared with UCS group for the palliation of GOO in patients with inoperable gastric cancer.
INTRODUCTION
The endoscopic self-expandable metallic stent (SEMS) has become a feasible alternative to surgery for the palliation of inoperable malignant gastric outlet obstruction (GOO) and has more favorable short-term clinical outcomes (1, 2, 3). However, the clinical efficacy of SEMS has been compromised by several unresolved problems (4, 5, 6, 7). For uncovered SEMS, long-term patency is mainly hindered by tumor ingrowth through the metal mesh and consequent luminal stenosis (which has a frequency of 12–30%) (8, 9, 10, 11). For covered SEMS, stent migration occurs at a frequency of 16–25% and is a major obstacle to stent patency (12, 13, 14). Despite much lower tumor ingrowth rates in covered stents, their high migration rates lead to comparable clinical outcomes between covered and uncovered SEMS for the palliation of malignant GOO. Currently, a few randomized trials have compared clinical outcomes between uncovered stents and partially or fully covered stents in patients with malignant GOO, and these trials have reported comparable stent patency between the two stents (13, 15, 16).
To reduce migration and achieve a higher stent patency rate, various modifications (using anti-migration designs) of covered SEMS have been attempted. Thus far, no prospective randomized trial has demonstrated that covered SEMS have superior clinical outcomes to uncovered SEMS for the palliation of malignant GOO (13, 15, 16, 17, 18).
Recently, we developed a new covered SEMS with anti-migration properties (WAVE stent). WAVE stent was designed to have reduced radial force and indentation in the central part of the SEMS, uncovered flared portions at both ends, and a lasso at the proximal end that enables optimization of the stent position after deployment. The aim of this study was to evaluate the efficacy and safety of the newly developed WAVE stent compared with uncovered SEMS for the palliation of malignant GOO in patients with inoperable gastric cancer.
METHODS
Study design
This study was a prospective, multicenter, double-arm, patient-blinded, randomized trial and was conducted at five tertiary care centers. The study was approved by the Institutional Review Boards of each of the participating centers and was conducted according to the guidelines described in the Declaration of Helsinki for biomedical research involving human subjects (Clinical trial registration number: ClinicalTrials.gov NCT01646476). Between July 2012 and July 2014, 147 patients who were admitted due to symptoms of GOO caused by inoperable gastric cancer were assessed for eligibility as described below. Of these 147 patients, 102 fulfilled the eligibility criteria and were enrolled in the study after providing written informed consent.
Eligibility criteria
Patients with an initial diagnosis of gastric cancer were assessed using the following inclusion criteria: (i) the presence of pathologically confirmed gastric adenocarcinoma that was inoperable due to distant metastasis or severe comorbidity; (ii) upper endoscopy or abdominal computed tomography findings that were consistent with GOO at the distal antrum, pylorus, or duodenal bulb; (iii) the presence of GOO symptoms (early satiety, nausea, or vomiting) and a Gastric Outlet Obstruction Scoring System (GOOSS) score ≤2 (no oral intake, 0; liquids only, 1; soft solids, 2; low residue or full diet, 3) (19); and (iv) aged 20–80 years. The exclusion criteria were as follows: (i) inability to provide informed consent; (ii) multiple-level bowel obstruction confirmed on radiographic studies such as small bowel series or abdominal computed tomography; (iii) previous history of stent insertion or endoscopic dilation for GOO treatment; (iv) prior gastric surgery; (v) inability to undergo an upper endoscopy; and (vi) Borrmann type IV advanced gastric cancer.
Randomization and masking
Patients who fulfilled the eligibility criteria were randomized in a 1:1 ratio to the uncovered SEMS (UCS) group (BONASTENT, Standard Sci Tech, Seoul, Korea) or the WAVE-covered SEMS (WCS) group (BONASTENT, Standard Sci Tech), using a centralized, web-based randomization system. The allocation sequence was computer generated, with a block size of four. Patients had no knowledge of the stent type to which they were allocated.
Features of WCS
The newly developed WCS is a partially covered stent made of a nitinol hook and a cross wire structure with a diameter of 20 mm (Figure 1). WCS has several features that prevent migration. The stent body is indented in the central portion and thus has a bumpy and wavy external appearance, providing mechanical resistance to migration. The central part of the stent was designed to have reduced radial force compared with its proximal and distal parts (244 gf for the central part and 284 gf for the proximal and distal parts). Reduction of the radial force in the central part of the stent may help to prevent migration by allowing fixation at the stricture site. Platinum radiopaque markers were sutured in place in order to identify the central and bumpy portion of the stent, aiding in accurate placement. The WCS is completely covered with a silicone membrane in the middle; both the proximal and distal ends are uncovered and flared. In addition, this stent has a lasso at the proximal end to facilitate repositioning after deployment. The stents are available in 6, 8, 10, 12, 14, and 16 cm lengths with diameters of 18/24 mm (body/flare) at full expansion.
Figure 1.
WAVE-covered self-expandable metallic stent with an anti-migration design (a), uncovered self-expandable metallic stent (b).
Procedures
SEMS placement was performed with a therapeutic endoscope (working channel ≥3.7 mm) using a through-the-scope method. All patients underwent procedures under conscious sedation with midazolam and pethidine. The length of the stricture was assessed either endoscopically or fluoroscopically; the length of the stent had to exceed that of the stricture by at least 3 cm. After the required stent length was determined, the stent was advanced through the endoscope over a guidewire until it passed across the distal end of the stricture. Then, the stent was deployed under continuous fluoroscopic control. For the WCS group, the stent was repositioned after deployment using the lasso under fluoroscopic guidance (if necessary), aligning the central portion of the stricture with the central portion of the stent, which reduced radial force and indentation. Endoscopists evaluated the fluoroscopic findings observed during stent placement to evaluate the location of the stent relative to the location of the ampulla of Vater. The stent occluded the ampulla of Vater if it bridged the distal half of the second portion of the duodenum (20). Stent positioning was confirmed endoscopically and fluoroscopically.
Follow-up after SEMS placement
If the SEMS insertion was technically successful without any immediate complications, the patient was allowed to drink clear water on the day of the procedure. The diet was advanced gradually to a low-residue diet within 2 days, provided that the patient tolerated the diet and obstructive symptoms did not recur. Bilirubin (normal range, 0.2–1.2 mg/dl) and alkaline phosphate (normal range, 40–120 IU/l) levels before and after stent placement were evaluated. Abdominal plain films were checked daily for at least 3 days after stent placement to confirm full expansion and proper positioning of the inserted SEMS. Relief of obstructive symptoms was assessed 72 h after stent placement using the GOOSS. After discharge, patients underwent scheduled follow-up at 8 weeks and 16 weeks after deployment of the SEMS at the hospital where the stent was placed. At the 8-week follow-up, symptoms were evaluated with the GOOSS. In addition, patients underwent upper endoscopy and abdominal plain film examination to check for stent dysfunction, including stenosis or migration. At the 16-week follow-up, the GOOSS score and abdominal plain films were checked. Whenever obstructive symptoms recurred after stent placement, patients underwent upper endoscopy and radiologic examinations (computed tomography or abdominal plain films). In addition to the scheduled 8-week and 16-week follow-ups, patients receiving chemotherapy underwent periodic follow-ups with abdominal computed tomography to assess treatment response. Data on chemotherapy after stent insertion were collected for subsequent analyses. Clinical data were recorded until the patient's death or until the censoring date of 30 November 2014 (the date of the last follow-up).
Definitions and end points
Technical success was defined as adequate placement of the SEMS across the stenotic area, as confirmed by a combination of endoscopy and fluoroscopy. Clinical success was defined as relief of obstructive symptoms with an improvement of at least one point in the GOOSS score at 72 h after technically successful SEMS placement. Failure of SEMS patency was defined as the recurrence of obstructive symptoms with a decrease in the GOOSS score due to stent dysfunction. Stent dysfunction included stent stenosis by tumor ingrowth or overgrowth, stent migration, collapse, and fracture. Stent stenosis was considered if the patient had recurrence of obstructive symptoms, and the endoscope could not be passed through the lumen of the stent. Stent migration was considered if the stent had moved from its initial position and did not cover the entire stenosis. The primary end point of the present study was 8-week stent patency after SEMS insertion; secondary end points included 16-week stent patency and overall stent patency.
Statistical analysis
The primary analysis was a superiority comparison between WCS and UCS for the primary end point of 8-week stent patency using an intention-to-treat (ITT) analysis. In addition, treatment efficacy data, including overall survival, overall patient survival without stent dysfunction, and duration of stent patency, were analyzed in the ITT population. We also performed a modified ITT (mITT) analysis. The mITT population included patients who underwent UCS or WCS placement and completed the 8-week follow-up for stent patency (21, 22). This mITT population (with secondary end point information) was analyzed for stent dysfunction pattern.
Based on a previous study of covered and uncovered SEMSs for palliation of malignant GOO (13), it was hypothesized that the 8-week stent patency rate in the WCS group would be superior, with a 29% difference in the patency rate compared with the UCS group (89% vs. 60%). To demonstrate a 29% difference in the stent patency rate using a statistical power of 80% and with the assumption of a two-sided error rate of 0.05, the protocol required at least 82 randomly assigned patients. After considering loss to follow-up, we determined that a sample size of 100 patients would be adequate.
Categorical data were analyzed with the χ2-test or Fisher's exact test. Continuous data were analyzed with the unpaired t-test. In each assigned group, the duration of stent patency was estimated using the Kaplan–Meier method. Patients who had not experienced recurrent obstructive symptoms due to stent dysfunction were censored at the date of the last follow-up or the date of death. The Kaplan–Meier method was used to evaluate overall survival, with living patients censored at the date of the last follow-up. The Kaplan–Meier method was also used to evaluate overall patient survival without stent dysfunction (the time to stent dysfunction or patient death); living patients without stent dysfunction were censored at the date of the last follow-up. The log-rank test was used to compare the UCS and the WCS groups. Predictive factors for stent patency were evaluated using the Cox proportional hazards model, which included patient age, gender, the location of the stricture site, the baseline GOOSS score, baseline performance status, disease stage, the type of stent, the length of the stent, and whether post-stent chemotherapy was administered. Stratified analyses with regard to the 8-week and 16-week stent patency were performed in subgroups that were pre-defined according to age, gender, the location of the stricture, the baseline GOOSS score, the length of the stent, and the receipt of post-stent chemotherapy. Linear modeling was used to evaluate the consistency of treatment effects among the subgroups by testing for interactions between the treatment group and the clinically relevant subgroups. P-values of <0.05 were considered statistically significant. Adjusted Bonferroni P-values were used for multiple comparisons with control for experimental errors due to multiple statistical tests.
RESULTS
Patient characteristics
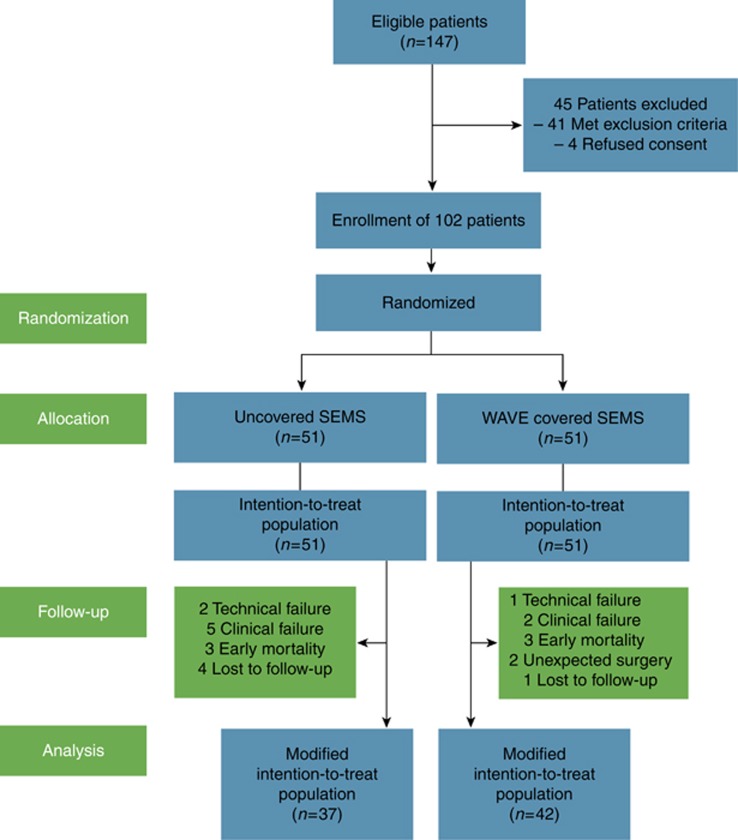
A total of 147 inoperable gastric cancer patients with symptomatic GOO were screened; 102 patients were randomly assigned to receive either a UCS (51 patients) or a WCS (51 patients). A flow diagram for the trial is provided in Figure 2. The baseline clinical and endoscopic characteristics are presented in Table 1. The UCS and WCS groups were well balanced in both the ITT and mITT populations. There were no significant differences between the two groups with respect to tumor location, stent length, baseline GOOSS distribution, Eastern Cooperative Oncology Group (ECOG) performance status, or the receipt of post-stent chemotherapy.
Figure 2.
Study flowchart. SEMS, self-expandable metallic stent.
Table 1. Demographic and clinical characteristics of the study population included in the intention-to-test and modified intention-to-treat analyses.
|
ITT population |
mITT population |
|||||
|---|---|---|---|---|---|---|
| UCS (n=51) | WCS (n=51) | P value | UCS (n=37) | WCS (n=42) | P value | |
| Age | ||||||
| Mean±s.d., years | 58.7±10.8 | 57.9±12.5 | 0.755 | 58.7±11.0 | 58.6±12.7 | 0.976 |
| ≥60 years (n, %) | 25 (49.0) | 25 (49.0) | 0.999 | 19 (51.4) | 21 (50.0) | 0.905 |
| Sex | ||||||
| Male (n, %) | 36 (70.6) | 34 (66.7) | 0.831 | 28 (75.7) | 28 (66.7) | 0.460 |
| Cancer stage IV (n, %) | 50 (98.0) | 51 (100) | 0.999 | 36 (97.3) | 42 (100) | 0.468 |
| Predominant symptom (n, %) | ||||||
| Vomiting with nausea | 42 (82.4) | 44 (86.3) | 0.786 | 31 (83.3) | 36 (85.7) | 0.999 |
| Early satiety and fullness | 9 (17.6) | 7 (13.7) | 6 (16.2) | 6 (14.3) | ||
| Location (n, %) | ||||||
| Distal antrum | 23 (45.1) | 23 (45.1) | 0.784 | 14 (37.8) | 19 (45.2) | 0.429 |
| Pyloric ring | 22 (44.1) | 24 (47.1) | 17 (45.9) | 20 (47.6) | ||
| Duodenal bulb | 6 (11.8) | 4 (7.8) | 6 (16.2) | 3 (7.1) | ||
| Length of stent | ||||||
| Mean±s.d., cm | 10.0±1.8 | 10.6±1.6 | 0.084 | 9.8±1.9 | 10.6±1.7 | 0.074 |
| ≥12 cm (n, %) | 25 (49.0) | 33 (64.7) | 0.110 | 18 (48.6) | 29 (69.0) | 0.072 |
| Baseline GOOSS | ||||||
| Mean±s.d. | 0.7±0.6 | 0.7±0.5 | 0.999 | 0.7±0.6 | 0.7±0.6 | 0.931 |
| 0 | 18 (35.3) | 17 (33.3) | 0.864 | 14 (37.8) | 14 (33.3) | 0.716 |
| ≥1 | 33 (64.7) | 34 (66.7) | 23 (62.2) | 28 (66.7) | ||
| Baseline ECOG performance status | ||||||
| Mean±s.d. | 1.9±0.7 | 1.7±0.6 | 0.185 | 2.0±0.8 | 1.7±0.7 | 0.117 |
| 1 | 15 (29.4) | 19 (37.3) | 0.340 | 11 (29.7) | 17 (40.5) | 0.213 |
| ≥2 | 36 (70.6) | 32 (62.7) | 26 (70.2) | 25 (59.5) | ||
| Post-stenting chemotherapy (n, %) | 26 (51.0) | 35 (68.6) | 0.106 | 25 (67.6) | 33 (78.6) | 0.314 |
ITT, intention-to-treat; mITT, modified intention-to-treat; UCS, uncovered stent; WCS, WAVE-covered stent.
Technical and clinical success
The UCS and the WCS groups demonstrated comparable results in terms of both the technical and clinical success rates. Technical success of UCS and WCS placement was achieved in 96.1% (49/51) and 98.0% (50/51) of the patients, respectively (P=0.558). Among patients undergoing technical success, five patients in the UCS group and two patients in the WCS group failed to achieve clinical success (P=0.240; Figure 2). Because all obstruction was related to distal gastric cancer only, we encountered no cases of biliary obstruction associated with stent placement bridging the ampulla of Vater. According to the laboratory tests, the pre-procedural and post-procedural bilirubin (1.60±0.14 vs.1.54±0.21 mg/dl) and alkaline phosphate levels (204.1±22.9 vs.217.5±17.8 mg/dl) did not differ.
8-week and 16-week stent patency and patterns of stent dysfunction
Before analysis of the primary outcome at 8 weeks after stent, gastric cancer-related death occurred in six patients (three patients in the UCS group and three patients in the WCS group, 5.9% each). In addition, two patients (3.9%) in the WCS group underwent unexpected palliative surgery due to massive tumor bleeding (Figure 2).
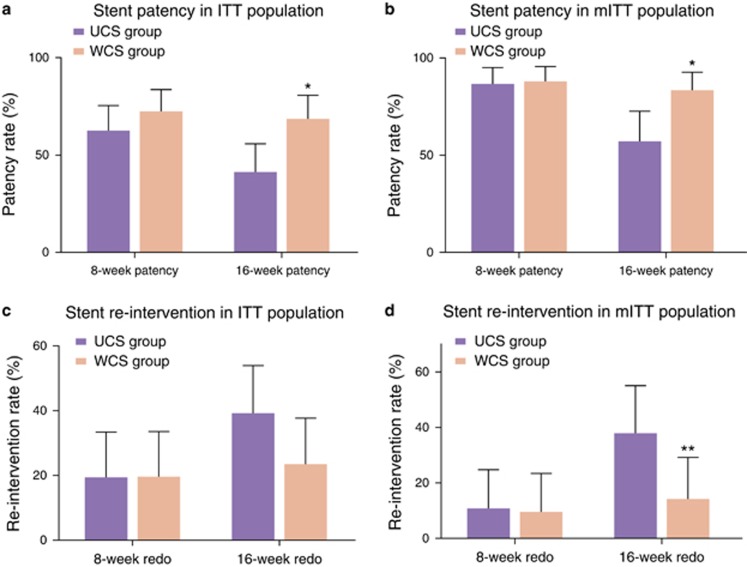
Figure 3 shows the 8-week and 16-week stent patency and re-intervention rates in the ITT and mITT populations. Table 2 and Figure 4 demonstrate the pattern of stent dysfunction up to the 8-week and the 16-week follow-ups in the mITT population. At the 8-week follow-up, both the stent patency and re-intervention rates were comparable between the UCS and the WCS groups. Stent patency rates were 62.7 and 72.5% in the UCS and the WCS groups in the ITT population (Figure 3a) and 86.5 and 88.1% in mITT population (Figure 3b), respectively (P=0.290 for ITT population and P=0.830 for mITT population). The re-intervention rates were 19.6% in both groups in the ITT analysis (Figure 3c) and 10.8% and 9.5% in the UCS and WCS groups, respectively, in the mITT analysis (Figure 3d; P=1.000 in ITT population and P=0.850 for mITT population). Up to the 8-week follow-up, stent migration rates were 5.4 and 9.5% in the UCS and the WCS groups, respectively, and statistically comparable between two groups (P=0.491). The rate of stent restenosis, including tumor ingrowth or overgrowth, was also comparable between the two groups (8.1% for the UCS group and 2.4% for the WCS group; P=0.261; Table 2 and Figure 4).
Figure 3.
Comparison of 8-week and 16-week stent patency and re-intervention rates between the uncovered stent and WAVE-covered stent groups. Stent patency rates are shown for the intention-to-treat population (a) and modified intention-to-treat population (b). Re-intervention rates are shown for the intention-to-treat population (c) and modified intention-to-treat population (d). Error bars represent 95% confidence intervals. *P<0.01; **P<0.05. ITT, intention-to-treat; mITT, modified intention-to-treat; UCS, uncovered stent; WCS, WAVE-covered stent.
Table 2. Patterns of stent dysfunction in the modified intention-to-treat population.
| Time since randomization | Adverse event |
Study population |
P value | |
|---|---|---|---|---|
| UCS (n=37) | WCS (n=42) | |||
| 8 Weeks | Restenosis (n, %) | 3 (8.1) | 1 (2.4) | 0.261 |
| Migration (n, %) | 2 (5.4) | 4 (9.5) | 0.491 | |
| Stent compression (n, %) | 0 | 0 | — | |
| Stent fracture (n, %) | 0 | 0 | — | |
| 16 Weeks | Restenosis (n, %) | 14 (37.8) | 3 (7.1) | 0.001 |
| Migration (n, %) | 2 (5.4) | 4 (9.5) | 0.491 | |
| Stent compression (n, %) | 0 | 0 | — | |
| Stent fracture (n, %) | 0 | 0 | — | |
mITT, modified intention-to-treat; UCS, uncovered stent; WCS, WAVE-covered stent.
Figure 4.
Cumulative probability of stent failure due to stent restenosis (a) and stent migration (b) in the modified intention-to-treat population. CI, confidence interval; HR, hazard ratio; UCS, uncovered stent; WCS, WAVE-covered stent.
At the 16-week follow-up, the WCS group had superior outcomes to UCS group in terms of stent patency and re-intervention rates, in contrast to the 8-week outcomes. Stent patency rates were 41.2 and 68.6% in the UCS and the WCS groups in the ITT population (Figure 3a) and 56.8 and 83.3% in the mITT population (Figure 3b), respectively (P=0.005 for the ITT population and P=0.009 for the mITT population). There was no significant difference between re-intervention rates between the two groups according to the ITT analysis after Bonferroni's correction (39.2% vs. 23.5% Figure 3c). The UCS group had a higher re-intervention rate than the WCS group in mITT analyses (37.8% vs. 14.3%, P=0.016; Figure 3d). The stent dysfunction pattern up to the 16-week follow-up also differed from the pattern of stent failure up to 8 weeks after stent placement. The UCS group had a significantly higher stent restenosis rate than the WCS group (37.8% vs. 7.1%, P=0.001). In contrast to the stent restenosis rate, stent migration rates up to the 16-week follow-up were comparable between the two groups (5.4% for the UCS group and 9.5% for the WCS group; P=0.491; Table 2 and Figure 4).
No stent-associated significant adverse events, such as bleeding or perforation, were reported during the follow-up period in either group.
Overall stent patency and survival
The mean total duration of follow-up was 141.7±66.7 days in the UCS group and 149.2±71.9 days in the WCS group. At the time of the final evaluation (30 November 2014), 25 patients (49.0%) in the UCS group and 19 patients (37.3%) in the WCS group had died. Supplementary Figure S1 online shows Kaplan–Meier curves for the duration of stent patency, overall survival, and overall survival without stent dysfunction in the ITT population. The WCS group had a significantly longer cumulative duration of stent patency compared with the UCS group (Supplementary Figure S1A). The overall survival time was comparable between the UCS and the WCS groups (Supplementary Figure S1B). Overall patient survival without stent dysfunction (the time to stent dysfunction or patient death) was longer in the WCS group than in the UCS group, with borderline statistical significance (Supplementary Figure S1C).
Predictive factors for stent patency
Table 3 shows the multivariate analysis of predictive factors for stent patency in the ITT population, using a Cox proportional hazards model. At the 8-week follow-up, only post-stent chemotherapy was identified as an independent predictor of stent patency. At the 16-week follow-up, stent type as well as post-stent chemotherapy were identified as independent predictors of stent patency. WCS placement reduced the risk of stent dysfunction, with a relative risk of 0.51 compared with UCS placement. Post-stent chemotherapy also reduced the risk of stent dysfunction. To evaluate the consistency of stent placement effects among the subgroups, we performed subgroup analyses of factors affecting the stent patency. The pre-specified subgroup analysis did not show heterogeneity of WCS treatment effects on either 8- or 16-week stent patency rates (Supplementary Figure S2A, B).
Table 3. Influence of covariates on stent failure at 8 weeks and 16 weeks in the Cox multivariate regression model.
| Covariate |
8 Weeks |
16 Weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | P value | Adjusted HR | 95% CI | P value | |||
| Lower | Upper | Lower | Upper | |||||
| Age | ||||||||
| <60 Years | 1 | 1 | ||||||
| ≥60 Years | 0.82 | 0.35 | 1.95 | 0.654 | 0.97 | 0.45 | 2.11 | 0.947 |
| Sex | ||||||||
| Male | 1 | 1 | ||||||
| Female | 0.99 | 0.41 | 2.39 | 0.989 | 0.85 | 0.38 | 1.9 | 0.687 |
| Location | ||||||||
| Distal antrum | 1 | 1 | ||||||
| Pylorus | 0.8 | 0.37 | 1.74 | 0.572 | 0.9 | 0.46 | 1.77 | 0.761 |
| Duodenal bulb | 1.56 | 0.4 | 6.12 | 0.523 | 1.51 | 0.45 | 5.12 | 0.508 |
| Baseline GOOSS | ||||||||
| 0 | 1 | 1 | ||||||
| 1 | 1.23 | 0.53 | 2.88 | 0.632 | 1.01 | 0.49 | 2.08 | 0.616 |
| 2 | 0 | 0 | — | 0.980 | 0.39 | 0.04 | 4.54 | 0.400 |
| Baseline ECOG performance status | ||||||||
| 1 | 1 | 1 | ||||||
| 2 | 1.03 | 0.45 | 2.35 | 0.951 | 1.46 | 0.67 | 3.15 | 0.341 |
| 3 | 0.42 | 0.1 | 1.88 | 0.258 | 0.46 | 0.13 | 1.61 | 0.224 |
| Stage | ||||||||
| III | 1 | 1 | ||||||
| IV | 0 | 0 | — | 0.992 | 2.22 | 0.21 | 23.29 | 0.507 |
| Type of stent | ||||||||
| Uncovered SEMS | 1 | 1 | ||||||
| Wave-covered SEMS | 0.72 | 0.34 | 1.55 | 0.401 | 0.51 | 0.26 | 0.99 | 0.047 |
| Length of stent | ||||||||
| <12 cm | 1 | 1 | ||||||
| ≥12 cm | 1.33 | 0.57 | 3.09 | 0.506 | 1.96 | 0.92 | 4.2 | 0.083 |
| Post-stenting chemotherapy | ||||||||
| Yes | 1 | 1 | ||||||
| No | 5.48 | 2.35 | 12.77 | <0.001 | 6.18 | 3.04 | 12.55 | <0.001 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; SEMS, self-expandable metallic stent.
DISCUSSION
To date, there have been many efforts to develop covered SEMS with anti-migration properties, with the goal of improving long-term stent patency. Despite these efforts, however, no prospective randomized trial has demonstrated that covered SEMS are superior to uncovered SEMS for the palliation of malignant GOO (13, 15, 16).
A few randomized trials, small comparative studies, and case series have been published regarding pyloric and duodenal stents for malignant GOO (Table 4). In accordance with the results of previous studies, we found that the 8-week stent patency rate was comparable between the WCS and the UCS groups in the ITT population. At the 16-week follow-up, however, the WCS group showed superior outcomes to the UCS group in both the stent patency rate and the re-intervention rate. This is the first prospective randomized study demonstrating superior clinical outcomes of covered SEMS to uncovered SEMS for the palliation of GOO in patients with inoperable gastric cancer.
Table 4. Various stent types reported in previous clinical trials.
| Stent | Manufacturer | Material | Length (cm) | Diameter shaft/flare (mm) | Covering | Reference |
|---|---|---|---|---|---|---|
| Wallstent | Boston Scientific | Elgiloy | 6, 9 | 20/22 | NC | (13, 25) |
| WallFlex | Boston Scientific | Nitinol | 6, 9, 12 | 22/27 | NC | (10, 13, 26, 27) |
| Evolution Duodenal | Cook Medical | Nitinol | 6, 9, 12 | 22/27 | NC | (9) |
| Niti-S D | Taewoong-Medical | Nitinol | 6, 8, 10, 12, 14, 15 | 18, 20, 22, 24 | NC | (16, 28, 29) |
| Niti-S S | Taewoong-Medical | Nitinol/silicone | 6, 8, 10, 12, 14, 15, 16 | 18/26, 20/28, 22/30, 24/32, 26/34, 28/36 | Covered | (13) |
| COMVI Stent | Taewoong-Medical | Nitinol/PTFE | 6, 8, 10, 12 | 18, 20, 22 | PC | (13, 15, 16, 30, 31) |
| Hanarostent NNN or NCN | MI Tech | Nitinol (or silicone) | 8, 9, 11, 14 | 20/25 or 20.26 | NC or PC | (32) |
| Hanarostent DPC | MI Tech | Nitinol/silicone | 9, 11, 13 | 20/40 (proximal)-22 (distal) | PC | (33) |
| Bonastent WAVE | Standard Sci Tech | Nitinol/silicone | 6, 8, 10, 12, 14, 16 | 18, 20, 22/25 | PC | — |
| Bonastent BP | Standard Sci Tech | Nitinol | 6, 8, 10, 12, 14, 16 | 18, 20, 22/25 | NC | — |
NC, not covered; PC, partially covered; PTFE, polytetrafluoroethylene.
Analyses of the 8-week and 16-week stent dysfunction patterns showed that the superior clinical outcomes in the WCS group were primarily driven by comparable stent migration rates and significantly lower stent restenosis rates compared with the UCS group. Stent migration rates remained stable over the follow-up period and were comparable between the two groups at both the 8-week and 16-week follow-up visits. Based on these results, it can be assumed that if patients undergoing WCS placement do not experience the stent migration in the early post-procedure phase, favorable long-term stent patency can be expected. These results also indicate that the anti-migration properties of the WCS efficiently and durably prevented stent migration and contributed to long-term overall stent patency and overall survival without stent dysfunction.
In the multivariate analysis, stent type and post-stent chemotherapy were identified as independent predictors of 16-week stent patency. Previous studies also reported comparable results regarding the protective effect of post-stent chemotherapy against stent dysfunction (23, 24, 25).
The strengths of this study were its prospective and randomized design and the scheduled follow-up protocol, with radiologic and endoscopic evaluations at 8 and 16 weeks. This standardized follow-up protocol enabled the adequate evaluation of long-term clinical outcomes after stent placement, as well as time-varying changes in the stent dysfunction pattern. This study has several limitations. First, we initially calculated the sample size to show a difference in the stent patency rate between the UCS and the WCS groups 8 weeks after stent deployment. However, 8-week stent patency rates, the primary end point of the study, were comparable between the two groups. In contrast to the 8-week results, there was a significant difference in 16-week stent patency rates between the UCS and the WCS groups. Although the difference reached statistical significance, the sample size may have been insufficient for assessing 16-week stent patency with adequate statistical power. In the present study, the WCS group demonstrated more favorable results compared with the UCS group not only in terms of 16-week stent patency, but also with respect to overall stent patency and overall patient survival without stent dysfunction. The consistency of the findings over the follow-up period suggests that the results of the present study are reliable. Second, our results may not be generalizable to GOO in pancreatic cancer patients, who have a poor prognosis and frequently have obstructions in the distal duodenal area.
In conclusion, WCS demonstrated significantly more durable long-term stent patency compared with UCS for the palliation of GOO in patients with inoperable gastric cancer. The superior clinical outcomes in the WCS group were primarily driven by comparable stent migration rates and significantly lower stent restenosis rates compared with the UCS group. Newly developed WCS could be considered as the promising stent option for the durable palliation of symptomatic GOO in patients with inoperable gastric cancer.
Study Highlights
Guarantor of the article: Sang Hyub Lee, MD.
Specific author contributions: S.H.L., B.H.M.: study concept and design. H.L., B.H.M.: analysis and interpretation of data. B.H.M., J.H.L., C.M.S., Y.K., H.C., H.L., and S.H.L.: acquisition of data. H.L.: drafting of the manuscript. B.H.M., J.H.L., C.M.S., Y.K., H.C., and S.H.L.: critical revision of the manuscript.
Financial support: None.
Potential competing interests: This work was supported by Standard Sci Tech, which provided the study stent. However, Standard Sci Tech had no role in the study design, data collection, analysis, decisions to publish or preparation of the manuscript. The final decision on content was exclusively retained by the authors. The study was conducted in compliance with all regulatory obligations and the Institutional Review Board of each investigational site.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Supplementary Material
References
- 1Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 2001;344:1681–1687. [DOI] [PubMed] [Google Scholar]
- 2Dormann A, Meisner S, Verin N et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy 2004;36:543–550. [DOI] [PubMed] [Google Scholar]
- 3Yim HB, Jacobson BC, Saltzman JR et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc 2001;53:329–332. [DOI] [PubMed] [Google Scholar]
- 4Brimhall B, Adler DG. Enteral stents for malignant gastric outlet obstruction. Gastrointest Endosc Clin N Am 2011;21:389–403. [DOI] [PubMed] [Google Scholar]
- 5Jeurnink SM, Steyerberg EW, van Hooft JE et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc 2010;71:490–499. [DOI] [PubMed] [Google Scholar]
- 6Jeurnink SM, van Eijck CH, Steyerberg EW et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol 2007;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Piesman M, Kozarek RA, Brandabur JJ et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol 2009;104:2404–2411. [DOI] [PubMed] [Google Scholar]
- 8Kim GH, Kang DH, Lee DH et al. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol 2004;39:1010–1014. [DOI] [PubMed] [Google Scholar]
- 9van den Berg MW, Haijtink S, Fockens P et al. First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy 2013;45:174–181. [DOI] [PubMed] [Google Scholar]
- 10van Hooft JE, Uitdehaag MJ, Bruno MJ et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc 2009;69:1059–1066. [DOI] [PubMed] [Google Scholar]
- 11van Hooft JE, van Montfoort ML, Jeurnink SM et al. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy 2011;43:671–675. [DOI] [PubMed] [Google Scholar]
- 12Jung GS, Song HY, Kang SG et al. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology 2000;216:758–763. [DOI] [PubMed] [Google Scholar]
- 13Kim CG, Choi IJ, Lee JY et al. Covered vs. uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc 2010;72:25–32. [DOI] [PubMed] [Google Scholar]
- 14Pan YM, Pan J, Guo LK et al. Covered vs. uncovered self-expandable metallic stents for palliation of malignant gastric outlet obstruction: a systematic review and meta-analysis. BMC Gastroenterol 2014;14:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Lim SG, Kim JH, Lee KM et al. Conformable covered vs. uncovered self-expandable metallic stents for palliation of malignant gastroduodenal obstruction: a randomized prospective study. Dig Liver Dis 2014;46:603–608. [DOI] [PubMed] [Google Scholar]
- 16Maetani I, Mizumoto Y, Shigoka H et al. Placement of a triple-layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig Endosc 2014;26:192–199. [DOI] [PubMed] [Google Scholar]
- 17Jeong JY, Han JK, Kim AY et al. Fluoroscopically guided placement of a covered self-expandable metallic stent for malignant antroduodenal obstructions: preliminary results in 18 patients. AJR Am J Roentgenol 2002;178:847–852. [DOI] [PubMed] [Google Scholar]
- 18Lee KM, Choi SJ, Shin SJ et al. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol 2009;44:846–852. [DOI] [PubMed] [Google Scholar]
- 19Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol 2002;97:72–78. [DOI] [PubMed] [Google Scholar]
- 20Kim SY, Song HY, Kim JH et al. Bridging across the ampulla of Vater with covered self-expanding metallic stents: is it contraindicated when treating malignant gastroduodenal obstruction? J Vasc Interv Radiol 2008;19:1607–1613. [DOI] [PubMed] [Google Scholar]
- 21Abraha I, Cherubini A, Cozzolino F et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. Br Med J 2015;350:h2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. Br Med J 2010;340:c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Cho YK, Kim SW, Hur WH et al. Clinical outcomes of self-expandable metal stent and prognostic factors for stent patency in gastric outlet obstruction caused by gastric cancer. Dig Dis Sci 2010;55:668–674. [DOI] [PubMed] [Google Scholar]
- 24Kim CG, Park SR, Choi IJ et al. Effect of chemotherapy on the outcome of self-expandable metallic stents in gastric cancer patients with malignant outlet obstruction. Endoscopy 2012;44:807–812. [DOI] [PubMed] [Google Scholar]
- 25Telford JJ, Carr-Locke DL, Baron TH et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc 2004;60:916–920. [DOI] [PubMed] [Google Scholar]
- 26Sasaki T, Isayama H, Maetani I et al. Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig Endosc 2013;25:1–6. [DOI] [PubMed] [Google Scholar]
- 27van Hooft J, Mutignani M, Repici A et al. First data on the palliative treatment of patients with malignant gastric outlet obstruction using the WallFlex enteral stent: a retrospective multicenter study. Endoscopy 2007;39:434–439. [DOI] [PubMed] [Google Scholar]
- 28Sato T, Hara K, Mizuno N et al. Gastroduodenal stenting with Niti-S stent: long-term benefits and additional stent intervention. Dig Endosc 2015;27:121–129. [DOI] [PubMed] [Google Scholar]
- 29Maetani I, Ukita T, Nambu T et al. Comparison of ultraflex and niti-s stents for palliation of unresectable malignant gastroduodenal obstruction. Dig Endosc 2010;22:83–89. [DOI] [PubMed] [Google Scholar]
- 30Isayama H, Sasaki T, Nakai Y et al. Management of malignant gastric outlet obstruction with a modified triple-layer covered metal stent. Gastrointest Endosc 2012;75:757–763. [DOI] [PubMed] [Google Scholar]
- 31Kim YW, Choi CW, Kang DH et al. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci 2011;56:2030–2036. [DOI] [PubMed] [Google Scholar]
- 32Havemann MC, Adamsen S, Wojdemann M. Malignant gastric outlet obstruction managed by endoscopic stenting: a prospective single-centre study. Scand J Gastroenterol 2009;44:248–251. [DOI] [PubMed] [Google Scholar]
- 33van den Berg MW, Walter D, Vleggaar FP et al. High proximal migration rate of a partially covered "big cup" duodenal stent in patients with malignant gastric outlet obstruction. Endoscopy 2014;46:158–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.