Abstract
Type 1 diabetes mellitus (T1D) is characterized by a heightened antibody (Ab) response to pancreatic islet self-antigens, which is a biomarker of progressive islet pathology. We recently identified a novel antibody to clade B serpin that reduces islet-associated T cell accumulation and is linked to the delayed onset of T1D. As natural immunity to clade B arises early in life, we hypothesized that it may influence islet development during that time. To test this possibility healthy young Balb/c male mice were injected with serpin B13 mAb or IgG control and examined for the number and cellularity of pancreatic islets by immunofluorescence and FACS. Beta cell proliferation was assessed by measuring nucleotide analog 5-ethynyl-2′-deoxyuridine (5-EdU) incorporation into the DNA and islet Reg gene expression was measured by real time PCR. Human studies involved measuring anti-serpin B13 autoantibodies by Luminex. We found that injecting anti-serpin B13 monoclonal Ab enhanced beta cell proliferation and Reg gene expression, induced the generation of ∼80 pancreatic islets per animal, and ultimately led to increase in the beta cell mass. These findings are relevant to human T1D because our analysis of subjects just diagnosed with T1D revealed an association between baseline anti-serpin activity and slower residual beta cell function decline in the first year after the onset of diabetes. Our findings reveal a new role for the anti-serpin immunological response in promoting adaptive changes in the endocrine pancreas and suggests that enhancement of this response could potentially help impede the progression of T1D in humans.
Keywords: antibody, beta cell (B-cell), pancreatic islet, serpin, Type 1 diabetes
Introduction
Type 1 diabetes mellitus (T1D)3 is an autoimmune condition that affects people of all ages. Although T1D was traditionally considered a pediatric disease, it has become clear that individuals over 20 years old can also develop detectable autoimmunity against pancreatic islets with concomitant sudden onset of insulin dependence (1). To date, the modifying factors that precipitate the clinical manifestation of autoimmune diabetes at different ages are largely unknown.
We have focused on factors that regulate the timing of clinical onset of T1D. Recent observations from our laboratory have revealed a novel autoantibody directed against the serpin B13 protease inhibitor (2, 3) and demonstrated that it partially protects against early onset autoimmune diabetes (4). In T1D susceptible nonobese diabetic (NOD) mice, elevated secretion of anti-serpin B13 autoantibody is associated with protection from diabetes before 16 weeks of age, whereas decreased secretion of this antibody (Ab) in humans is associated with T1D onset before age 5 years (4). These observations suggest an inverse relationship between the serpin B13 autoantibody response and the appearance of the clinical features of T1D. We further linked the serpin Ab to reduced autoimmune inflammation in pancreatic islets, likely due to enhanced extracellular cleavage of key cell surface molecules expressed in T and B cells (5). Of note, serpins have been implicated as anti-apoptotic agents that improve islet survival (6, 7), although in a model of prevention of autoimmune diabetes by limited apoptosis, transgenic intraislet expression of serpin-like protein CrmA reduces rather than enhances preventive effect (8).
Notwithstanding the anti-inflammatory impact of serpin B13 Ab, our previous studies suggested that an additional mechanism may account for the protective effect of this immunological response in the setting of T1D. First, we found that the natural Ab response to serpin B13 arises early in life but declines in 8- to 12-week-old mice and the first 5 to 10 years in humans (4). This early transient Ab response to serpin B13 suggests that anti-serpin autoantibodies have limited, if any, effect on intraislet inflammation that develops after early childhood. Rather serpin Ab may affect cell growth and/or death in non-inflamed islets before autoimmunity strikes, ultimately protecting endocrine pancreatic tissue. The published reports on the enhanced regenerative potential of pancreatic tissue at a young age lend support to this possibility (9–12). Second, our previous finding of serpin B13 expression in pancreatic exocrine ducts suggests that the immune response to this molecule may regulate the relationship between distinct pancreatic tissue compartments. In this hypothetical scenario, a ductal immunological response to serpin B13 could intensify functional cross-talk with the endocrine tissue and facilitate the renewal of insulin-producing cells. Research by other groups also hints at the possibility of deriving beta cells and their progenitors from pancreatic ductal cells or adjacent regions, at least in the setting of acute injury in animal models (13, 14) or chronic human pancreatic disease (15, 16).
The present study is the first attempt to elucidate serpin Ab-mediated regulation of endocrine islet cells under normal conditions. To ensure clinical relevancy, we employed a monoclonal Ab (mAb) as an enhancing agent and applied it to the animals in the third week of life when the natural response to serpin B13 is at its maximum. We found that both the number of pancreatic islets and beta cell proliferation are significantly increased after exposure to serpin mAb. Moreover, to translate our findings to human we examined the impact of serpin B13 Abs in children in the first year after the onset of T1D and found that this Ab response is associated with slower progression of beta cell defect. Together with our previous reports, these observations have implications for a better understanding of how anti-serpin activity may fine-tune the balance between the destructive forces of autoimmune inflammation and adaptive changes in the islets, and point to this activity as a potential modifying factor of the natural history of diabetes.
Experimental Procedures
Human Subjects
Serum samples (n = 54) and supporting data (e.g. the age, gender, body weights, insulin regimens, hemoglobin A1c levels, and fasting and stimulated C-peptide concentrations in a mixed-meal tolerance test at baseline and 3, 6, and 12 months after diagnosis) from new-onset, placebo-treated patients with T1D were obtained from the TrialNet Biosample Repository. The male to female ratio was 1.84, the age of subjects was 10.15 ± 2.78 years, and body weight was 39.57 ± 13.31 kg. At baseline, 19 subjects were considered serpin Ab strongly (++) positive (n = 19; male to female ratio, 2.17; body weight, 35.92 ± 2.90 kg; age, 9.635 ± 0.58 years), another 19 subjects were considered serpin Ab weakly (+) positive (n = 19; male to female ratio, 1.42; body weight, 42.75 ± 9.34 kg; age, 11.3 ± 2.18 years), and the remaining 16 subjects were considered serpin Ab negative (n = 16; male to female ratio, 2.22; body weight, 39.26 ± 3.44 kg; age, 10.2 ± 0.61 years). The subjects had been previously enrolled in Type 1 Diabetes TrialNet protocols TN02 MMF/DZB (n = 14), TN08 GAD-Alum Vaccine (n = 15), TN09 CTLA4 (n = 15), and TN14 anti-IL1β (n = 10). The University of Rochester institutional review board approved the study. Insulin dose-adjusted glycosylated hemoglobin A1C (IDAAC) was calculated, as A1C (percent) + [4 × insulin dose (units/kg/24 h)], as previously described (17).
Animals
Male Balb/c and female NOD mice were purchased from the Jackson Laboratory. The University Committee on Animal Resources at the University of Rochester approved all mouse experiments.
Antibodies
The mouse mAb against serpin B13 (clone B29) was described previously (4) and control IgG was TIB92 (ATCC). The guinea pig anti-insulin (DAKO) polyclonal Ab was used to stain the pancreatic islets. Alexa Fluor 488 (or Alexa Fluor 594)-conjugated goat anti-guinea pig IgG was used as the secondary antibody.
Other Reagents
Fluoroshield mounting medium with DAPI (Abcam) was used to stain nuclei. Streptozotocin (STZ, 10 mg/ml; Sigma) dissolved in 0.1 m sodium citrate buffer (pH 4.5, J. T. Baker) was used to induce diabetes. Mouse serpin B13 (GenScript) was used to immunize Balb/c mice.
Collagenase P and DNase I (Roche Applied Science) were used to isolate islets, and Cellstripper (Corning) was used to dissociate islet cells. A FOXP3 intracellular staining kit (Biolegend) was used for cell fixation and permeabilization.
Immunofluorescence Microscopy (IF)
The pancreata were fixed in 2% paraformaldehyde for 2 h at 4 °C, then incubated in 30% sucrose buffer, embedded in optimal cutting tissue media (O.C.T.), and instantly frozen in a dry ice/ethanol bath. For studies examining islet section number, size, and cellularity, pancreata of Balb/c mice were processed in a fashion similar to that described elsewhere (18). Briefly, each tissue block was cut through into 4-μm sections every 100 μm, producing 12 to 18 representative layers per animal. The frozen sections representing every other layer were blocked in 5% bovine serum albumin (BSA) for 30 min at room temperature followed by incubation with the primary Ab for 1 h. Following two washes in PBS and exposure to the secondary Ab for 45 min, the slides were mounted with DAPI mounting medium and coverslipped. To detect insulin, tissue sections were stained with anti-insulin guinea pig polyclonal Ab (1:100) followed by Alexa Fluor® 488 (or Alexa Fluor 594)-conjugated goat anti-guinea pig IgG (1:200). IF images were taken with Olympus BX41 microscope. The sections were viewed through a 4 × 0.1 NA objective to determine the size and number of pancreatic islet sections or a 20 × 1.0 NA objective to determine their cellularity. The images were processed using Image-Pro Plus 6.0 and Adobe Photoshop 7.0 software.
Islets Morphometry
Numbers of pancreatic islet sections and their sizes were assessed from images of IF slides labeled with insulin Ab. All insulin positive clusters with a surface area of at least 200 pixels (image resolution of 1360 × 1024 pixels, 72 dpi) were analyzed. The data were then converted to depict the size of islet section in diameter using the following equation,
 |
where (d0) is an islet section diameter of 58 μm corresponding to an islet section area (A0) of 200 pixels (using 4 × 0.1 NA objective) or 5000 pixels (using 20 × 1.0 NA objective).
Generating Mathematical Formulas Predicting Islet Cellularity
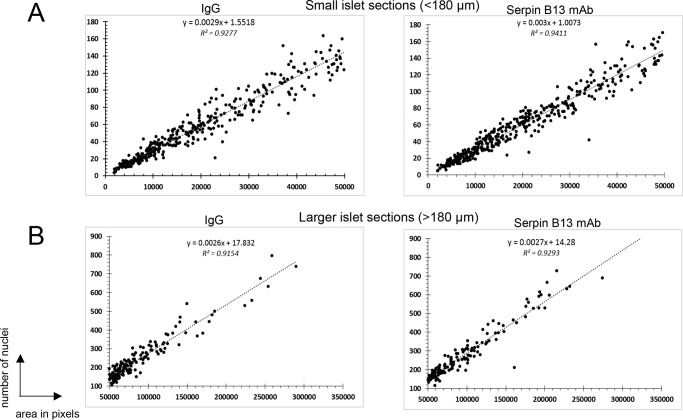
Two representative pancreatic sections from each of 9 IgG-treated and 9 serpin B13 mAb-treated 8-week-old Balb/c mice were stained for insulin and mounted with DAPI. The actual cellularity of islet sections was determined based on the number of DAPI-stained nuclei in areas that were also positive for insulin Ab. To generate formula predicting cellularity of small islet sections (<180 μm in diameter), we examined ∼40 to 45 pancreatic sections per animal (366 islet sections in IgG group and 403 islet sections in serpin B13 mAb group). To generate formula predicting cellularity of larger islets (>180 μm in diameter), we examined ∼16 pancreatic sections per animal (145 islet sections in IgG group and 145 islet sections in serpin B13 mAb group). The trend formulas predicting the expected number of beta cell nuclei (y) in relationship to islet section size expressed in pixels (x) were graphed in scatter plots using Microsoft Excel 2013. The formula were then adjusted to ×4 magnification under which islet images were captured. Specifically, x value in the formula was multiplied by the value of 25 corresponding to a square difference between ×4 and ×20 objectives.
Determining Beta Cell Mass
Islet beta cell mass was calculated as the percentage surface area occupied by insulin-positive cells multiplied by the pancreas weight expressed in milligrams.
Beta Cell Proliferation
Proliferation of beta cells was assessed by measuring incorporation of the nucleotide analog, 5-ethynyl-2′-deoxyuridine (5-EdU) (Carbosynth) into DNA during the synthesis phase of the cell cycle. 5-EdU was dissolved in drinking water at 1 mg/ml and given to mice for 2 weeks (at 21 days after the last injection of serpin B13 mAb or IgG). To ensure persistent exposure, drinking water containing 5-EdU was changed every 3 days and kept in the dark at all times. The animals were then sacrificed, and their pancreata were sectioned and stained for insulin followed by incubation with a “click” mixture to detect 5-EdU. Briefly, each tissue section was incubated for 30 min at room temperature with a mixture containing 97 μl of 1× PBS, 0.5 μl of aqueous solution 0.1 m CuSO4 (Sigma-Aldrich), 2.5 μl of aqueous solution 1 m (+) sodium l-ascorbate (Sigma), and 0.08 μl of 6-FAM azide (Lumiprobe). The slides were then thoroughly washed two times and left in PBS for an additional 30 min. Finally, the slides were mounted with DAPI medium and coverslipped. We screened 57,027 beta cells in 511 islet sections in the IgG group (n = 9 animals) and 65,086 beta cells in 548 islets sections in the serpin B13 mAb-treated group (n = 9 animals). More specifically, the numbers of islet sections according to their diameter in the IgG group were as follows: 153 islet sections <90 μm, 213 islet sections 90–180 μm, 113 islet sections 180–270 μm, and 32 islet sections >270 μm. The numbers of islet sections according to their diameter in the anti-serpin B13 mAb group were as follows: 152 islet sections <90 μm, 251 islet sections 90–180 μm, 106 islet sections 180–270 μm, and 39 islet sections >270 μm.
Quantitative Real-time Polymerase Chain Reaction
Pancreatic islet RNA was prepared by using TRIzol reagent (Invitrogen). Approximately 1 μg of islet RNA from each group was reverse transcribed into cDNA using oligo(dT) as a primer and an iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR assays were performed on a Rotor-Gene 3000 System platform using cDNA and the iQ SYBR Green Supermix reagent (Bio-Rad). β-Actin (or GAPDH for human samples) amplification was used as the internal control to normalize the data. Three individual samples were amplified from each group in duplicates. The primer sequences are provided in Table 1.
TABLE 1.
PCR primer mouse and human (h) sequences used in this study
| Forward | Reverse | |
|---|---|---|
| β-Actin | GCTTCTTTGCAGCTCCTTCG | CTTTGCACATGCCGGAGCC |
| Reg1 | AGACGCCAATGTCTGGACTG | GTTAGGAGACCCAGTTGCCC |
| Reg2 | GCCAACGCCTATGGTTCCTA | CTCTCCTTAACCAGCGAGGC |
| Reg3a | CTGGTCTGCCAGAAGAGACC | GGTAGTTGTCCACTCTGCCG |
| Reg3b | TGCCTTAGACCGTGCTTTCT | TCGGGATGTTTGCTGTCTGAT |
| Reg3d | AGGGGAGTTTGCCCAATGAA | TGAGGCCTGAGACAAAGCTG |
| Reg3g | GTACCCTGTCAAGAGCCTCA | TGTGGGGAGAATGTTCCCTT |
| Reg4 | GGCTCTGAGGGCCTTGAAAT | GCACAGCTGGGTCTCAAGAT |
| Arx | TCTCTTCCTCCGGATACCCC | CTGGCCCTGGCATCTGTTTA |
| Brn4 | CAGCTGCCTCGAATCCCTAC | TCGCTAAGACTGGTCACCCA |
| Hlxb9 | CTCATCTGAGGACGACTCGC | CCAGGTAGCCATCTTTCGCA |
| Insm1 | AGAACTGTGCCTTCACTCGG | GGAGTCACAGCGAGAAGACC |
| Ins2 | GTCAAGCAGCACCTTTGTGG | GTCTGAAGGTCACCTGCTCC |
| Irx1 | GCGATCTCGGATACGGGC | GCGGAGGGCAACACTAACT |
| Irx2 | TTCCCGTCCTACGTGGGCT | GTGGGGTACGGGTTCTTTCG |
| Isl1 | TTCTCCGGATTTGGAGTGGC | CACGCATCACGAAGTCGTTC |
| MafA | GCTGGTATCCATGTCCGTGC | CTCTCCAGAATGTGCCGCT |
| Neurod1 | AAAGCCCCCTAACTGACTGC | CGGCACCGGAAGAGAAGATT |
| Ngn3 | CGGATGACGCCAAACTTACA | GTTACCCGCTTGGGAGACTG |
| NKx2.2 | GGAGAGCCACGAATTGACCA | TCATCGTTGGTGTCCGGAAG |
| Nkx6.1 | GGCTGTGGGATGTTAGCTGT | TCATCTCGGCCATACTGTGC |
| Pax4 | GACGGACTCAGCAGTGTGAA | CAAGACACCTGTGCGGTAGT |
| Pax6 | CGGGAAAGACTAGCAGCCAA | GGCCTGTCTTCTCTGGTTCC |
| Pdx1 | CTTAACCTAGGCGTCGCACA | TGGTCCCAGGAAAGAGTCCT |
| GAPDH (h) | TCCTGTTCGACAGTCAGCC | CGCCCAATACGACCAAATCC |
| REG1A (h) | ACCGGACCATCTCTCCAACT | AGGGTTCCAAAGACTGGGGT |
| REG1B (h) | TGCCTATCGCTCCTACTGCT | AGGCCAATCCAGACATTGCT |
| REG3A (h) | AGGACTCACCCTGGAAGAGA | AAGCCTTAGGCCGTATGACA |
| REG3G (h) | AGCCTGTCAAGAAGCACAGG | AGTCAGGTAGGGCAGTTTGG |
Luminex
A Luminex-based technology was used to measure serpin B13 autoantibodies in NOD mice and humans (blind samples), as described previously (4). Specifically, serum samples were divided into 2 halves, then incubated overnight at 4 °C with either soluble serpin B13 or serpin B8 as specific and nonspecific competitor, respectively. After incubation, the beads were precoated with individual antigens, then added to the samples, which were incubated for 2 h at room temperature on a shaker (final serum dilution of serum: 1:10), and stained using a mixture of biotinylated mouse anti-human κ chain and λ chain mAbs (BD Biosciences) (dilution: 1:300). The final step was to incubate the beads with streptavidin (dilution: 1:200) for 10 min at room temperature. Fluorescence intensity was measured using Luminex 200 and Bio-Rad Bioplex software and data were expressed as fluorescence intensity units after subtracting fluorescence intensity due to serum binding activity in the presence of beads precoated with a control lysate expressing green fluorescence protein. The samples were evaluated based on the level of fluorescence intensity and the degree of inhibition of binding to serpin B13-coated beads with soluble serpin B13 compared with soluble serpin B8. Serum samples, in which binding activity to Luminex bead-bund serpin B13 was >1000 IF units or 500 to 1000 IF units, and the degree of inhibition of that binding with soluble serpin B13 was >25% were considered strongly (++) or weakly (+) positive, respectively. Serum samples, in which binding activity to Luminex bead-bound soluble serpin B13 was <500 were considered negative.
Islet Isolation
Mouse pancreatic islets were isolated using a collagenase P/DNase I digestion method followed by handpicking under a stereomicroscope. Islet sizes that were higher or lower than 200 μm in diameter were determined by using MR400 microscopic calibration slide (AmScope). For FACS analysis islet cell suspensions were obtained by treating the islets with Cellstriper buffer for 5 min at 37 °C. Human pancreatic islets were obtained from the Integrated Islet Distribution Program.
Isolation of Pancreatic Ductal Cells
Ductal cells were isolated according to the protocol developed by Reicher et al. (19). Briefly, the pancreata from 8-week-old male Balb/c mice were digested with collagenase IV (Sigma). Next, the pancreatic cell suspensions were labeled with DBA lectin-FITC (Vector Labs)(1:400) followed by MicroBeads-conjugated anti-FITC (Miltenyi Biotec) (1:10). The DBA lectin-positive cells were separated from DBA lectin-negative cells using MS columns (Miltenyi Biotec) and magnetic field. The isolated DBA lectin-positive cells were referred to as the ductal cells, although the remaining cells were considered to be non-ductal. Ductal or non-ductal cells (3 × 104) were lysed in 100 μl of PBS containing 0.5% BSA, 2 mm EDTA and, either control IgG or anti-serpin B13 mAb (8 μg/ml), and using high speed (13,300 rpm/5 min) centrifugation for several times. After incubating on ice for 30 min, the lysates were added to human islets.
Treatment of Human Islets
The aliquots of human islets (100 islets per sample) were incubated in 96-well flat bottom tissue culture plates. The islets were cultured at 37 °C for 24 h in medium containing lysate extract (mixed with the original medium at a ratio of 1:1) of either pancreatic ductal or non-ductal cells. After incubation, the islets were harvested and processed for RNA isolation.
Fluorescence-activated Cell Sorting (FACS) Analysis
A FOXP3 intracellular staining kit and anti-insulin Ab (1:400) was used to determine the total and beta cell numbers in pancreatic islets. After acquiring all events, the live cells were gated on forward and side scatter light dot plots and their numbers (including positively insulin-stained fractions) were divided by the number of isolated islets to determine the average total cellularity (or beta cell number) per islet.
Enzyme-linked Immunosorbent Assay (ELISA)
The Mercodia Ultrasensitive Mouse Insulin ELISA kit was used to measure plasma insulin according to the manufacturer's recommendations.
Treatment of Mice with Anti-serpin B13 mAb
Balb/c male mice were intraperitoneally injected on days +13, +15, +18, and +21 of life with 2.5 or 25 μg of serpin B13 mAb or IgG control per injection (total dose of 10 or 100 μg). Alternatively, the above injection regimen (4 × 25 μg) was repeated two additional times at 2 and 4 months of age. The animals were sacrificed at 8 weeks of age for pancreatic islet analysis by FACS or IF, or at 9 months of age for IF analysis only.
Treatment of Mice with Purified Serpin B13
Balb/c male mice were intraperitoneally injected on days +5 and +10 with 10 μg of mouse serpin B13 diluted in PBS or PBS alone (control), then injected with a single dose of STZ and monitored for blood glucose levels.
Diabetes Induction and Monitoring
Balb/c male pups (10 days old) were intraperitoneally injected with a single dose of STZ (100 mg/kg of body weigh). Eight-week-old Balb/c mice were intraperitoneally injected with two doses of STZ, first with 100 mg/kg body weight after an overnight fast, and then with 50 mg/kg body weight 1 day later. Glucose levels were measured in tail blood 1 week after the last injection of STZ.
Glucose Tolerance Test
After overnight fasting, 12-week-old mice were intraperitoneally injected with 10% d-glucose solution (10 μl/g body weight). A glucometer was used to monitor glucose levels using tail blood collected before glucose injection and then after injection at 30-min intervals for 2 h.
Statistics
Statistical analyses were performed using t test, linear regression analysis, and two-way analyses of variance with Dunnett multiple comparison. A p value <0.05 was considered significance. Data are presented as mean ± S.E.
Results
Treatment with Serpin B13 mAb Induces Morphometric Changes in the Endocrine Pancreas, Effect on Islet Number and Cellularity
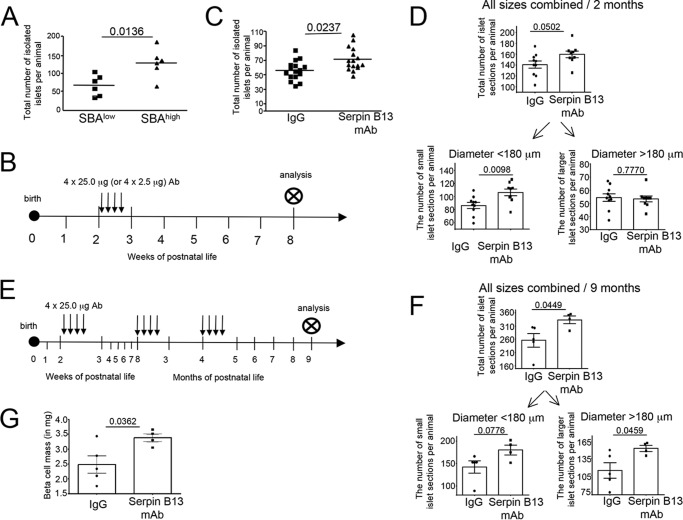
The initial experiments involving screening young NOD female mice for secretion of autoantibodies against serpin B13 allowed us to isolate small groups of animals with relatively low (e.g. <500 units of fluorescence intensity; SBAlow) and high (e.g. >3000 units of fluorescence intensity; SBAhigh) levels of these autoantibodies and compare the numbers of pancreatic islets. In all six experiments performed, the number of islets that were isolated by the collagenase/DNA digestion method was higher in SBAhigh NOD mice compared with SBAlow NOD mice (p = 0.0136, Fig. 1A). To validate this finding, we performed a similar analysis in Balb/c mice by injecting them with serpin B13 mAb during the third week of life (Fig. 1B). We decided to inject antibody at an early age in the Balb/c mice for two reasons. First, not every mouse is strongly positive for serpin antibody at a young age. According to our previous work, at this age less than 20% of the colony demonstrates high serum binding activity to serpin, whereas the remaining animals show little to no serpin binding activity. Therefore, injecting anti-serpin Ab in the third week of life allowed us to generate a uniform baseline of serpin antibody within our Balb/c mouse cohort. Second, by injecting antibody at an early age we were able to take advantage of the relatively high pancreatic islet regenerative potential during this time, thereby maximizing our chances of identifying potential adaptive responses to our treatment. Examination of 8-week-old Balb/c mice (e.g. 5 weeks after the last mAb injection) revealed an increase in the number of isolated pancreatic islets in the serpin B13 mAb group (p = 0.0237, Fig. 1C). Therefore, exposure to both the endogenous autoantibodies to serpin B13 (in NOD mice) and exogenous serpin B13 mAb (in Balb/c) increases the number of pancreatic islets that can be isolated from the pancreas.
FIGURE 1.
Increase in the number of pancreatic islets in mice exposed to serpin B13 antibodies. A, 3-week-old NOD female mice with distinct levels of endogenous serpin B13 Ab were sacrificed, and their pancreata were subjected to pancreatic islet isolation. B, scheme of Ab injection in Balb/c mice depicted in C and D. 2-Week-old Balb/c male mice injected with serpin B13 mAb or IgG control were sacrificed at 8 weeks of age and their pancreata were subjected to pancreatic islet isolation (C) or examined by IF for insulin-positive cell cluster sections measuring >58 μm in diameter (D). E, scheme of Ab injection in Balb/c mice depicted in F and G. Balb/c male mice injected at 2 weeks, 8 weeks, and 4 months of age with serpin B13 mAb or IgG control were sacrificed at 9 months of age and their pancreata were examined by IF exactly as in D (F) or analyzed for beta cell mass as described under ”Experimental Procedures“ (G). Data are presented as mean ± S.E.
To distinguish between enhanced isolation efficiency and a genuine increase in total islet number following serpin B13 mAb injection, we performed microscopic examinations of pancreata from 8-week-old Balb/c male mice that had been treated in the third week of life with the experimental Ab or control IgG (Fig. 1B). We noted a marked difference in the proportion of individual islet section sizes: the small islet sections measuring 58 to 180 μm in diameter accounted for almost 60% of all mouse pancreas islet sections (data not shown). Based on these observations we analyzed all islet sections regardless of their size as well as individual subgroups with distinct diameter ranges (e.g. <180 μm and >180 μm). We observed an overall, but not significant, increase in the number of islet sections in the serpin B13 mAb group (160 ± 6.0 islet sections/pancreas) compared with those that received IgG (141.2 ± 6.5 islet sections/pancreas). Further subgroup analysis revealed a significantly increased number of small islet sections in the experimental group compared with the control group (Fig. 1D, lower left, p = 0.0098), whereas the numbers of larger (>180 μm) islet sections per pancreas were similar in both animal groups. The beta cell mass remained unchanged in 8-week-old mice (data not shown).
To examine whether an observed increase in the output of small islets is followed by additional adaptive changes later in life, we performed microscopic examination of pancreata from 9-month-old Balb/c male mice that received serpin B13 mAb or IgG control during the first, second and forth month of life (Fig. 1E). We observed that at 9 months of age, serpin B13 mAb-treated mice had on average 77 more islets compared with IgG-treated animals. Specifically, there was an overall significant increase in the number of all islet sections in the serpin B13 mAb group (333.5 ± 14.43 islet sections/pancreas) compared with those that received IgG (256 ± 25.71 islet sections/pancreas) (p = 0.0449) (Fig. 1F, upper). At this later time point, the number of larger islet sections was more up-regulated compared with the number of small islet sections (Fig. 1F, lower). Importantly, beta cell mass was significantly increased after treatment with serpin B13 mAb compared with control IgG in 9-month-old animals (e.g. 2.484 ± 0.2896 versus 3.382 ± 0.1318 mg, p = 0.0362, Fig. 1G).
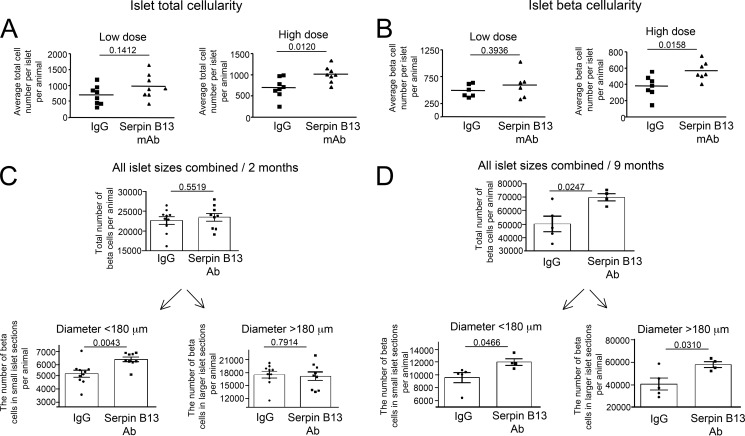
We next focused on beta cell cellularity to further characterize islet adaptive changes in response to anti-serpin activity. FACS analyses confirmed that Balb/c mice exhibited dose-dependent elevations, in terms of both islet total (Fig. 2A, p = 0.012) and beta (Fig. 2B, p = 0.0158) cellularity, following treatment with serpin B13 mAb. We also performed IF microscopy to determine any changes in the total beta cellularity in the pancreas in response to serpin B13 mAb. Toward this goal we applied mathematical formulae that predict islet cellularity according to the size of islet microscopic section (Fig. 3). Specifically, an examination of 8-week-old Balb/c male mice (treated with serpin B13 mAb according to the scheme depicted in Fig. 1B) revealed that in comparison with the IgG-treated group, their small islet sections combined had higher total number of beta cells per pancreas (5,200 ± 288.5 versus 6,364 ± 188.9 (p = 0.0043, Fig. 2C lower left)), although no such difference was noted for larger islet sections. In 9-month-old Balb/c male mice (treated with serpin B13 mAb exactly as depicted in Fig. 1E), the difference was much more evident for both all islet sections regardless of their size (e.g. 49,999 ± 5,755 versus 69,757 ± 2,673 beta cells per animal, p = 0.0247; Fig. 2D, upper) as well as for individual islet section subgroups (e.g. 9,539 ± 787.8 versus 11,958 ± 518.5 beta cells per animal for small islet sections (p = 0.0466, Fig. 2D, lower left) and 40,460 ± 5,304 versus 57,799 ± 2,618 beta cells per animal for larger islet sections (p = 0.0310, Fig. 2D, lower right). Of note, beta cell size slightly decreased (less than 2%) as a result of treatment with serpin Ab (data not shown).
FIGURE 2.
Increased pancreatic islet cellularity in serpin B13 mAb-treated mice. A and B, Balb/c male mice were injected with a low (4 × 2.5 μg) or high (4 × 25 μg) dose of serpin B13 mAb or IgG control, as described in the legend to Fig. 1B. The animals were sacrificed at 8 weeks old, and their pancreata were prepared for insulin staining and FACS. The average total cellularity (A) and beta cell number per islet (B) are shown. The islets from experiments depicted in Fig. 1C were used for the analysis. C and D, Balb/c male mice were injected with serpin B13 mAb or IgG control (4 × 25 μg), as described in the legend to Fig. 1, B (2-month follow up), and E (9-month follow up), respectively. The data depicted in C and D are based on mice that were also used for the analysis depicted in Fig. 1, D and F, respectively. Data are presented as mean ± S.E.
FIGURE 3.
Linear regression analysis of beta cellularity according to islet section size in IgG- and serpin B13 mAb-treated mice. Pancreatic tissue blocks were obtained from the same Balb/c male mice that were used in the experiments depicted in Fig. 1. Tissue sections were stained with an anti-insulin Ab and DAPI, and nuclei in small and larger beta cell cluster sections were quantified. A, analysis of small islet sections. A total of 20,429 nuclei in the IgG-treated group and 23,497 nuclei in the serpin B13 mAb-treated group were examined to generate the formulae. B, analysis of larger islet sections. A total of 36,598 nuclei in the IgG-treated group and 41,589 nuclei in the serpin B13 mAb-treated group were examined to generate the formulae.
Collectively, our findings suggest that the initial increase in the number and cellularity of small-sized islets (e.g. within several weeks after exposure to serpin B13 antibodies) is followed by similar increases in larger islets (e.g. during 4 to 8 months after treatment with serpin B13 mAb) and ultimately leads to a significant increase in beta cell mass in the pancreas.
Serpin B13 Ab Treatment Induces Beta Cell Proliferation and Up-regulation of Reg Gene Expression in Pancreatic Islets
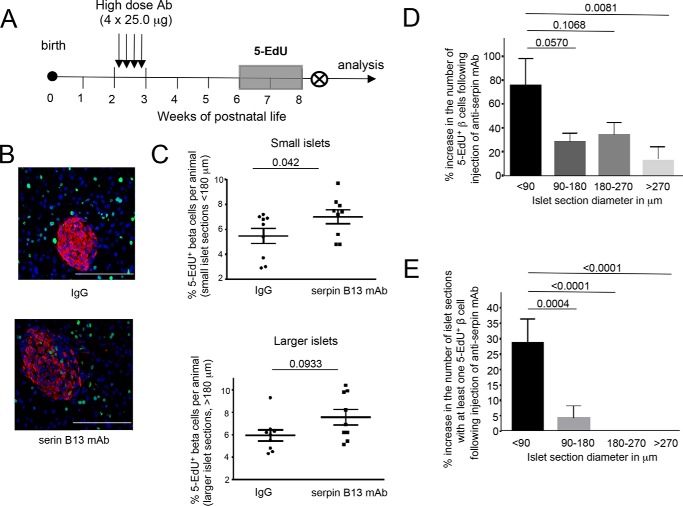
Increased beta cellularity in the pancreas following injection of serpin B13 mAb could be caused by enhanced beta cell proliferation, improved survival due to increased resistance to cell death, or de novo cell formation. In this study we focused on the first possibility. To examine beta cell proliferation in mice exposed to serpin mAb, we measured nucleotide analog 5-EdU incorporation into the newly synthesized DNA in replicating cells. After feeding mice with drinking water containing 5-EdU for 2 weeks (weeks 4 and 5 after the last IgG or serpin mAb injection), animals were sacrificed at 8 weeks of age (the end of week 5 after the last injection (Fig. 4A)) and their pancreata were examined for 5-EdU incorporation in insulin-positive clusters, as described under ”Experimental Procedures.“ The analysis of 57,027 beta cells in 511 islets in the IgG-treated group (n = 9) and 65,086 beta cells in 548 islets in the serpin B13 mAb-treated group (n = 9) revealed 3,239 and 4,629 5-EdU+ cells, respectively, indicating a 43% overall increase in beta cell proliferation in the latter group. This enhancement of beta cell proliferation by anti-serpin affected predominantly small islets (p = 0.042 versus p = 0.0933; Fig. 4, B and C). In particular, beta cell clusters <90 μm in diameter showed a strong increase in the frequency of 5-EdU+ beta cells (Fig. 4D) and in the fraction of islets containing at least one 5-EdU+ beta cell (Fig. 4E). Of note, we also examined mice exposed to 5-EdU during weeks 2 and 3 after the last IgG or serpin mAb injection and found no significant up-regulation in beta cell proliferation.
FIGURE 4.
Increased beta cell 5-EdU incorporation in serpin B13 mAb-treated mice. A, strategy for Ab injection and 5-EdU incorporation. B, representative staining of pancreatic islets for Edu. Red, insulin; green, EdU; blue, DAPI. C, the percentages of 5-EdU beta cells in islet sections per animal in small and larger islets. D, percent increase in the number of 5-EdU+ beta cells and E, pancreatic islet sections with at least one 5-EdU+ beta cell following serpin B13 mAb injection. Data are presented as mean ± S.E.
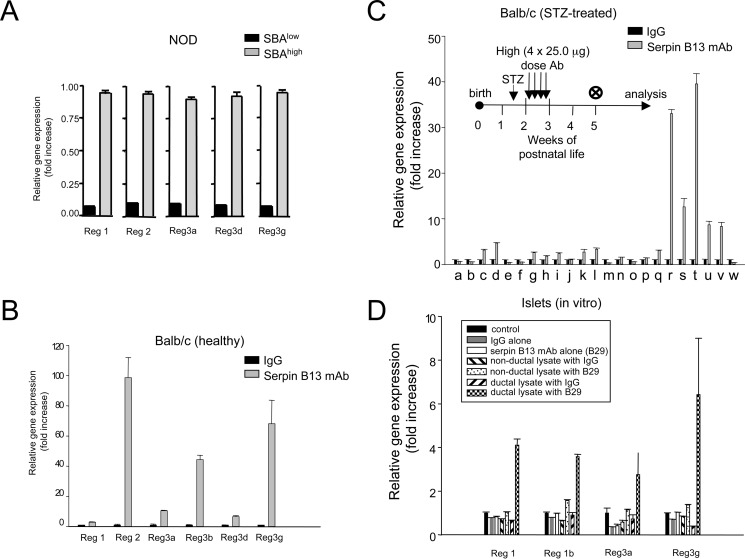
Next, we measured expression of genes associated with the development of islet endocrine cells and beta cell renewal to independently examine pancreatic islet regeneration (20–23). We found that young NOD mice with elevated endogenous serpin B13 autoantibodies (Fig. 5A), as well as both healthy (Fig. 5B) and STZ-treated (Fig. 5C) male Balb/c mice injected with serpin B13 mAb according to the scheme depicted in Figs. 1B and 5C, inset, respectively, showed marked up-regulation of several members of the Reg gene family. However, the expression of Reg 4 and other genes linked to cell differentiation and insulin expression regulation remained unchanged (Fig. 5C). Importantly, increased expression of Reg genes in Balb/c mice was noted predominantly in the small (<200 μm in diameter; Fig. 5B) rather than larger (>200 μm in diameter; data not shown) islets matching other adaptive changes that occur in small islets within several weeks after completion of treatment with serpin mAb (Figs. 1D, lower left; 2C, lower left; and 4C, upper). Together, measurements of 5-EdU incorporation and Reg gene expression suggest that serpin B13 antibody impacts beta cell adaptive changes.
FIGURE 5.
Effect of serpin antibodies on Reg gene expression in pancreatic islets. Female NOD mice (A) with or without endogenous serpin autoantibodies, as well as healthy (B) or STZ-treated (C) male Balb/c mice injected with serpin mAb or control IgG (exactly as depicted in Figs. 1B and 5C, inset, respectively) were subjected to quantitative PCR to assess in their islets expression of Reg and other genes (a, Arx; b, Brn4; c, Hlxb9; d, Insm1; e, Irx1; f, lrx2; g, Isl1; h, MafA; i, Nkx2.2; j, Pax4; k, Pax6; l, Neurod1; m, Ins2; n, Ngn3; o, Nkx6.1; p, Pdx1; q, Reg1; r, Reg2; s, Reg3a; t, Reg3b; u, Reg3d; v, Reg3g; and w, Reg4). Analysis depicted in B is confined to islets with <200 μm diameter. D, Reg gene expression in human islets incubated with serpin B13 mAb (or control IgG) alone, or an extract of pancreatic ductal (or nonductal) epithelium containing these antibodies. Data are presented as mean ± S.E.
To address whether the effect of serpin antibody on beta cells is direct or indirect we performed an in vitro study with human islets. We found that adding antibody to islets did not change the expression of REG genes. However, adding serpin antibody to the islets together with an extract of the pancreatic ductal epithelium led to a significant up-regulation of REG genes, although similar treatment with an extract of non-ductal pancreatic cells failed to induce these changes (Fig. 5D). Therefore, adaptive changes in human islets are similar to those observed in the mouse, and are likely secondary to serpin Ab acting on the exocrine pancreas.
Both Active and Passive Immunization of Balb/c Mice with Serpin B13 Ameliorates Effect of Streptozotocin on Diabetes
Encouraged by our observations of islet adaptive changes following treatment with serpin mAb, we decided to examine the impact of serpin B13 vaccine in diabetes induced with STZ. In this regard we took advantage of a model previously described by others, in which young diabetic Balb/c male mice spontaneously recover from STZ-induced diabetes (24). We found that during the initial 2 weeks after immunization there was no difference in STZ-induced hyperglycemia between animals injected with serpin B13 and control cohort treated with PBS. On the other hand, during the following 3 months vaccinated mice had blood glucose levels reaching on average between 200 and 300 mg/dl, whereas in PBS-injected animals these levels were ∼400 mg/dl (Fig. 6). These data suggest that islet adaptation caused by early enhancement of anti-serpin immunity can be beneficial in the setting of metabolic stress including STZ-dependent diabetes.
FIGURE 6.
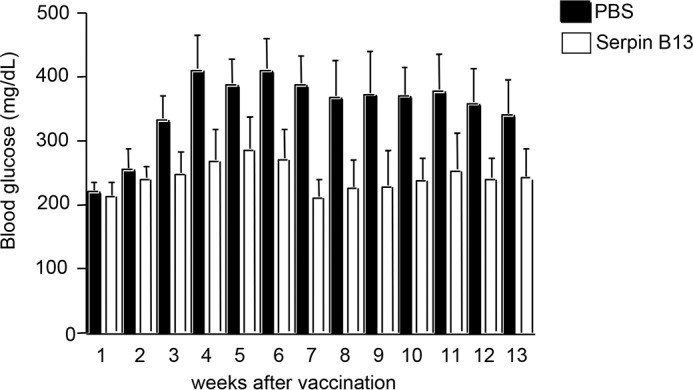
Amelioration of STZ-induced diabetes in Balb/c mice treated with serpin vaccine. Balb/c male pups were intraperitoneal injected on two occasions (days +5 and +10) with 10 μg of mouse serpin B13 diluted in PBS (open, n = 8) or PBS alone (black, n = 7) and injected with a single dose of 100 mg/kg of STZ on day +10 to induce diabetes. Mice were then monitored for blood glucose levels at 1-week intervals, starting on day +17, as indicated.
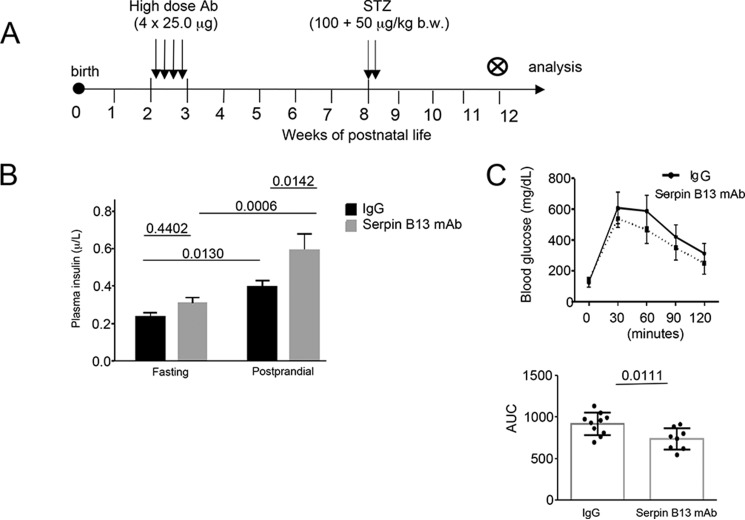
To further examine the impact of the serpin Ab response in STZ model of diabetes we performed experiments according to the regimen depicted in Fig. 7A. We noted that mice that had been exposed to serpin B13 mAb and later developed STZ-induced diabetes, secreted more insulin than the control diabetic mice that had received IgG (p = 0.0059) (Fig. 7B). Moreover, treatment with the serpin B13 mAb also resulted in improved glucose tolerance test performance (p = 0.0111) (Fig. 7C). Thus, islet adaptation caused by early and transient enhancement of anti-serpin activity can be beneficial in the setting of metabolic stress including insulin-dependent diabetes.
FIGURE 7.
Glucose control in Balb/c mice subjected to serpin B13 mAb treatment. A, strategy for Ab and STZ injection. B, plasma insulin levels at fasting and 1 h after glucose intraperitoneal injection (postprandial). C, blood glucose levels at baseline and every 30 min for 2 h after glucose injection (top). The data also are expressed as the area under the curve (AUC) for all time points combined (bottom). IgG group: n = 10; serpin Ab group: n = 8.
The Serpin B13 Ab Response Is Associated with a Slower Decline in Beta Cell Function in Humans with Recent Onset T1D
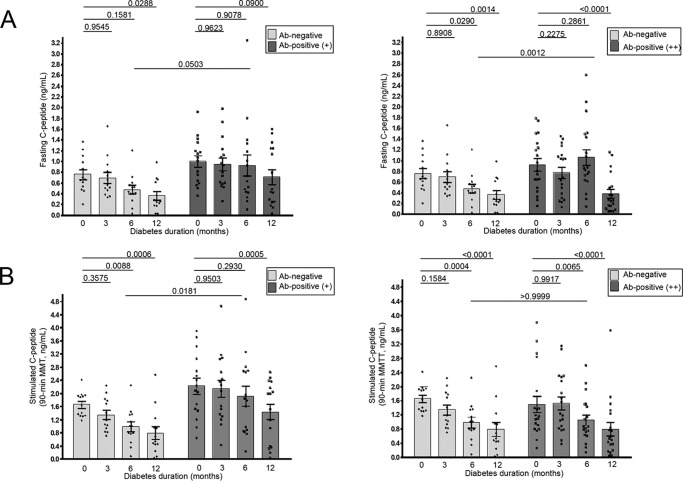
To assess whether anti-serpin B13 autoantibodies promote beta cell health in humans, we examined the impact of this Ab response on the residual beta cell function in pediatric patients with a recent diagnosis of T1D who were previously enrolled as the placebo subjects in several TrialNet double-blind placebo-controlled immuno-intervention protocols. First, to determine their beta cell function we used IDAA1C (17), which is validated by a noninvasive manner to establish whether a subject with T1D is in remission, as commonly occurs during the first year following the onset of T1D. As expected, children who were serpin Ab negative at baseline showed a steady increase in their IDAA1C levels at the 3-, 6-, and 12-month follow-up (slope value of 0.2295 ± 0.04719, Fig. 8). Interestingly, the slope value for serpin B13 Ab positive children was two times lower suggesting a slower ongoing loss of remaining beta cell mass in these subjects (Fig. 8). In this regard, analysis of serpin B13 Ab weakly (+) positive patients revealed a similar trend to that observed in Ab strongly (++) positive subjects (slope values for these two groups were 0.114 ± 0.05502 and 0.1086 ± 0.0382, respectively). Importantly, the subjects with anti-serpin Abs showed a tendency to maintain their residual beta cell function for 6 (Fig. 9, A, right, and B, left) and 12 (Fig. 9A, left) months after the onset of T1D. In contrast, children with no significant expression of serpin Ab demonstrated earlier decline in beta cell function, which was noticeable at these follow up points. Moreover, children with serpin Abs had higher fasting C-peptide concentrations, regardless of the amplitude of their anti-serpin activity (e.g. 0.4710 versus 0.9244 ng/ml (p = 0.0503, Fig. 9A, left) and 0.4710 versus 1.056 ng/ml (p = 0.0012, Fig. 9A, right), both at 6 months follow up). Also, subjects with weak (but not strong) expression of serpin B13 Ab showed elevated stimulated C-peptide levels compared with negative subjects (e.g. 0.9838 versus 1.914 ng/ml at 6 months follow up (p = 0.0181); Fig. 9B, left).
FIGURE 8.
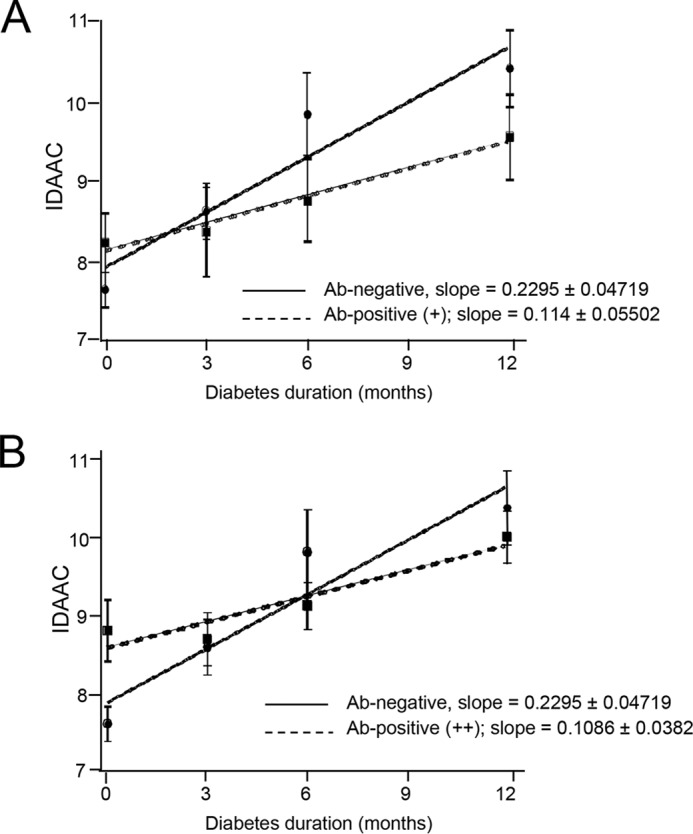
Impact of antibodies to serpin B13 on IDAA1C in recent-onset T1D patients. Fifty-four placebo subjects previously recruited to Type 1 Diabetes TrialNet protocols were examined for baseline secretion of serpin B13 antibodies and analyzed for IDAA1C at baseline and follow-up at 3, 6, and 12 months. The negative samples were compared with samples that showed weak (+) (A) or strong (++) (B) binding to serpin B13. Linear regression was used for the analysis. Data are presented as mean ± S.E.
FIGURE 9.
Impact of antibodies to serpin B13 on C-peptide response in recent-onset T1D patients. Serpin B13 Ab was measured at baseline and its impact examined with regard to C-peptide concentration, either fasting (A) or stimulated at 90 min during mixed-meal tolerance test (MMTT) (B). Two-way analyses of variance with Dunnett's was used to compare C-peptide at baseline versus the 3-, 6-, and 12-month follow up.
Discussion
Serpin protease inhibitors play a fundamental role in preserving the structural and functional integrity of cells and tissues in the body (25, 26). Autoantibody-mediated dysregulation of these functions can have serious clinical complications (27, 28). In contrast to other autoimmune conditions where the presence of serpin autoantibodies is indicative of disease, we found that the presence of the immunoglobulin response to serpin B13 is protective in T1D (4). Our previous results demonstrated that serpin B13 Abs reduce lymphocytic infiltration of pancreatic islets (4, 5). In the present study, we found that serpin B13 can also influence adaptive changes in pancreatic tissue by increasing output of small islets soon after exposure to serpin Ab, and then during the following months, causing a similar increase in the number of larger islets.
To explain these adaptive changes, we focused on the impact of serpin B13 mAb on beta cells proliferation, reasoning that the newly formed islets could represent preexisting tiny clusters of replicating beta cells. Total beta hypercellularity also implied proliferation as the mechanism of expansion. Indeed, quantification of cells positive for both 5-EdU and insulin revealed that anti-serpin activity enhanced beta cell proliferation, especially in small pancreatic islets. Our discovery of high Reg gene expression in pancreatic islets lends further support to the hypothesis that enhanced humoral immunity to serpin molecules can stimulate adaptive changes because Reg proteins have been implicated in the processes of islet proliferation and regeneration. For example, Reg1 is important in islet cell proliferation, and its expression is increased during islet regeneration (21), whereas Reg3δ has been shown to stimulate the differentiation of putative pancreatic stem cells in diabetes (22).
Although our results are somewhat expected because we used young animals with relatively well preserved beta cell proliferation (29) it is possible that an immune response to serpin molecules may influence the endocrine cell adaptation by additional mechanisms. Indeed, observed by us relatively modest up-regulation in beta cell proliferation may not fully account for noticeable changes in islet numbers and their cellularity following injection of serpin B13 mAb. Moreover, we previously reported that the target of our serpin Ab is expressed in pancreatic ducts (5). In addition, others have demonstrated that ductal epithelium or an adjacent niche plays a role in endocrine progenitor cell activation (30, 31). All these observations suggest that de novo beta cell formation may occur following ductal stimulation with serpin antibodies. However, the proposal that pancreatic exocrine ducts are a source of beta cells in animals exposed to serpin antibody has not been directly assessed in this study. Such investigations should employ genetic lineage tracing of ductal cells in mice with an inducible Cre recombinase-driven promoter that can act as a duct marker (32, 33).
The exact role of increased numbers of small islets in our system remains to be elucidated. Small islets that emerge after exposure to serpin Abs could represent a dormant pool that can be activated during increased metabolic demand (34). Because pancreatic endocrine cells are thought to have a long lifespan (35), it is possible that the newly formed islets that arise in young mice following serpin B13 Ab injection can survive for a considerable period of time and are responsible for further beta cell and islet expansion that was noted in our study (Fig. 1F). A related question is how late can serpin B13 mAb treatment induce adaptive changes in islets? Although our serpin B13 mAb treatment coincided with the second wave of natural islet neogenesis that occurs between the second and third week of life in rodents (12), it is equally important to know whether a similar treatment of aged mice (e.g. >1 year old) can overcome their severely compromised islet regenerative potential (36, 37) and up-regulate the number of islets or induce other islet adaptive changes.
The studies of NOD mice expressing high levels of serpin B13 autoantibodies indicated an almost 50% increase in the number of pancreatic islets, although this value in Balb/c mice that received serpin B13 mAb was only 20%. Although mouse strain difference could account for partial resistance to serpin B13 mAb, other reasons should also be considered. For example, the limited response to our mAb may reflect a weak effect of this antibody binding on serpin B13 conformation. A passive immunization with a mAb that interacts with a critical fragment of serpin B13 (e.g. the hinge region in the reactive site loop that allows serpins to neutralize target proteases (2 and 3)) is an alternative that should be tested. Notably, this strategy to influence protease activity is in accordance with our earlier report that Ab serpin B13 reduces autoimmune inflammation in T1D, likely through enhanced cleavage of key cell-surface molecules on lymphocytes residing in pancreatic islets (5). However, it should be stressed that the role of proteases has not been addressed in this study, and whether proteolytic activity contributes to islet adaptive changes remains to be determined.
The translational aspect of our project suggests that monitoring anti-serpin activity in humans helps predict progression of changes in the well being of beta cells throughout the first year of T1D. This conclusion is supported by examination of both C-peptide secretion profiles and IDAA1C levels as a surrogate measure. However, it is unclear why only patients with weak (+), but not strong (++) secretion of serpin B13 Ab demonstrated significantly higher stimulated C-peptide response compared with negative controls at the 6-month follow up. One possible interpretation of this finding could be the potentially different kinetics of immunological responses to serpin molecules. More specifically, weakly positive Ab at baseline may represent a declining response that was at its maximum long before the onset of diabetes. Consequently, patients with long-lasting immunity to serpin molecules would have pancreatic islets better prepared to resist their final destruction compared with other patients. By the same token, a strong presence of serpin Ab at baseline may reflect a more recent immunological response, thereby offering the patients little opportunity to develop islet adaptive changes immediately after diabetes onset. In future, comparing anti-serpin activity in at-risk progressors versus non-progressors, or following this activity in subjects with ongoing diabetes for 2 to 5 years after disease onset, would give us a better insight into the long-term impact of serpin Ab on diabetes. Addressing this and other questions will require examinations of serum samples from well defined subject cohorts and should be attainable through continued collaboration with other groups that possess these precious collections of human specimens.
Author Contributions
Y. K. and C.-W. L. contributed equally to this work. Y. K. and C.-W. L. contributed to study design, researched and analyzed the data, and reviewed and edited the manuscript. E. M. and R. B. researched the data. N. J. contributed to study design, and reviewed and edited the manuscript. J. C. designed the experiments, analyzed the data and wrote the manuscript.
Acknowledgments
We thank Sarah Muller and the Type 1 Diabetes TrialNet for help in providing human serum samples and supporting data. We also thank Dr. Jerry Palmer for coordinating review of our manuscript by the TrialNet Publications and Presentations Subcommittee prior to its submission for publication.
This work was supported by the Juvenile Diabetes Research Foundation Grant 17-2013-428 (to J. C.). The authors declare that they have no conflicts of interest with the contents of this article.
- T1D
- type 1 diabetes mellitus
- NOD
- nonobese diabetic
- STZ
- streptozotocin
- IF
- immunofluorescence
- 5-EdU
- 5-ethynyl-2′-deoxyuridine.
References
- 1.Karjalainen J., Salmela P., Ilonen J., Surcel H. M., and Knip M. (1989) Comparison of childhood and adult type I diabetes mellitus. N. Engl. J. Med. 320, 881–886 [DOI] [PubMed] [Google Scholar]
- 2.Abts H. F., Welss T., Mirmohammadsadegh A., Köhrer K., Michel G., and Ruzicka T. (1999) Cloning and characterization of hurpin (protease inhibitor 13): a new skin-specific, UV- repressible serine proteinase inhibitor of the ovalbumin serpin family. J. Mol. Biol. 293, 29–39 [DOI] [PubMed] [Google Scholar]
- 3.Welss T., Sun J., Irving J. A., Blum R., Smith A. I., Whisstock J. C., Pike R. N., von Mikecz A., and Ruzicka T., Bird P. I., and Abts H. F. (2003) Hurpin is a selective inhibitor of lysosomal cathepsin L and protects keratinocytes from ultraviolet induced apoptosis. Biochemistry 42, 7381–7389 [DOI] [PubMed] [Google Scholar]
- 4.Czyzyk J., Henegariu O., Preston-Hurlburt P., Baldzizhar R., Fedorchuk C., Esplugues E., Bottomly K., Gorus F. K., Herold K., and Flavell R. A. (2012) Enhanced anti-serpin antibody activity inhibits autoimmune inflammation in type 1 diabetes. J. Immunol. 188, 6319–6327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldzizhar R., Fedorchuk C., Jha M., Rathinam C., Henegariu O., and Czyzyk J. (2013) Anti-serpin antibody-mediated regulation of proteases in autoimmune diabetes. J. Biol. Chem. 288, 1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millet I., Wong F. S., Gurr W., Wen L., Zawalich W., Green E. A., Flavell R. A., and Sherwin R. S. (2006) Targeted expression of the anti-apoptotic gene CrmA to NOD pancreatic islets protects from autoimmune diabetes. J. Autoimmun. 26, 7–15 [DOI] [PubMed] [Google Scholar]
- 7.Zhang B., Lu Y., Campbell-Thompson M., Spencer T., Wasserfall C., Atkinson M., and Song S. (2007) α1-Antitrypsin protects beta-cells from apoptosis. Diabetes 56, 1316–1323 [DOI] [PubMed] [Google Scholar]
- 8.Hugues S., Mougneau E., Ferlin W., Jeske D., Hofman P., Homann D., Beaudoin L., Schrike C., Von Herrath M., Lehuen A., and Glaichenhaus N. (2002) Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity 16, 169–181 [DOI] [PubMed] [Google Scholar]
- 9.Kassem S. A., Ariel I., Thornton P. S., Scheimberg I., and Glaser B. (2000) Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49, 1325–1333 [DOI] [PubMed] [Google Scholar]
- 10.Meier J. J., Butler A. E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R. A., and Butler P. C. (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perl S., Kushner J. A., Buchholz B. A., Meeker A.K., Stein G. M., Hsieh M., Kirby M., Pechhold S., Liu E. H., Harlan D. M., and Tisdale J. F. (2010) Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 95, E234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finegood D. T., Scaglia L., and Bonner-Weir S. (1995) Dynamics of beta-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 44, 249–256 [DOI] [PubMed] [Google Scholar]
- 13.Wang R. N., Klöppel G., and Bouwens L. (1995) Duct- to islet-cell differentiation and islet growth in the pancreas of duct ligated adult rats. Diabetologia 38, 1405–1411 [DOI] [PubMed] [Google Scholar]
- 14.Bonner-Weir S., Baxter L. A., Schuppin G. T., and Smith F. E. (1993) A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes 42, 1715–1720 [DOI] [PubMed] [Google Scholar]
- 15.Jones L. C., and Clark A. (2001) Beta-cell neogenesis in type 2 diabetes. Diabetes 50, S186–187 [DOI] [PubMed] [Google Scholar]
- 16.Phillips J. M., O'Reilly L., Bland C., Foulis A. K., and Cooke A. (2007) Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes 56, 634–640 [DOI] [PubMed] [Google Scholar]
- 17.Mortensen H. B., Hougaard P., Swift P., Hansen L., Holl R. W., Hoey H., Bjoerndalen H., de Beaufort C., Chiarelli F., Danne T., Schoenle E. J., Aman J., and Hvidoere Study Group on Childhood Diabetes (2009) New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 32, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rankin M. M., Wilbur C. J., Rak K., Shields E. J., Granger A., and Kushner J. A. (2013) β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes 62, 1634–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichert M., Takano S., Heeg S., Bakir B., Botta G. P., and Rustgi A. K. (2013) Isolation, culture and genetic manipulation of mouse pancreatic ductal cells. Nat. Protoc. 8, 1354–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Hoof D., D'Amour K. A., and German M. S. (2009) Derivation of insulin-producing cells from human embryonic stem cells. Stem Cell Res. 3, 73–87 [DOI] [PubMed] [Google Scholar]
- 21.Terazono K., Yamamoto H., Takasawa S., Shiga K., Yonemura Y., Tochino Y., and Okamoto H. (1988) A novel gene activated in regenerating islets. J. Biol. Chem. 263, 2111–2114 [PubMed] [Google Scholar]
- 22.Okamoto H. (1999) The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. J. Hepatobiliary Pancreat. Surg. 6, 254–262 [DOI] [PubMed] [Google Scholar]
- 23.Pittenger G. L., Taylor-Fishwick D., and Vinik A. I. (2009) The role of islet neogeneis-associated protein (INGAP) in pancreatic islet neogenesis. Curr. Protein Pept. Sci. 10, 37–45 [DOI] [PubMed] [Google Scholar]
- 24.Hartmann K., Besch W., and Zühlke H. (1989) Spontaneous recovery of streptozotocin diabetes in mice. Exp. Clin. Endocrinol. 93, 225–230 [DOI] [PubMed] [Google Scholar]
- 25.Irving J. A., Pike R. N., Lesk A. M., and Whisstock J. C. (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 10, 1845–1864 [DOI] [PubMed] [Google Scholar]
- 26.Luke C. J., Pak S. C, Askew Y. S, Naviglia T. L., Askew D. J., Nobar S. M., Vetica A. C., Long O. S., Watkins S. C., Stolz D. B., Barstead R. J., Moulder G. L., Brömme D., and Silverman G. A. (2007) An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell 130, 1108–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson J., Sim R. B., Whelan A., and Feighery C. (1986) An IgG autoantibody which inactivates C1-inhibitor. Nature 323, 722–724 [DOI] [PubMed] [Google Scholar]
- 28.Cacoub P., Frémeaux-Bacchi V., De Lacroix I., Guillien F., Kahn M. F., Kazatchkine M. D., Godeau P., Piette J. C. (2001) A new type of acquired C1 inhibitor deficiency associated with systemic lupus erythematosus. Arthritis Rheum. 44, 1836–1840 [DOI] [PubMed] [Google Scholar]
- 29.Kushner J. A. (2013) The role of aging upon β cell turnover. J. Clin. Invest. 123, 990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X., D'Hoker J., Stangé G., Bonné S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D., Bouwens L., Scharfmann R., Gradwohl G., and Heimberg H. (2008) Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 [DOI] [PubMed] [Google Scholar]
- 31.Wu F., Guo L., Jakubowski A., Su L., Li W. C., Bonner-Weir S., Burkly L. C. (2013) TNF-like weak inducer of apoptosis (TWEAK) promotes beta cell neogenesis from pancreatic ductal epithelium in adult mice. PLoS ONE 8, e72132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solar M., Cardalda C., Houbracken I., Martín M., Maestro M. A., De Medts N., Xu X., Grau V., Heimberg H., Bouwens L., and Ferrer J. (2009) Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 [DOI] [PubMed] [Google Scholar]
- 33.Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R., Sharma A., and Bonner-Weir S. (2008) Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. U.S.A. 105, 19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir G. C., and Bonner-Weir S. (2011) Sleeping islets and the relationship between β-cell mass and function. Diabetes 60, 2018–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cnop M., Hughes S. J., Igoillo-Esteve M., Hoppa M. B., Sayyed F., van de Laar L., Gunter J. H., de Koning E. J., Walls G. V., Gray D. W., Johnson P. R., Hansen B. C., Morris J. F., Pipeleers-Marichal M., Cnop I., and Clark A. (2010) The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 53, 321–330 [DOI] [PubMed] [Google Scholar]
- 36.Teta M., Long S. Y., Wartschow L. M., Rankin M. M., and Kushner J. A. (2005) Very slow turnover of beta-cells in aged adult mice. Diabetes 54, 2557–2567 [DOI] [PubMed] [Google Scholar]
- 37.Saisho Y., Butler A. E, Manesso E., Elashoff D., Rizza R. A., and Butler P. C. (2013) β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 36, 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]