Abstract
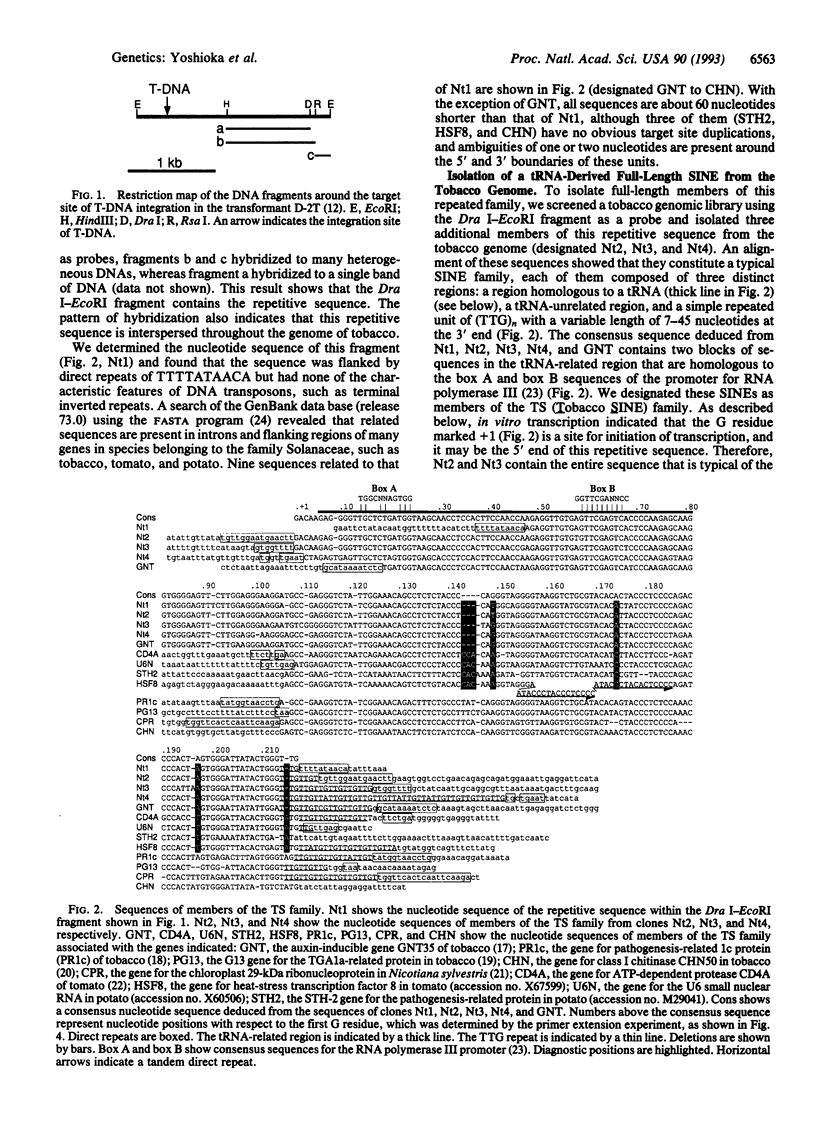
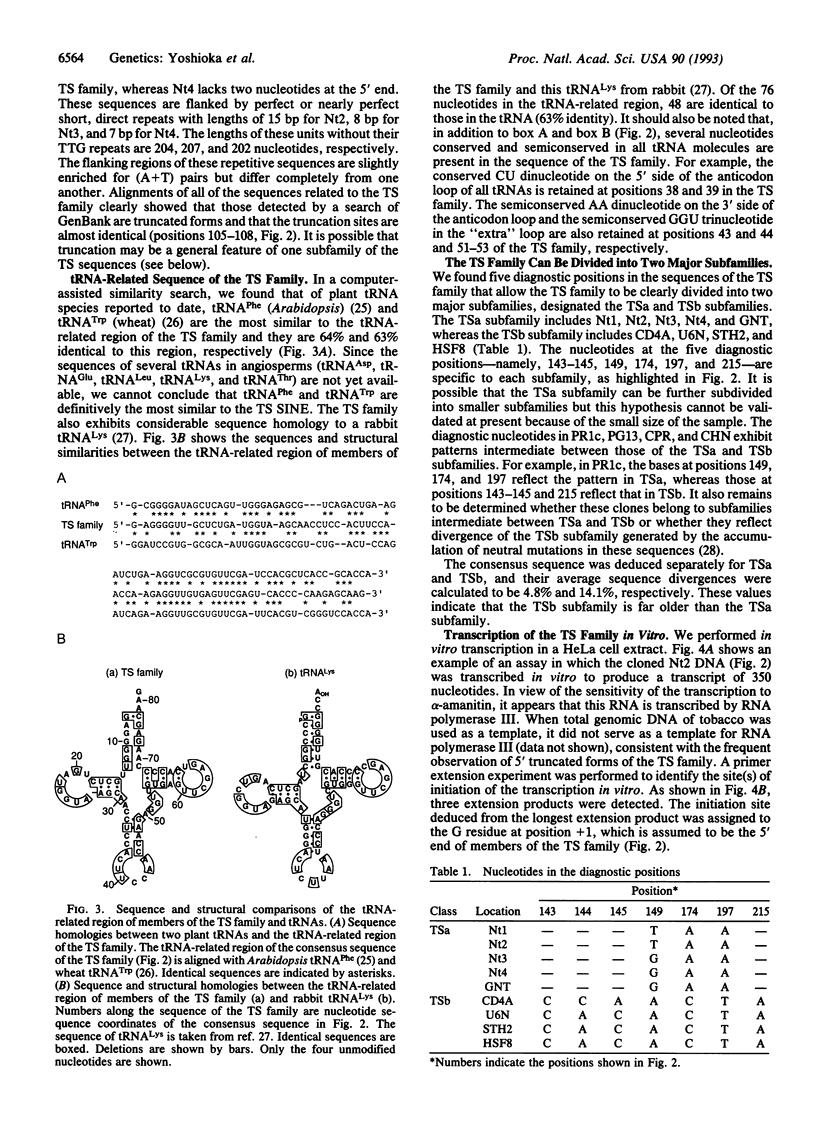
We have characterized a family of tRNA-derived short interspersed repetitive elements (SINEs) in the tobacco genome. Members of this family of SINEs, designated TS, have a composite structure and include a region structurally similar to a rabbit tRNA(Lys), a tRNA-unrelated region, and a TTG repeat of variable length at the 3' end. Southern blot hybridization, together with a search of the GenBank data base, showed that various plants belonging to the families Solanaceae and Convolvulaceae contain sequences homologous to the TS family in the introns and flanking regions of many genes, whereas Arabidopsis in the family Cruciferae and several species of monocoytledonous plants do not. The TS family is widely involved in structural and genetic variations in the genomes of many plants that belong to the order Tubiflorae. All of nine sequences identified in a data base search are truncated at their 5' regions and lack the tRNA-related region of the TS family. We characterized the entire sequence of the members of the TS family and found that this family can be categorized as a member of a group of SINEs with a tRNA(Lys)-like structure, as can several animal SINEs. The TS family can be divided into two major subfamilies by analysis of diagnostic positions, and one of the subfamilies is clearly younger than the other. Amplification of many copies of the full sequence of the younger subfamily occurred during the recent evolution of the tobacco lineage. We also discuss mechanisms that could be involved in the generation of SINEs in animals and also in plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akama K., Tanifuji S. Sequence analysis of three tRNA(Phe) nuclear genes and a mutated gene, and one gene for tRNA(Ala) from Arabidopsis thaliana. Plant Mol Biol. 1990 Aug;15(2):337–346. doi: 10.1007/BF00036919. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Baron W. F., Stout D. B., Davidson E. H. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels G. R., Deininger P. L. Repeat sequence families derived from mammalian tRNA genes. 1985 Oct 31-Nov 6Nature. 317(6040):819–822. doi: 10.1038/317819a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Dunbar E., Anderson R., Pearce S. R., Hartley R., Kumar A. Ty1-copia group retrotransposons are ubiquitous and heterogeneous in higher plants. Nucleic Acids Res. 1992 Jul 25;20(14):3639–3644. doi: 10.1093/nar/20.14.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm H., Katagiri F., Chua N. H. The tobacco transcription activator TGA1a binds to a sequence in the 5' upstream region of a gene encoding a TGA1a-related protein. Mol Gen Genet. 1991 Oct;229(2):181–188. doi: 10.1007/BF00272154. [DOI] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P. Structure and function of tryptophan tRNA from wheat germ. Nucleic Acids Res. 1984 Jun 25;12(12):4997–5003. doi: 10.1093/nar/12.12.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Squires C., Pichersky E., Carrington M., Hobbs M., Mattick J. S., Dalrymple B., Kuramitsu H., Shiroza T., Foster T. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci U S A. 1990 May;87(9):3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandbastien M. A., Spielmann A., Caboche M. Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature. 1989 Jan 26;337(6205):376–380. doi: 10.1038/337376a0. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Fukuchi A., Kikuchi F. Retrotransposon families in rice. Mol Gen Genet. 1992 May;233(1-2):209–216. doi: 10.1007/BF00587581. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Hirochika R. Ty1-copia group retrotransposons as ubiquitous components of plant genomes. Jpn J Genet. 1993 Feb;68(1):35–46. doi: 10.1266/jjg.68.35. [DOI] [PubMed] [Google Scholar]
- Konieczny A., Voytas D. F., Cummings M. P., Ausubel F. M. A superfamily of Arabidopsis thaliana retrotransposons. Genetics. 1991 Apr;127(4):801–809. doi: 10.1093/genetics/127.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. B., McDonnell D. P., Ramsey W. J. Analysis of repetitive sequence elements containing tRNA-like sequences. Nucleic Acids Res. 1985 Jun 25;13(12):4239–4252. doi: 10.1093/nar/13.12.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Ito Y., Hosoi T., Takahashi Y., Machida Y. Integration of Agrobacterium T-DNA into a tobacco chromosome: possible involvement of DNA homology between T-DNA and plant DNA. Mol Gen Genet. 1990 Dec;224(3):309–316. doi: 10.1007/BF00262423. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Pruitt R. E. Arabidopsis thaliana and Plant Molecular Genetics. Science. 1985 Sep 20;229(4719):1214–1218. doi: 10.1126/science.229.4719.1214. [DOI] [PubMed] [Google Scholar]
- Mochizuki K., Umeda M., Ohtsubo H., Ohtsubo E. Characterization of a plant SINE, p-SINE1, in rice genomes. Jpn J Genet. 1992 Apr;67(2):155–166. doi: 10.1266/jjg.67.155. [DOI] [PubMed] [Google Scholar]
- Muratani K., Hada T., Yamamoto Y., Kaneko T., Shigeto Y., Ohue T., Furuyama J., Higashino K. Inactivation of the cholinesterase gene by Alu insertion: possible mechanism for human gene transposition. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11315–11319. doi: 10.1073/pnas.88.24.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima K., Koishi R., Matsuo M., Okada N. Several short interspersed repetitive elements (SINEs) in distant species may have originated from a common ancestral retrovirus: characterization of a squid SINE and a possible mechanism for generation of tRNA-derived retroposons. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6260–6264. doi: 10.1073/pnas.90.13.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima M., Harada N., Matsuoka M., Ohashi Y. The nucleotide sequence of pathogenesis-related (PR) 1c protein gene of tobacco. Nucleic Acids Res. 1990 Jan 11;18(1):182–182. doi: 10.1093/nar/18.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Rochon D., Siegel A. Chloroplast DNA transcripts are encapsidated by tobacco mosaic virus coat protein. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1719–1723. doi: 10.1073/pnas.81.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Rogers J. Molecular biology. CACA sequences - the ends and the means? Nature. 1983 Sep 8;305(5930):101–102. doi: 10.1038/305101a0. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Okada N. Rodent type 2 Alu family, rat identifier sequence, rabbit C family, and bovine or goat 73-bp repeat may have evolved from tRNA genes. J Mol Evol. 1985;22(2):134–140. doi: 10.1007/BF02101691. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Talkington C. A., Nishioka Y., Leder P. In vitro transcription of normal, mutant, and truncated mouse alpha-globin genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7132–7136. doi: 10.1073/pnas.77.12.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas D. F., Ausubel F. M. A copia-like transposable element family in Arabidopsis thaliana. Nature. 1988 Nov 17;336(6196):242–244. doi: 10.1038/336242a0. [DOI] [PubMed] [Google Scholar]
- Voytas D. F., Cummings M. P., Koniczny A., Ausubel F. M., Rodermel S. R. copia-like retrotransposons are ubiquitous among plants. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7124–7128. doi: 10.1073/pnas.89.15.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. R., Andersen L. B., Saulino A. M., Gregory P. E., Glover T. W., Collins F. S. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991 Oct 31;353(6347):864–866. doi: 10.1038/353864a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Ye L. H., Li Y. Q., Fukami-Kobayashi K., Go M., Konishi T., Watanabe A., Sugiura M. Diversity of a ribonucleoprotein family in tobacco chloroplasts: two new chloroplast ribonucleoproteins and a phylogenetic tree of ten chloroplast RNA-binding domains. Nucleic Acids Res. 1991 Dec 11;19(23):6485–6490. doi: 10.1093/nar/19.23.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren M., Neuhaus J. M., Shinshi H., Ryals J., Meins F., Jr The structure and regulation of homeologous tobacco endochitinase genes of Nicotiana sylvestris and N. tomentosiformis origin. Mol Gen Genet. 1992 Apr;232(3):460–469. doi: 10.1007/BF00266251. [DOI] [PubMed] [Google Scholar]
- van der Zaal E. J., Droog F. N., Boot C. J., Hensgens L. A., Hoge J. H., Schilperoort R. A., Libbenga K. R. Promoters of auxin-induced genes from tobacco can lead to auxin-inducible and root tip-specific expression. Plant Mol Biol. 1991 Jun;16(6):983–998. doi: 10.1007/BF00016071. [DOI] [PubMed] [Google Scholar]




