Abstract
Background and Objectives:
Alopecia areata (AA) is a common, non-scarring type of hair loss, affecting approximately 2.1% of the population, many modality of treatment recommended like steroid injection, topical Immunotherapy and several systemic therapies. The aim of this study was to compare intralesional steroid injection and cryotherapyoutcomes in AA.
Materials and Methods:
In an analytical-descriptive study, 120 AA patients treated with intralesionalsteroid injection and 120 AA patients treated with cryotherapy were randomly selected. These two groups matched for location, duration and size of lesion and also matched for age and gender. From March 2011 to September 2013, the effect and complications of the therapies after 3, 6, 9 and 12 weeks were assessed and results were compared between the two groups.
Results:
Mean age of patients in steroid injection group was 30.2 ± 6.8 and in cryotherapy group was 31.8 ± 7.1. Sexual distribution in both groups was 56.7% and 43.3 % for male and female, respectively. Location of disease in 80% was in scalp and 20% was in face in both groups. The time of beginning response in steroid group was 4.13 ± 2.13 weeks and in cryotherapy group was 6.14 ± 0.29 weeks, difference between two groups was significant (P = 0.001). In term of clinical response at the end of study, in steroid group,20 patients (16.7%) no response, 32 patients (26.7%), moderate response and68 patients (56.7%) had a complete response, and also in cryotherapy group was, 52 patients (43.3%) no response, 40 patients (33.3%) moderate response and 28 patients (23.3%) had a complete response. There was significant different in complete response rate and steroid injection was more effective than cryotherapy(P < 0.05).
Conclusion:
As the cryotherapy isa considerable treatment of AA, alsothis study proposes intralesional injection of corticosteroid, as a replacement of AA therapy; particularly the short-term complications are not significantly different.
Keywords: Alopecia areata, cryotherapy, intralesional steroid injection
INTRODUCTION
Alopecia areata (AA) is an autoimmune disorder cause of inflammation-inducednon-scarring type of hair loss, which consists of 1% patients referred to the dermatology clinic.1 AA due to the complications affects the patients’ quality of life and reduces their self-esteem.2 The life-time risk of AA is about 2.1% and affects both children and adults with any kind of hair colour and there is no significant difference in terms of gender.3
The precise aetiology of AA is still obscure. Current evidence suggests that AA is a T-cell mediated autoimmune disease directed against a putative autoantigen of the hair follicle.4 AA is generally associated with an increased risk of other autoimmune diseases like autoimmune thyroid disease.5,6 Histologically, lesional biopsies of AA demonstrate a peri-follicular and intra-follicular mononuclear cell infiltrate around anagen phase hair follicles. The infiltrate consists mostly of activated lymphocytes, in particular CD4+ T cells as well as dendritic cells and macrophage.4,7
There is many kind of treatment modality in localisedkind of AA. Injectable forms of corticosteroids are first line of AA therapy, and also topical use of steroids is widely used. Others are topical sensitisationwith Anthralin, minoxidil and cryotherapy. In extensive form of AA, systemic treatments like corticosteroids, cyclosporine and methotrexate can be used.8,9
The tissue response to cryotherapy ranges from an inflammatory reaction to mild or severe destruction. The response to cryo can be divided to two mechanisms: Early effect and delayed effect. The early effect is due to formation of ice crystal which can distract cells due to direct effect or osmotic effect. The delayed effect included vascular damage, apoptosis and immunological effect, vascular constriction, platelet aggravation, ischaemia, increased permeability. So because of those effects it can be effective in AA.10 None of the available treatments of AA have not been approved by Food and Drug Administration (FDA) and do not have a protective role.11
Aim of this study was to compare two treatment modalitiesof AA including intralesional steroid injection and cryotherapy.
MATERIALS AND METHODS
In a descriptive-analytical study from March 2011 to September 2013, we randomly selected using Rand list (version 1.2) software and investigated 120 AA patients treated with intralesional steroid injection and 120 AA patients treated with cryotherapy who had referred to dermatology clinic of Sina Educational Centre of Tabriz (which is a referral center for dermatology in the North West of Iran).
These two groups matched for location, duration and size of lesion and also matched for age and gender. The manner of cryotherapy wassuperficial cryotherapy using liquid nitrogen spray two cycles each one 3-5 second and steroid manner was injecting 5 mg/mltriamicinoloneacetonideper session. Patients in both groups were treated with mentioned methods, forfour sessions with an interval of 3 weeks. Response and complications were inspected among clinical treatment in 3, 6, 9 and 12 weeks. Clinical response was defined as hair regrowth in the manner of without response (0-30% regrowth), mild response (30-60% regrowth), moderate (60-90% regrowth) and complete response (90-100% regrowth).
Collected information has been presented as mean ± standard deviation (mean ± SD) and also as frequency and percentage. Implemented software program was SPSS™ version 16. To compare quantitative variables Student T-test was used and for qualitative variables Chi-square and Fischer exact test if needed, have been used. In all cases of study, results were statistically considered as significant with P ≤ 0.05.
RESULTS
Mean age of patients in steroid injection group was 30.2 ± 6.8 and in cryotherapy group was 31.8 ± 7.1. In both groups 68 (56.7%) Of patients were male and 52 (43.3%) were female. Age range of patients in cryotherapy group was 14-40 years and in steroid injection were 17-38 years. Duration of disease in 83.3% of patient is shorter the 6 month and in 16.7% of patient longer than 6 month in both groups. Location of disease in 80% was in scalp and 20% was in face in both groups.
The time of beginning response in steroid group was 4.13 ± 2.13 weeks and in cryotherapy group was 6.14 ± 0.29 weeks, difference between two groups was significant (P = 0.001).
Clinical response 3 weeks after each session of therapy in both groups was according to Table 1.
Table 1.
Clinical response 3 weeks after each session of therapy
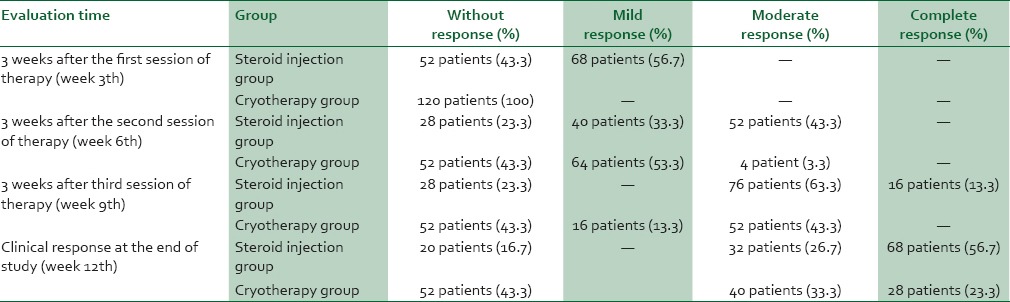
There was significant different in complete response rate between two groups and steroid injection was more effective than cryotherapy (P < 0.05). Local corticosteroid injection efficiency was 83.3% and cryotherapy 56.7%. Local corticosteroid injection was significantly more effective than cryotherapy (P = 0.05).
Complications in steroid injection group were in 13.3% patients (75% pain and 25% atrophy) and in cryotherapy group were in 6.7% patients (50% bullae and 50% erythema), difference was not significant (P = 0.671).
DISCUSSION
The main aetiology for the occurrence of AA is not clear, the evidence is in favour of an autoimmune disease. Disease changes with genetic factors, and is exacerbated by stress. Conventional treatments of AA include intralesional, photochemotherapy and systemic steroid therapy.12 Cryotherapy can also be useful in the treatment of AA.13
In this study, the treatment of local injection of low dose corticosteroid (5 mg per ml) use in AA patients compared with cryotherapy. The response to treatment was determined based on the regrowth of hair in the 3, 6, 9 and 12 weeks after treatment. The response at the local corticosteroid injection group was significantly better than the group treated with cryotherapy (P < 0.05). The average time for onset of hair growth after treatment with topical corticosteroid injection group was significantly shorter than in the group treated with cryotherapy (P = 0.001). The efficiency of topical corticosteroid injection in AA treatment, based on the average or total hair regrowth, was 80% and significantly better comparing withcryotherapy 56.7% (P = 0.05).
A descriptive study of 10 patients concluded that intralesional corticosteroid injection is a safe and effectiveness treatment of extensive AA,14 and several review studies suggest the intalesional steroid injection as an effective and almost safe treatment of AA.15,16,17,18
In a retrospective study of 153 patients with AA treated by cryotherapy, positive therapeutic responses were in 68.6% of patients. This study suggests that cryotherapy can be the first line of treatment in mild form of AA especially in children due to less of pain and side effects.13 In recent study efficiency cryotherapy was 56.7% and also complications of cryotherapy just were in 6.7% patients. Also, a study reported the use ofsuperficial cryotherapy to increase eyebrow hair growth due to AA.19
In a cohort of 290 patients of AA, after 1-2 intralesional steroid injections using 61% of patients were cured.20 In our analytical-descriptive study, local corticosteroid injection efficiency was 83.3% and complications of that were only in 13.3% patients (75% pain and 25% atrophy) and significant complications were not observed.
Considering different complications of therapies designated for AA treatment reported in various studies.21,22,23,24,25 We recommend do this study in more cases to evaluate perfect effect and complication of these two methods and perform a randomisedclinical trial about this topic.
CONCLUSIONS
As the cryotherapy is a considerable treatment of AA, also this study proposes intralesional injection of corticosteroid, as a replacement of AA therapy; particularly the short-term complications are not significantly different.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287–90. doi: 10.1016/j.jaad.2004.10.873. [DOI] [PubMed] [Google Scholar]
- 2.Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: Does stress play a role? J Invest Dermatol. 2009;129:1324–6. doi: 10.1038/jid.2009.111. [DOI] [PubMed] [Google Scholar]
- 3.Mirzoyev SA, Schrum AG, Davis MD, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141–2. doi: 10.1038/jid.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019–27. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishak RS, Piliang MP. Association between alopecia areata, psoriasis vulgaris, thyroid disease, and metabolic syndrome. J Investig Dermatol Symp Proc. 2013;16:S56–7. doi: 10.1038/jidsymp.2013.22. Nature Publishing Group; 2013. [DOI] [PubMed] [Google Scholar]
- 6.Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149:789–94. doi: 10.1001/jamadermatol.2013.3049. [DOI] [PubMed] [Google Scholar]
- 7.McElwee KJ, Freyschmidt-Paul P, Hoffmann R, Kissling S, Hummel S, Vitacolonna M, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(-) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJmouse model. J Investig Dermatol. 2005;124:947–57. doi: 10.1111/j.0022-202X.2005.23692.x. [DOI] [PubMed] [Google Scholar]
- 8.Miteva M, Tosti A. Treatment options for alopecia: An update, looking to the future. Expert Opin Pharmacother. 2012;13:1271–81. doi: 10.1517/14656566.2012.685160. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro J. Current treatment of alopecia areata. JInvestig Dermatol Symp Proc. 2013;16:S42–4. doi: 10.1038/jidsymp.2013.14. Nature Publishing Group; 2013. [DOI] [PubMed] [Google Scholar]
- 10.James WD, Berger T, Elston D. Andrew's diseases of the skin: Clinical dermatology. Elsevier Health Sciences. 2011;10:403–15. [Google Scholar]
- 11.Shapiro J. Dermatologic therapy: Alopecia areata update. Dermatol Ther. 2011;24:301. doi: 10.1111/j.1529-8019.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 12.Park KY, Jang WS, Son IP, Choi SY, Lee MY, Kim BJ, et al. Combination therapy with cyclosporine and psoralen plus ultraviolet a in the patients with severe alopecia areata: A retrospective study with a self-controlled design. Ann Dermatol. 2013;25:12–6. doi: 10.5021/ad.2013.25.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong SP, Jeon SY, Oh TH, Lee WS. A retrospective study of the effect of superficial cryotherapy on alopecia areata. Korean Journal of Dermatology. 2006;44:274–80. [Google Scholar]
- 14.Chang KH, Rojhirunsakool S, Goldberg LJ. Treatment of severe alopecia areata with intralesional steroid injections. J Drugs Dermatol. 2009;8:909–12. [PubMed] [Google Scholar]
- 15.Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355–63. doi: 10.1111/j.1529-8019.2011.01419.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito T. Advances in the management of alopecia areata. J Dermatol. 2012;39:11–7. doi: 10.1111/j.1346-8138.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam, Keerthi, Saritha M, Karthikeyan K. Hair: Therapy and Transplantation. 2014;4:1–3. [Google Scholar]
- 18.Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166:916–26. doi: 10.1111/j.1365-2133.2012.10955.x. [DOI] [PubMed] [Google Scholar]
- 19.Jeon SY, Ahn BK, Lee S, Lee WS. Superficial Cryotherapy of Alopecia Areata in Eyebrows. Korean Journal of Dermatology. 2004;42:1024–7. [Google Scholar]
- 20.Ranawaka RR. An observational study of alopecia areata in Sri Lankan adult patients. Ceylon Med J. 2014;59:128–31. doi: 10.4038/cmj.v59i4.7865. [DOI] [PubMed] [Google Scholar]
- 21.Amirnia M, Sinafar S, Sinafar H, Nuri M. Assessment of Zinc and Copper Contents in Scalp Hair and Serum and Superoxide Dismutase, Glutathione Peroxidase and Malondialdehyde in Serum of Androgenetic Alopecia and Alopecia Areata Patients. Medical Journal of Tabriz University of Medical Sciences. 2011;33:7–13. [Google Scholar]
- 22.Ostadrahimi A, Esfahani A, Jafarabadi MA, Ziaei JE, Movassaghpourakbari A, Farrin N. Effect of Beta Glucan on Quality of Life in Women with Breast Cancer Undergoing Chemotherapy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Advanced pharmaceutical bulletin. 2014;4:471. doi: 10.5681/apb.2014.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moghimipour E, Salimi A, Leis F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Advanced pharmaceutical bulletin. 2012;2:141. doi: 10.5681/apb.2012.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herizchi QH, Majidi J, Sheikh NM, Fadaii R. Results of compelete blood cell count, Fasting blood suger, Rheumatoid factor, C-reactive protein, anti- nuclear abntibody and thyroid function tests in patients with Alopecia Areata. Medical Journal of Tabriz University of Medical Sciences. 2011;33:82–5. [Google Scholar]
- 25.Fathi-Azarbayjani A, Ng KX, Chan YW, Chan SY. Lipid Vesicles for the Skin Delivery of Diclofenac: Cerosomes vs. Other Lipid Suspensions. Advanced pharmaceutical bulletin. 2015;5:25. doi: 10.5681/apb.2015.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


