Abstract
Background:
Malaria remains a major cause of under-five morbidity and mortality in Nigeria, and prompt diagnosis occupies a strategic position in its management. Malaria rapid diagnostic test (RDT), a nontechnical, easy to perform test promises to meet this need. It is important to locally document the usefulness of the use of RDT in making prompt malaria diagnosis in children.
Objective:
To determine the prevalence of malaria and evaluate the diagnostic performance of malaria RDT kit in febrile under-five children presenting to a Tertiary Health Facility in Gusau, North-Western Nigeria.
Materials and Methods:
A cross-sectional study of children aged 6-59 months, evaluated for malaria in a tertiary health facility from August 2012 to January 2013. Information was obtained from care providers of all subjects with fever and a presumptive diagnosis of malaria. All subjects were investigated using Giemsa stain microscopy and Carestart™ malaria RDT.
Results:
The prevalence of malaria in 250 febrile under-five children was 54%. Three-quarter (79%) of the children received inappropriate nonrecommended antimalaria prior to their presentation, including 20% who received chloroquine. The overall sensitivity of RDT was 40.3%. The specificity, positive and negative predictive values were 89.6%, 81.8%, and 56.5%, respectively.
Conclusion:
Use of RDT should be encouraged for screening and diagnosis using a protocol such that febrile children with positive RDT results are confirmed as having malaria while those with negative results are further evaluated using microscopy.
Keywords: Malaria, parasite density, prevalence, rapid diagnostic test, sensitivity, specificity
INTRODUCTION
Malaria is associated with high morbidity and mortality,1,2 with over 90% of Nigerians at risk1,2,3,4 In 2010, 42% of children under the age of 5 years had malaria diagnosed by microscopy.4 Geopolitical zonal variation exists in prevalence of malaria with 48% in the North-West.4,5,6 The Nigerian malarial control policy on malaria recommends universal diagnostic testing by microscopy or rapid diagnostic test (RDT) in all suspected cases and the use of artemisinin-based combination therapy (ACT) for treatment of malaria.7,8,9 Microscopy requires a laboratory set up and a trained microscopist, which limits its applicability as both requirements are lacking in most primary health care settings. In these settings, therefore, RDT provides a parasite based rapid, early and accurate diagnosis of malaria.10,11,12 The 2009-2013 national malaria control strategic plan aims to reduce malaria-related mortality in Nigeria by 50% and targets 80% parasitological diagnosis for patients 5 years and above using RDT by the end of 2013.1,2,3
RDT detects plasmodial antigens in the blood by immunochromatographic assay with monoclonal antibodies directed against target antigens impregnated on a test strip. Histidine-rich protein antigen 2 (HRP-2) is the most common malarial antigen and is specific for Plasmodium falciparum.6,10,12 RDT requires only a few drops of blood, little expertise and takes only 5-15 min to perform.6,10,12 Although most commercially available RDTs have sensitivity >95% at parasite density count of 200 parasite/μl of blood this, however, declines with low-level parasite density.10 When in good condition some RDT products achieve sensitivity, which approximates to that of microscopy (100 parasite/μl).13,14,15 RDT sensitivity remains varied. In Lagos Ben - Edet et al.16 have documented low RDT sensitivity while other researchers17,18,19 in contrast have also documented high RDT sensitivity.
MATERIALS AND METHODS
This prospective study was conducted on 250 children aged 6-59 months who were consecutively evaluated for malaria over a 6 months period, from August 2012 to January 2013. At the paediatric outpatient department (POPD) and Emergency Paediatric Unit (EPU) of a Federal Medical Centre. Consecutive patients who were presented to these units of the hospital with history of fever and a provisional diagnosis of malaria were recruited for the study. Consent was obtained from the care provider of a patient who met the inclusion criteria of the study after being informed of the details of the study.
The study inclusion criteria were:
Age 6-59 months
The presence of fever ≥37.5°C measured by axillary temperature recording using mercury glass thermometer.
Information was obtained from the care provider using a proforma and all subjects were tested using both the standard Giemsa stain microscopy and malaria RDT. Biodemographic information obtained include age, gender, presenting symptoms, duration of fever, other symptoms, history of treatment received before the presentation, and all patients were examined, and clinical findings were documented. Axillary temperature was measured using a mercury glass thermometer.
The recruited subjects all had investigations both Giemsa stain microscopy and the RDT conducted by a trained and experienced Laboratory scientist following standard methods while the RDT tests were conducted using the (Access Bio, Inc., NJ, USA) guide. The manufacturer's recommendations for maintaining the integrity of the test kits were strictly followed. Kits were maintained at optimal temperature T = (1-40°C).
The study was approved by the Ethical and Research Committee of the Federal Medical Centre.
RESULTS
A total of 250 children between the ages of 6 and 59 months were studied. One hundred and forty-one (56.4 %) of the children were males whereas the remaining 109 (43.4%) were females giving a male:female ratio of 1.3:1. There were 187 (74. 8%) children who were aged 36 months and below the other 63 (25.2%) were aged 37-59 months. The major presenting symptoms for which the children presented necessitating evaluation for malaria are as depicted in Table 1. 62.8% of the subjects had temperatures between 38°C and 41°C.
Table 1.
Frequency table of presenting symptoms in children with clinical diagnosis of malaria
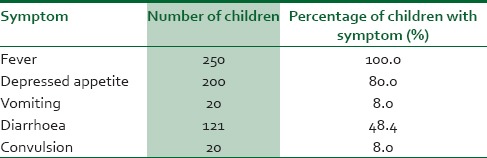
One hundred and forty-two (57%) of the subjects had received some form of antimalarial treatment before presentation and of these, 21% have received ACT [Figure 1], whereas the remaining majority (79%) received other nonrecommended antimalarials.
Figure 1.
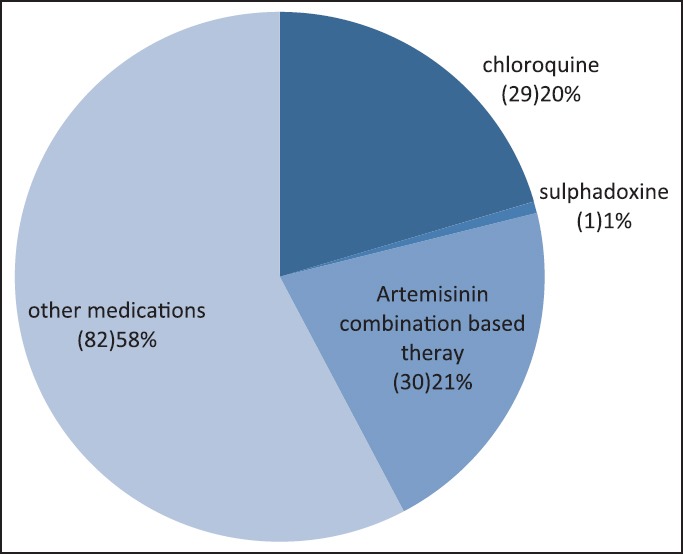
Medications received by subjects before presentation to Federal Medical Centre
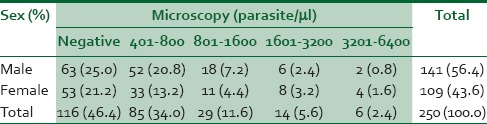
The prevalence of malaria in this group of febrile children with a clinical diagnosis of malaria, using the microscopy test (Gold standard), was 53.6% where 134 of the 250 subjects were positive. The results of the microscopy test for 245 (98%) subjects were obtained within 24 h of requesting for the test. The remaining 5 (2%) malaria parasite microscopy tests were obtained between 24 and 36 h.
Table 2 shows parasite density in studied children. Sixty-six (26.4%) of the 250 children who were screened using the RDT had positive results while the remaining were negative. Of the 66 subjects with positive RDT 12 did not have malaria (they had negative microscopy) while on the other hand 80 subjects who had negative RDT had positive microscopy test. There were a total of 92 discordant results of which 12 were false positive, whereas 80 were false negative results. Half (6) of the 12 children with false-positive results had received antimalarial medications (ACT in 50% 3 of the children).
Table 2.
Malarial parasite microscopy results (parasite density) and sex of children with clinical diagnosis of malaria
The overall sensitivity of RDT was 40.3% while the sensitivity for the different parasite densities were 100% for 3201-6.400 parasites/μl, 85.7% for 1601-3200 parasites/μl, 62% for 801-1600 parasites/μl, and 27% for 401-800 parasites/μl [Table 3]. The proportions of the 130 children with malaria who had 3201-6400, 1601-3200, 801-1600, 401-800 parasites/μl were 0.04% (6/134), 10.4% (14/134), 21.6% (29/134), and 63.4% (85/134), respectively, with children with low parasite density (801-1600 and 401-800) accounting for 85.1% (114/134) of the children with malaria.
Table 3.
Microscopy versus RDT in febrile children with clinical diagnosis of malaria
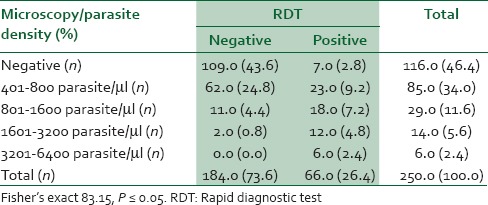
Table 4.
RDT compared with age, sex, prior use of antimalaria and convulsion in febrile children with clinical diagnosis of malaria
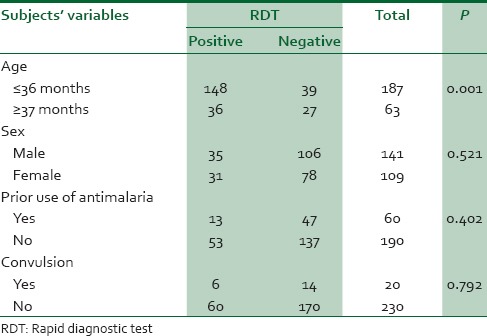
Overall the specificity of the RDT was 89.6% while the positive and negative predictive values were 81.8% and 56.5%, respectively. The association between the parasite density and positivity of the RDT test was statistically significant P = 0.0001 [Table 3]. Age ≤36 was significant associated with RDT positivity P = 0.01. The prior use of antimalarial medications did not significantly influence the result of RDT P = 0. 402.
DISCUSSION
The prevalence of malaria in febrile children presumptively diagnosed to have malaria was 54% meaning that the chance that a child presenting with fever has malaria was a little above 0.5. This is instructive and may support the practice by clinicians to evaluate febrile under-five children for malaria in our environment where it is already known to be endemic. The prevalence was similar to the 56.9% documented by Ikeh and Teclaire5 in Jos, but much higher than the 12% reported from Tanzania20 and 16.9% reported by Oladosu and Oyibo21 in Lagos.
About half of the children studied (57%) received some antimalarial medications for the treatment of presumed malaria before the presentation and only 21% of these received ACT. This depicts that despite the publicized national guideline for the treatment of malaria, majority of community treatment of malaria continues to be suboptimal, and inappropriate with use of nonrecommended medications such as chloroquine with an almost equal the proportion of children receiving ACT treated with chloroquine (20%) before presentation.
The sensitivity, specificity, positive predictive value and negative predictive value were 40.3%, 89.6%, 81.8% and 56.5%, respectively. The low sensitivity obtained in this study was comparable to that (42.31%) documented in Enugu17 and that (69.6%) documented by Ben - Edet et al.16 in Lagos. This, however, contrasts the finding of Sani et al.6 in Sokoto were they found a sensitivity of 93.0% and 93.1% by Nwuba et al.18 in Ibadan. These contrasting findings; however, when further analyzed revalidates the general principle of the functionality of RDTs, which facts remain inability to detect parasites at low densities (<200-400/μl depending on the RDT). This becomes glaring when we look at the varying sensitivity of the RDT at different parasite densities for the different studies. In our study, the sensitivities for parasite densities of 401-800, 801-1600, 1601-3200, and >3200 parasites/μl were 27%, 62%, 85.7% and 100%, respectively. The proportion of children with low parasite densities (401-1600 parasites/μl) in our study, however, constituted a greater (85.1%) proportion of the children with malaria. This category of children with low parasite densities also accounted for a high proportion of those with negative RDT results and hence low sensitivity which was contributory to and invariably greatly influenced the overall sensitivity of the RDT. Conversely in comparison to the study of Sani et al.6 in Sokoto, the proportion of children with low parasite density was small (<10 %) while those with high parasite densities were in the majority (>90%). These facts (degree of parasitemia and the proportion of children with low versus high parasite densities) appear to more than any other factors influence the sensitivity and by extension other efficacy determining measures of RDTs and as well findings of the various authors.
The specificity in this study was high (90%), however, 12 children had discordant results, whence their RDT was positive, microscopy was negative implying the children did not have malaria, but tested RDT positive (false positive). Reasons for false positivity have been documented to include delayed clearance of antigen from the circulation up to 2 to 4 weeks even when treatment was successful; meaning such febrile patients perhaps had other infection other than malaria. Six of the children, who had false positive results received antimalarial medication, though the dose, and interval between therapy and presentation with fever was not ascertained, successful treatment and delayed clearance of Falciparum antigen could be responsible for the false positive results and therefore means that these children probably had other infection responsible for their fever. The 80 children who had false negative results could have been infected with Plasmodium species other than Falciparum or caused by other non-HRP II containing P. falciparum. The prior use of antimalaria did not influence the result of RDT neither did the sex of the child nor did convulsion.
CONCLUSION
The prevalence of malaria among febrile under-five children in Zamfara was 55% with majority (79%) of the prehospital/community treatment of malaria still suboptimal with the use of nonrecommended antimalarials. The overall sensitivity of RDT was low, but high in children with high Plasmodium parasite density.
Recommendation
The use of RDT should be highly encouraged both as a screening and diagnostic tool with protocol such that febrile children who have positive results are confirmed as having malaria while those with negative results are further evaluated with microscopy. Urgent, robust, and targeted efforts need be directed toward education on the national guidelines for the treatment of malaria and its implementation to ensure optimal treatment of malaria.
ACKNOWLEDGMENT
We appreciate the support of Medical Lab Scientist Umar Mustapha, the head of Parasitology Unit of FMC, Gusau Zamfara State and that of Medical Lab Scientist Abdullahi Mohammed for reading the slides and we also appreciate all parents, guardians and caregiver for consenting to participation of their children in the study.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Nigeria malaria fact sheet. Federal Ministry of Health. December 2011. [Last accessed on 2014 Aug 3]. Available from: http://nigeria.usembassy.gov .
- 2.Malaria control in Nigeria. A strategy for behavior change communication. 2004-2005. Federal Ministry of Health. 2004. [Last accessed on 2014 Aug 6]. Available from: http://www.jhpiego.org/files .
- 3.Nigeria Malaria Operation Plan FY 2014. USAID President Malaria Initiative. [Last accessed on 2014 Aug 6]. Avilable from: http://www.pmi.gov/docs/default-source .
- 4.Nigeria, Malaria Indicator Survey (MIS) 2010. Final Report, 2012. [Last accessed on 2014 Aug 3]. Available from: http://www.microdata.worldbank.org .
- 5.Ikeh EI, Teclaire NN. Prevalence of malaria parasitaemia and associated factors in febrile under-5 children seen in Primary Health Care Centres in Jos, North Central Nigeria. Niger Postgrad Med J. 2008;15:65–9. [PubMed] [Google Scholar]
- 6.Sani UM, Jiya NM, Ahmed H. Evaluation of a malaria rapid diagnostic test among febrile children in Sokoto, Nigeria. Int J Med Med Sci. 2013;3:433–40. [Google Scholar]
- 7.FMOH. National Antimalaria Treatment Policy. Federal Ministry of Health National Malaria and Vector Control Division Abuja-Nigeria. 2005 [Google Scholar]
- 8.2nd ed. Geneva: World Health Organization; 2010. Guidelines for the Treatment of Malaria. [PubMed] [Google Scholar]
- 9.WHO. Briefing on Malaria Treatment Guidelines and Artemisinin Monotherapies. World Health Organization. 2006 [Google Scholar]
- 10.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT) Am J Trop Med Hyg. 2007;77(6 Suppl):119–27. [PubMed] [Google Scholar]
- 11.Malaria Consortium. Diagnosis of malaria in resource poor settings and rapid diagnositic tests. [Last accessed on 2014 Mar 22]. Available from: http://www.malariaconsortium.org/pages/115.htm .
- 12.Rdt Info. Appropriate use of rapid tests. [Last accessed on 2013 Oct 22]. Available from: http://www.rapid-diagnostics.org/appropriateuse.htm .
- 13.Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, et al. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis. 2005;192:870–7. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira AM, Skarbinski J, Ouma PO, Kariuki S, Barnwell JW, Otieno K, et al. Performance of malaria rapid diagnostic tests as part of routine malaria case management in Kenya. Am J Trop Med Hyg. 2009;80:470–4. [PubMed] [Google Scholar]
- 15.Bisoffi Z, Sirima BS, Angheben A, Lodesani C, Gobbi F, Tinto H, et al. Rapid malaria diagnostic tests vs. clinical management of malaria in rural Burkina Faso: Safety and effect on clinical decisions. A randomized trial. Trop Med Int Health. 2009;14:491–8. doi: 10.1111/j.1365-3156.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 16.Ben - Edet AE, Lesi FE, Mafe AG, Grange AO. Diagnosis of Plasmodium falciparum Malaria in children using the immuno – chromatograhic diagnostic technique. Niger J Paediatr. 2004;31:71–8. [Google Scholar]
- 17.Tagbo O, Henrietta UO. Comparison of clinical, microscopic and rapid diagnostic test methods in the diagnosis of Plasmodium falciparum malaria in Enugu, Nigeria. Niger Postgrad Med J. 2007;14:285–9. [PubMed] [Google Scholar]
- 18.Nwuba RI, Anumudu CI, Omosun YO, Sodeinde O, Nwagwu M. Evaluation of a rapid immunochromatographic card test for Plasmodium falciparum in Ibadan, Nigeria. Afr J Med Med Sci. 2001;30:123–4. [PubMed] [Google Scholar]
- 19.Bisoffi Z, Sirima SB, Menten J, Pattaro C, Angheben A, Gobbi F, et al. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar J. 2010;9:192. doi: 10.1186/1475-2875-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazigo HD, Meza W, Ambrose EE, Kidenya BR, Kweka EJ. Confirmed malaria cases among children under five with fever and history of fever in rural western Tanzania. BMC Res Notes. 2011;4:359. doi: 10.1186/1756-0500-4-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oladosu OO, Oyibo WA. Over diagnosis and overtreatment of Malaria in children that presented with fever in Lagos, Nigeria. Int Scholar Res Network ISRN Infect Dis 2013. Article ID 914675, 6 pages. http://dx.doi.org/10.5402/2013/914675 .


