Abstract
Background and Aims:
Heat-related illness (HRI) due to high ambient temperatures is a common feature during the Indian summer. HRI often results in Intensive Care Unit (ICU) admissions and are associated with significant morbidity and mortality. However, published report on the effects of HRI among the Indian population is lacking. This study was undertaken to identify the profile of patients admitted to ICU with clinical features of HRI and study their clinical outcomes.
Methods:
This was a retrospective case series of patients admitted with features of HRI during the summer of 2012 in our multidisciplinary ICU. Data on demographics, co-morbid illness, admission severity of illness (Acute Physiology and Chronic Health Evaluation II [APACHE II]), organ failure scores (Sequential Organ Failure Assessment [SOFA]) and neuroimaging studies were collected. Outcome data studied included mortality, ICU length of stay (LOS), ventilator days and hospital LOS. Statistical analysis was performed using Student's t-test, Chi-square test and multivariate analysis.
Results:
Twenty-six patients met the diagnostic criteria for HRI. Fifteen were males. The mean age was 53.12 ± 18.6 years. Mean APACHE II score was 19.6 ± 7.7 and mean SOFA score was 7.5 ± 2.6. The common presenting symptoms were fever with neurological impairment (100%) and gastrointestinal symptoms (30%). Major organ systems involvement include neurological (100%), renal (57%), hepatic (34%) and coagulation abnormalities (26%). Most common metabolic abnormality noted was hyponatraemia (73%). Magnetic resonance imaging findings suggestive of heat stroke were seen in 5 of 26 patients. Mortality rate was 34%. 8 of 17 survivors had residual neurological impairment.
Conclusion:
HRI carries a high mortality and significant neurological morbidity.
Keywords: Heat-related illness, heat stroke, magnetic resonance imaging, mortality in heat-related illness, neurological impairment
INTRODUCTION
Heat-related illness (HRI) is a spectrum of disorders ranging from minor heat cramps, heat exhaustion to life-threatening heat stroke.[1] Heat stroke is defined clinically as a core body temperature above 40°C and accompanied by central nervous system (CNS) abnormalities such as delirium, convulsions, or coma.[2] Heat stroke, the extreme form of HRI is associated with significant mortality and morbidity, mortality in these patients range between 10% to 50%.[3] Heat stroke is often associated with multiple organ system involvement and survivors may sustain permanent neurologic damage.[4]
Heat stroke may be divided into exertional and non-exertional (classical) heat stroke. While exertional heat stroke is associated with exercise, classic heat stroke is typically seen in debilitated patients during high ambient temperature and humidity.[1,5] Risk factors for classical heat stroke include - old age, alcoholics, antihypertensive or psychiatric medications, neurological disease and dehydrating illness.[5,6,7]
India is a tropical country with high temperature during summer. The year 2012 recorded the highest temperatures in last 10 years in Chennai [Figure 1]. Due to the unusually high temperatures recorded in the summer of 2012, there was spurt in such patients admitted to our Intensive Care Unit (ICU).
Figure 1.
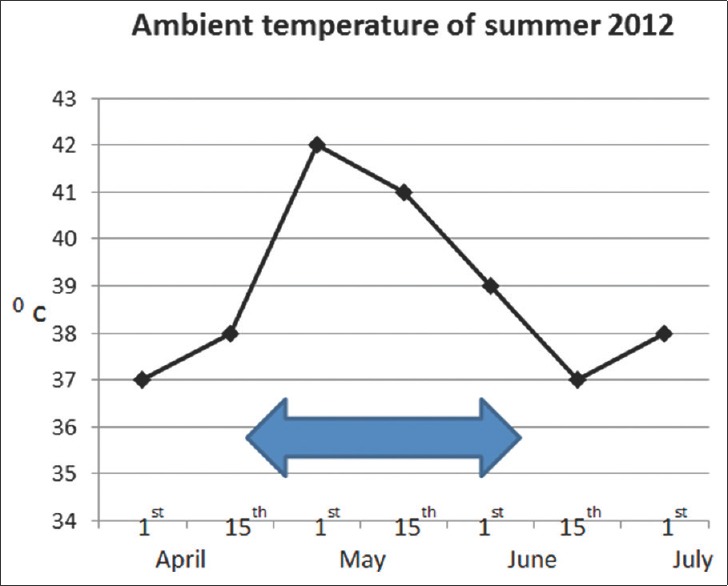
Ambient temperature of summer 2012. Arrow indicates admission time frame of patients to Intensive Care Unit with heat-related illness
METHODS
The aim of the study was to identify the profile of patients admitted to our multidisciplinary ICU with clinical features of HRI and analyse their clinical outcomes.
This was a retrospective case series, of patients admitted with features of HRI during the period from April to June 2012 in our 25 bedded multidisciplinary ICU. After obtaining Institutional Ethics Committee approval, we included all patients admitted with complaints of fever with CNS abnormalities such as delirium, convulsions, acute alteration of consciousness, or coma and absence of any other evident cause for the fever or acute onset of CNS dysfunction.
We excluded patients showing features of CNS infection, stroke and other causes of encephalopathy. All the patients were managed in the ICU in a similar manner as mandated by their severity of illness which included appropriate organ support, fluid resuscitation, electrolyte management and active cooling as required. Patients also received other supportive measures such as, stress ulcer, deep vein thrombosis prophylaxis, glycaemic control, nutritional support and analgesia/sedation. The patients were evaluated to rule out any underlying infections including CNS infection, stroke or encephalopathy as deemed necessary.
Data collected from patient's medical records, included demographics, co-existing illness, current medications, admission vitals and Glasgow coma scale (GCS) score. Other laboratory parameters required to calculate Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were also captured. These two scores were calculated using the worst parameters recorded during the first 24h of ICU admission. Details regarding cerebrospinal fluid analysis, echocardiography and neuroimaging studies obtained wherever indicated were also captured. Outcome data on mortality, ICU length of stay (LOS), number of ventilator days, discharge GCS and SOFA score were recorded.
Values for continuous data were expressed as mean ± standard deviation (SD). Categorical variables were reported as proportions. Continuous variables with normal distribution were compared using Student's t-test whereas those not normally distributed were analysed using Mann-Whitney U-test. Categorical data were analysed using Pearson Chi-square test or Fischer's exact test. Multivariate logistic regression models were used, to determine predictors of mortality.
RESULTS
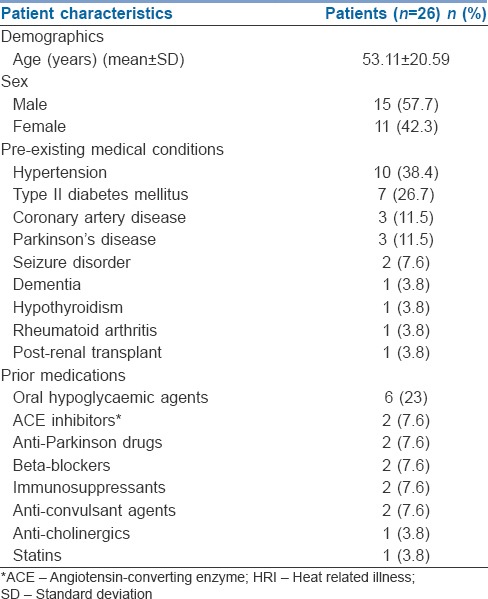
During the study period, 30 patients presented with features of HRI as described above, of whom two patients had features suggestive of CNS infection and another two patients had features of acute stroke in neuroimaging studies. Excluding these patients, data from the remaining 26 patients were analysed. Demographic characteristics of patients are presented in [Table 1]. The mean age of our patients was 53.11 ± 20.59 years (±SD). 20 patients (77%) had at least one co-existing illness, of which hypertension and diabetes mellitus were the most common [Table 1]. 18 (69.2%) patients were on prior medications at admission.
Table 1.
Demographics, pre-existing medical conditions and prior medications of patients with HRI
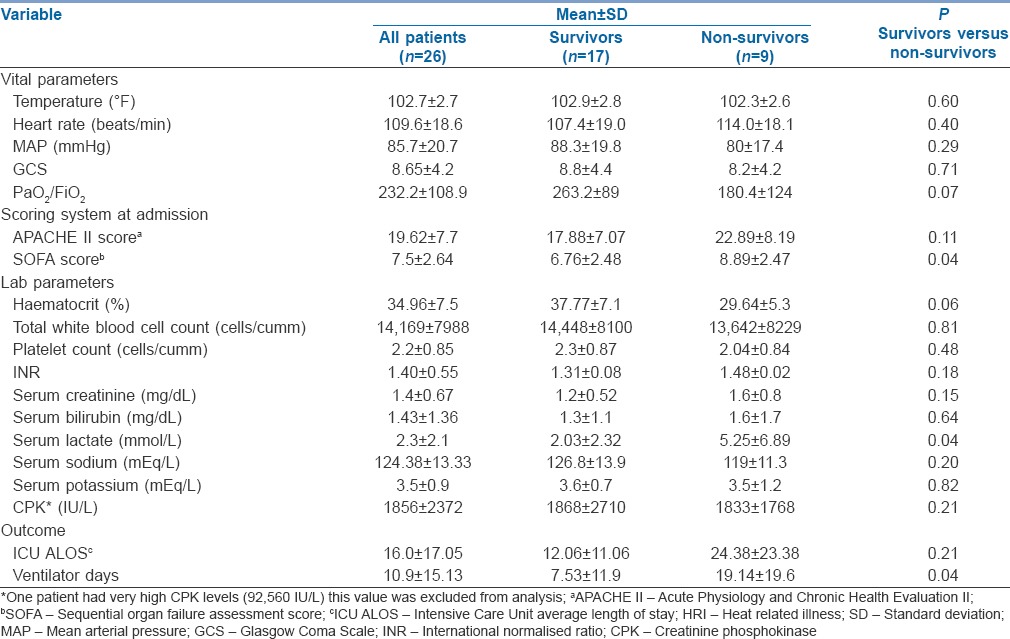
Patients admitted to ICU with HRI were critically ill as indicated by high admission APACHE II (19.62 ± 7.7, mean ± SD) and SOFA (7.5 ± 2.64, mean ± SD) scores [Table 2]. High SOFA scores on admission are reflective of significant organ system involvement at admission. Organ system involvement includes CNS (100%), renal (57%), liver (34%), coagulation (26%), respiratory (26%) and heart (23%).
Table 2.
Characteristics of patients with HRI
In line with our inclusion criteria, all our patients were febrile (temperature 102.7 ± 2.7° F, mean ± SD). More than 30% of patients presented with seizures and more than a third were comatose on admission [Figure 2]. Apart from CNS involvement, 30% of patients also had gastrointestinal (GI) symptoms such as nausea, vomiting (8/26) and diarrhoea (4/26).
Figure 2.
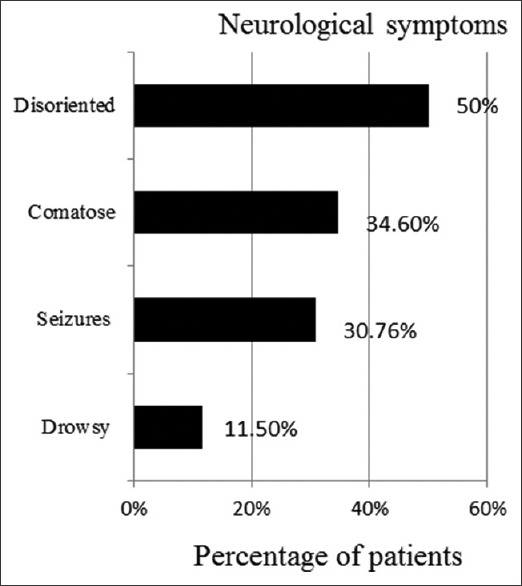
Spectrum of neurological involvement in heat-related illness
Seventy-three percentage (19/26) of our patients had hyponatraemia (serum sodium <135 mEq/L) on admission (mean serum sodium 124.38 ± 13.33 mEq/L [±SD]) and most responded to resuscitation with normal saline [Figure 3]. However, hypertonic saline (3%) was used in seven patients who had persistent neurological symptoms (GCS 11.2 ± 1.79, mean ± SD) and hyponatraemia (serum sodium 111 ± 4.0 mEq/L, mean ± SD). Almost 50% of patients had hypokalaemia (serum potassium <3.5 mEq/L) (mean serum potassium 3.5 ± 0.9 mEq/L [±SD]). Creatinine phosphokinase (CPK) levels were elevated in all patients (1856 ± 2372 IU/L, mean ± SD). One patient had CPK levels as high as 92,560 IU/L on admission; this patient had a previous history of heat stroke, had myoglobinuria and renal failure requiring haemodialysis. 15 (57%) patients had elevated serum creatinine; five of these patients had progressively worsening renal function requiring haemodialysis. Liver enzymes were raised in 50%(13/26) patients (aspartate aminotransferase [AST] 198.50 ± 395.6 IU/L and alanine aminotransferase [ALT] 97.5 ± 110 IU/L, mean ± SD) with mildly deranged coagulation profile (International normalised ratio 1.40 ± 0.55) with no obvious bleeding tendencies.
Figure 3.
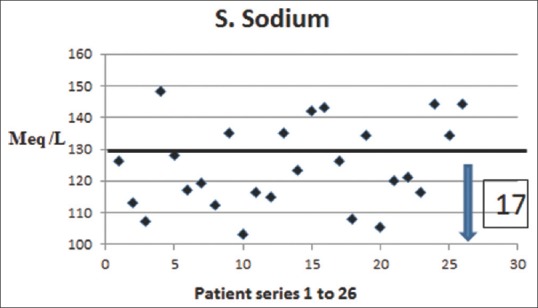
Serum sodium levels in patients with heat stroke
Neuroimaging was performed in patients who were comatose or had focal neurological deficit post-resuscitation and stabilisation. Computerised tomography scan of brain was done in 11 (11/26) patients, while magnetic resonance imaging (MRI) of brain was done in 12 (12/26) patients. Diffusion-weighted MRI features suggestive of heat stroke [Figure 4] were seen in five patients. These included diffusion restriction involving cerebellum (4/5), basal ganglia and thalamus (3/5), hippocampus (2/5), cerebral cortex and subcortical white matter (2/5). There were corresponding increased T2/T2 fluid-attenuated inversion recovery signal intensities in these regions. Features of diffuse cerebral oedema (3/5) and diffuse cerebellar oedema (4/5) were also seen in these patients.
Figure 4.
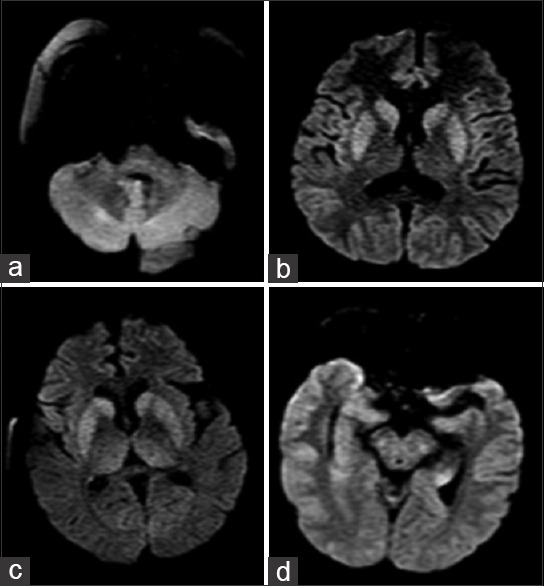
Diffusion-weighted magnetic resonance imaging showing restricted diffusion involving (a) cerebellum (b) thalamus (c) basal ganglia and (d) hippocampus
Sixteen patients (61.5%) required mechanical ventilation to protect airway due to low GCS. Six patients (23%) who remained in shock (mean arterial pressure <70 mmHg) despite adequate fluid resuscitation required vasopressor support.
Nine out of twenty six patients died (34%). Mean ICU LOS was 16.0 ± 17.05 days (±SD)(range 2-60 days). Mean ventilator days were 10.16 ± 12.46 days (±SD) [Table 2]. The mean discharge GCS of our patients was 13.17 ± 1.8, (±SD). Nine out of 17 patients were discharged with normal GCS. Of the remaining eight patients, four had GCS scores <10. These four patients had a prolonged ICU stay (28 ± 5 days, mean ± SD). The remaining four patients had a discharge GCS of 14, of whom two had pre-existing, Parkinsonism, one had dementia and another had previous stroke. Raised serum lactate, high admission SOFA scores and prolonged ventilator days were risk factors for mortality in univariate analysis; however, multivariate analysis by logistic regression did not identify any risk factors of mortality.
DISCUSSION
HRI following periods of sustained heat wave have been reported previously[4,8,9,10,11]; however, in our study, we had cases admitted over a period of 2 months corresponding to high ambient temperature. Risk factors for HRI include old age, alcohol abuse, neurological disease, psychiatric illness and patients on long-term medications.[1,2,5,6,7] In our study, the mean age of patients was 53.1 years. This was a strikingly younger group as compared to the patients in the studies by Argaud et al.[11] (79.6 ± 9.9 years) and Misset et al.[12] (67.2 ± 14.1 years). In these two studies, nearly 30-50% of patients had significant functional limitations and in our study, most of the patients were functionally active prior to admission. Hence, our patients may have been exposed to high ambient temperatures in an open environment making them more prone to HRI.
Exposure to high temperatures lead to elevation of body temperature, which causes activation of various physiological compensatory mechanisms which include increased cutaneous circulation and concomitant vasoconstriction of the renal and splanchnic circulation.[1] Heat stress causes cell damage by apoptosis, release of inflammatory mediators and endothelial damage leading to multi organ failure.
The Purkinje cells in cerebellum are most susceptible to injury due to hyperthermia. Most common sites of CNS involvement of heat stroke include cerebellum, basal ganglia, thalamus, hippocampus and fronto-parietal cortex[13,14,15,16,17] All our patients had neurological involvement of varying degrees. MRI done in our patients revealed similar pattern of involvement. MRI features were due to cerebral ischaemia (UK English) resulting from auto regulatory mechanisms that divert blood flow towards periphery to dissipate excessive heat. None of our patients showed features of intracerebral haemorrhage.
Apart from CNS symptoms, 30% of patients also presented with GI symptoms such as nausea, vomiting and diarrhoea. This might be due to the compensatory splanchnic vasoconstriction that occurs secondary to hypovolaemia.[3,18] Studies have shown that even though thermal stress results in an increased cardiac output, splanchnic flow is reduced because of increased splanchnic vascular resistance, resulting in gut ischaemia with focal necrosis, resulting in GI symptoms.[4] A high prevalence of elevated liver aminotransferase levels has been reported in exertional heat stroke and has been attributed to ischaemia and direct thermal injury.[19,20]
Coagulation abnormalities have been described in exertional heat stroke. The primary event seems to be related to direct thermal injury of vascular endothelium, initiating platelet aggregation and activation of the clotting cascade along with increased fibrinolysis and disseminated intravascular coagulation.[19,20,21] Even though our patients had mildly elevated liver enzymes and slightly deranged coagulation parameters, they didn't show any obvious bleeding tendencies. Argaud et al.[11] during the French heat wave reported 40% incidence of raised ALT levels in their study compared to the 50% in our study.
Previously reported incidence of renal failure in HRI range from 30% to 35%.[4,22] All but 5 of the 15 patients with elevated serum creatinine improved their renal function with fluid resuscitation, this points to volume depletion as a cause for renal involvement. Three of the five patients requiring haemodialysis had myoglobinuria. Suggested mechanisms of renal injury in patients with heat stroke include direct thermal injury, renal hypoperfusion secondary to compensatory renal artery vasoconstriction and rhabdomyolysis.
Haemodynamic failure requiring vasopressor support was seen in 23% of our patients compared to 43% reported by Argaud et al.[11] Misset et al.[12] reported an even higher rate of vasopressor use (65.2%). Shock in heat stroke is due to the inability of the cardiovascular system to increase cardiac output in the context of heat-related dehydration and vasoplegia enhanced by proinflammatory response.[3,5] Cardiovascular dysfunction could be exacerbated in patients with pre-existing cardiovascular disease and patients on long-term antihypertensive medications.[3]
Our patients had a mortality rate of 35%. Previously reported mortality in HRI ranges between 20% and 62%.[4,12] Misset et al.[12] reported a mortality rate of 62% in 345 patients following 2003 heat stroke in France. Compared to ours, their patients were elderly (67.2 ± 14.1 vs. 53.11 ± 20.59 years), were sicker as reflected by lower GCS (5.7 vs. 8.6) and higher rate of mechanical ventilation (99% vs. 64%). Various predictors of mortality have been previously described in heat stroke which includes long-term use of antihypertensive medication, anuria at admission, coma, cardiovascular failure, high Simplified Acute Physiology Score (SAPS II), high body temperature and prolonged prothrombin time.[11,12]
We did not find any independent risk factors for mortality in our study.
Previous studies have described significant neurological impairment in survivors of heat stroke. Dematte et al.[4] described 33% of their survivors having moderate to severe neurological impairment which was higher than the rate of 23.52% (4 of 17 with GCS <10) observed in our study. This difference may be due to the greater sickness of their patients (APACHE II 28 ± 6 vs. 19.62 ± 7.7 mean ± SD) as compared to our patients. However, survivors regained normal function of other organ systems in our study.
Due to the retrospective nature of our study we faced certain limitations. History of intensity of heat exposure, or exertion prior to admission and information regarding cooling methods employed were not reliable. Similarly there was a lack of clarity on the degree of neurological involvement in patients with pre-existing neurological disease. We also did not have information on long-term follow-up of patients after hospital discharge.
CONCLUSIONS
Patients admitted to ICUs with Heat Related Illness had multiple organ system dysfunction and were associated with significant neurological morbidity, and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lugo-Amador NM, Rothenhaus T, Moyer P. Heat-related illness. Emerg Med Clin North Am. 2004;22:315–27, viii. doi: 10.1016/j.emc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 3.Bouchama A. Heatstroke: A new look at an ancient disease. Intensive Care Med. 1995;21:623–5. doi: 10.1007/BF01711537. [DOI] [PubMed] [Google Scholar]
- 4.Dematte JE, O’Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, et al. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173–81. doi: 10.7326/0003-4819-129-3-199808010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Grogan H, Hopkins PM. Heat stroke: Implications for critical care and anaesthesia. Br J Anaesth. 2002;88:700–7. doi: 10.1093/bja/88.5.700. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson JG. Exertional heat stroke-predisposing factors, clinical features, treatment and prevention. In: Hopkins PM, Ellis FR, editors. Hyperthermic and Hypermetabolic Disorders. 1st ed. Cambridge: Cambridge University Press; 1996. pp. 20–41. [Google Scholar]
- 7.Yamashita S, Uchida Y, Kojima S, Sakaguchi H, Kimura E, Maeda Y, et al. Heatstroke in patients with Parkinson's disease. Neurol Sci. 2012;33:685–7. doi: 10.1007/s10072-011-0842-7. [DOI] [PubMed] [Google Scholar]
- 8.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, et al. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335:84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- 9.Khogali M, Weiner JS. Heat stroke: Report on 18 cases. Lancet. 1980;2:276–8. doi: 10.1016/s0140-6736(80)90232-9. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Heat – related illnesses and deaths-United States, 1994-1995. MMWR Morb Mortal Wkly Rep. 1995;44:465–8. [PubMed] [Google Scholar]
- 11.Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167:2177–83. doi: 10.1001/archinte.167.20.ioi70147. [DOI] [PubMed] [Google Scholar]
- 12.Misset B, De Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, et al. Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: A national multiple-center risk-factor study. Crit Care Med. 2006;34:1087–92. doi: 10.1097/01.CCM.0000206469.33615.02. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin CT, Kane AG, Auber AE. MR imaging of heat stroke: External capsule and thalamic T1 shortening and cerebellar injury. AJNR Am J Neuroradiol. 2003;24:1372–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Van Stavern GP, Biousse V, Newman NJ, Leingang JC. Downbeat nystagmus from heat stroke. J Neurol Neurosurg Psychiatry. 2000;69:403–4. doi: 10.1136/jnnp.69.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudhakar PJ, Al-Hashimi H. Bilateral hippocampal hyperintensities: A new finding in MR imaging of heat stroke. Pediatr Radiol. 2007;37:1289–91. doi: 10.1007/s00247-007-0612-0. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero WR, Varghese S, Savitz S, Wu TC. Heat stress presenting with encephalopathy and MRI findings of diffuse cerebral injury and hemorrhage. BMC Neurol. 2013;13:63. doi: 10.1186/1471-2377-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albukrek D, Bakon M, Moran DS, Faibel M, Epstein Y. Heat-stroke-induced cerebellar atrophy: Clinical course, CT and MRI findings. Neuroradiology. 1997;39:195–7. doi: 10.1007/s002340050392. [DOI] [PubMed] [Google Scholar]
- 18.Yarbrough B, Vicario S. Heat illness. In: Marx J, editor. Rosen's Emergency Medicine. Concepts and Clinical Practice. 5th ed. St. Louis, Mo: Mosby; 2002. pp. 1997–2009. [Google Scholar]
- 19.Hassanein T, Razack A, Gavaler JS, Van Thiel DH. Heatstroke: Its clinical and pathological presentation, with particular attention to the liver. Am J Gastroenterol. 1992;87:1382–9. [PubMed] [Google Scholar]
- 20.Kew M, Bersohn I, Seftel H, Kent G. Liver damage in heatstroke. Am J Med. 1970;49:192–202. doi: 10.1016/s0002-9343(70)80075-4. [DOI] [PubMed] [Google Scholar]
- 21.Bouchama A, Hammami MM, Haq A, Jackson J, al-Sedairy S. Evidence for endothelial cell activation/injury in heatstroke. Crit Care Med. 1996;24:1173–8. doi: 10.1097/00003246-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Knochel JP. Exertional heat stroke-pathophysiology of heat stroke. In: Hopkins PM, Ellis FR, editors. Hyperthermic and Hypermetabolic Disorders. 1st ed. Cambridge: Cambridge University Press; 1996. pp. 42–62. [Google Scholar]


