INTRODUCTION
Oral cavity cancers are one of the most common cancers in Indian males. Consequently, maxillofacial cancer surgery constitutes a large part of surgical oncology practice in India. Manipulation and excision of mandible, maxilla and tongue are extremely noxious stimuli and severe hypertension and tachycardia during these procedures is not unusual. Management includes deepening the plane of anaesthesia by increasing the inhalational anaesthetic concentration and addition of intravenous (IV) opioids.
Traditionally, strong analgesics such as opioids have been used intra-operatively whereas non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol are most commonly given at the end of surgery as part of multimodal approach to post-operative analgesia.[1,2] NSAIDs are highly effective in controlling bone pain and have analgesic effects in various conditions especially where tissue inflammation contributes to pain.[3,4] Concerns regarding the deleterious effects of NSAIDs on platelet function and renal function in conditions of renal hypoperfusion are some of the reasons why NSAIDs are not preferred as intra-operative analgesics. In cancer patients, many commonly used chemotherapeutic drugs have known nephrotoxic effects. Drugs such as cisplatinum and ifosfamide cause tubular damage whereas bevacizumab and gemcitabine injure renal vasculature. Hence, there is a tendency to restrict use of NSAIDs in these patients.
We hypothesized that addition of single dose of diclofenac at the time of induction would exert opioid sparing effect during intra-operative period and reduce surges in blood pressure (BP) and pulse rate during noxious stimuli.
METHODS
This prospective, double-blind, randomised, placebo-controlled study was conducted in a tertiary care cancer hospital. Institutional Review Board approval was obtained prior to the study. Eligible subjects were enrolled in the study after written informed consent.
The subjects included 100 adult patients more than 18 years of age posted for maxillofacial surgery with reconstructive surgery. Patients known to have allergy to the study drugs, bleeding disorders, pregnancy, acid-peptic disorder, heart ailments such as congestive cardiac failure, liver and kidney diseases were excluded from the study. Patients were also excluded if they were already on treatment with NSAIDs, opioids, anticoagulants, methotrexate, cyclosporin, lithium or phenytoin.
The patients recruited to the study were randomised prior to anaesthesia to either Group 1 (Placebo Group) to receive 100 cc normal saline after induction of anaesthesia and Group 2 (Diclofenac Group) to receive diclofenac sodium 1 mg/kg in 100 cc normal saline IV after induction of anaesthesia.
We selected sample size of 100 based on convenience. Patients were randomised based on the date of surgery. Patients operated on odd days were recruited to Group 1 whereas those operated on even days were recruited to Group 2. Patients, attending doctors including the anaesthesiologist and the recovery room staff were blinded to the study group.
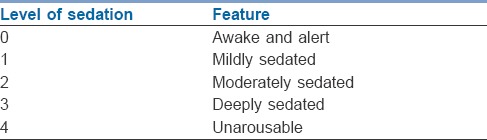
Primary outcome was difference in opioid consumption between two groups and secondary end points were pain score and sedation score in recovery. Pain scores using visual analogue scale [VAS] and sedation score, using University of Michigan Sedation Scale [Table 1][5,6] were measured by recovery nurse as part of vital signs monitoring on arrival and every hour and reported to investigator/recovery area physician. Study investigators independently recorded pain and sedation score 4th hourly. In post-operative period, rescue analgesic buprenorphine 3 μ/kg IM was given at VAS ≥4. If patient had pain, he/she would also use sign language to communicate with attending physician or nurse.
Table 1.
Sedation scale
Anaesthesia was induced with fentanyl 2 μ/kg followed by thiopentone 3-5 mg/kg. Muscle relaxation was achieved with succinylcholine 2 mg/kg or vecuronium bromide 0.1 mg/kg as indicated. Anaesthesia was maintained with O2 + N2 O (40:60) and isoflurane 1.5% dialed concentration for first 15 min which was reduced to 0.8% thereafter and total gas flows of 1 to 1.5 L/min. Both groups received continuous infusion of IV fentanyl 1 μ/kg/h as the standard analgesic. During surgery, BP and pulse rate above 20% of baseline values were considered as signs of inadequate analgesia and were treated with an additional bolus dose of IV fentanyl 1 μ/kg, which could be repeated at 5 min interval as required since peak action of fentanyl occurs at 3-5 min.[7] Fall in BP was treated with fluid boluses. Bradycardia was treated by stopping surgical manipulations and if bradycardia persisted, injection atropine 0.6 mg IV was administered. At the end of surgery, fentanyl infusion was stopped. The neuromuscular block was reversed with IV neostigmine 0.05 mg/kg and IV glycopyrrolate 8 μg/kg. The endotracheal tube was left in situ overnight for airway maintenance.
All patients received standard post-operative care. The time of first demand for analgesic after admission to PACU was noted.
RESULTS
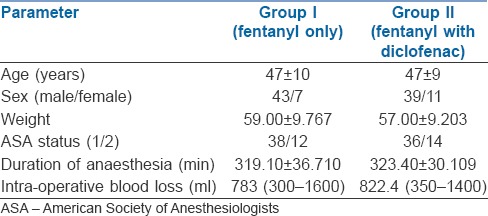
The demographic variables were comparable in both the groups [Table 2]. Majority of patients were male (86% and 78% in Group 1 and Group 2, respectively). The average age in both groups was 47 years (30-65 years) and 74% of the patients belonged to the American Society of Anaesthesiologists I physical status. The mean duration of surgery was around 320 min (210-370 min).
Table 2.
Demographic data
Patients in the control group consumed significantly more IV fentanyl boluses intra-operatively than in the placebo (2.84 vs. 0.74, P < 0.01) mainly during the phase of incision and time of removal of specimen that requires bone cuts [Figure 1]. Patients in the Diclofenac Group also had better pain and sedation scores on arrival to recovery (time 0) which was statistically significant. Mean pain intensity at rest which was measured on VAS at arrival was 3.30 ± 0.65 in Placebo Group compared to 2.10 ± 0.30 in the Diclofenac Group which was significant (P < 0.01). Sedation scores on arrival in recovery room were 2.58 ± 0.57 and 1.66 ± 0.63 in the Placebo and Diclofenac Group, respectively (P < 0.01). Time to demand for first dose of analgesia in post-operative period was significantly shorter for group 1 (114.5 min vs. 252.6 min P < 0.01) [Figure 2].
Figure 1.
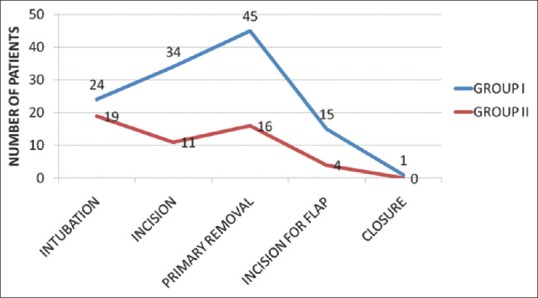
Comparison of number of patients requiring fentanyl boluses intra-operatively
Figure 2.
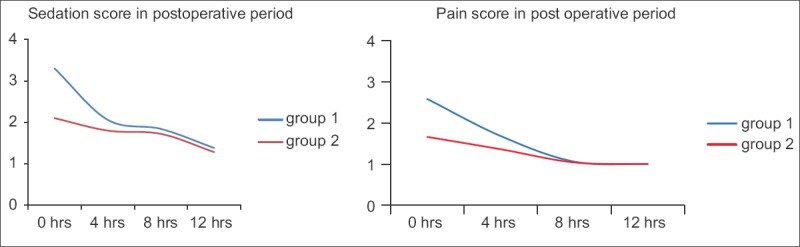
Comparison of pain score and sedation score in immediate post-operative period
There was no difference in pulse rate at induction, incision and removal of tumour; there was significant difference in mean arterial pressure at incision (P < 0.01), at primary tumour removal (P < 0.007) and at incision for reconstructive surgery (P = 0.011). None of the patients in either group required any other measures to control the BP [Figure 3].
Figure 3.
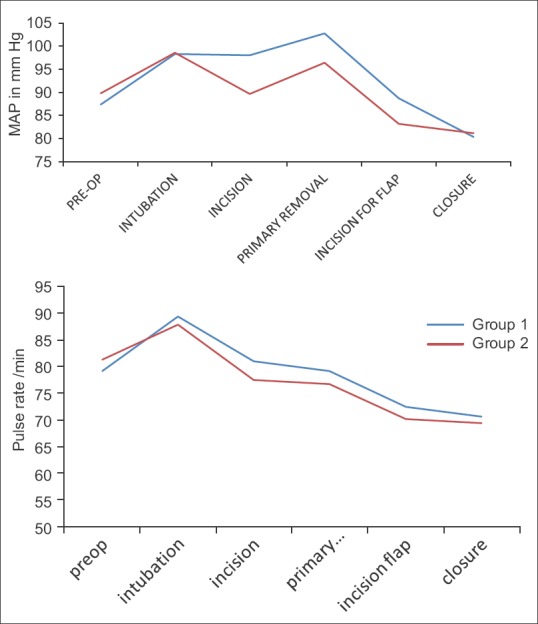
Comparison of mean arterial pressure and mean pulse during surgery between two groups
DISCUSSION
Our study showed that diclofenac administered at the beginning of surgery reduced opioid required to control the haemodynamic response to surgical stimulation.
During balanced anaesthesia, optimum analgesia reduces the dose of anaesthetic agents and muscle relaxants resulting in a better post-operative recovery. Better analgesia also helps to maintain cardiovascular stability. Succinylcholine and vecuronium used in the study have different effects on pulse rate, but the effect is short lasting and would not affect results as interval between induction and incision usually exceeds 15 min. NSAIDs are highly effective in conditions especially where tissue inflammation contributes to pain while lacking most of the side effects of opioids. They have been used as part of pre-emptive analgesia and shown to have an opioid sparing effect in many studies in post-operative pain management.[8,9,10] We could not find any study on pre-emptive use of NSAIDs with opioid sparing effects in intra-operative period. There may also be benefits in cancer outcome as recent studies have shown association between use of opioids and cancer outcome.[11,12] Studies have suggested that opioid receptor antagonists may inhibit opiate and vascular endothelial growth factor-induced angiogenesis.[13]
The main concern is the effect of NSAIDs on platelet aggregation thereby increasing the surgical bleeding. However, studies looking for a relationship between the use of NSAIDs and perioperative bleeding have failed to show strong association. This study also did not show increased bleeding or increased need for transfusion in the Diclofenac Group. Average intra-operative blood loss in the Placebo Group was 783 ml (300-1600) compared to 822.4 ml (350-1400) (P = 0.551) in the Diclofenac Group.
This was a quasi-randomised study which is not ideal method of randomization. However, since surgeons were not part of this study, they were blind towards methodology. Similarly, assessors and anaesthesiologist conducting case and patient were not aware about the method of randomisation, minimizing chance of bias.
CONCLUSION
Diclofenac when given as an intra-operative analgesic reduced fentanyl consumption in the intra-operative period with extension of analgesia into the early post-operative period. Patients receiving diclofenac were significantly less sedated than those in the Placebo Group.
Financial support and sponsorship
Tata Memorial Hospital, Mumbai, Maharashtra, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rashwana D, El-Rahmawyb GF. Multimodal analgesia after upper limb orthopedic surgeries: Patient controlled intravenous low dose tramadol analgesia with or without intravenous acetaminophen - A comparative study. Egypt J Anaesth. 2013;29:231–4. [Google Scholar]
- 2.Danou F, Paraskeva A, Vassilakopoulos T, Fassoulaki A. The analgesic efficacy of intravenous tenoxicam as an adjunct to patient-controlled analgesia in total abdominal hysterectomy. Anesth Analg. 2000;90:672–6. doi: 10.1097/00000539-200003000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Buggy DJ, Wall C, Carton EG. Preoperative or postoperative diclofenac for laparoscopic tubal ligation. Br J Anaesth. 1994;73:767–70. doi: 10.1093/bja/73.6.767. [DOI] [PubMed] [Google Scholar]
- 4.Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;2:CD004768. doi: 10.1002/14651858.CD004768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K, Naughton N. Depth of sedation in children undergoing computed tomography: Validity and reliability of the University of Michigan Sedation Scale (UMSS) Br J Anaesth. 2002;88:241–5. doi: 10.1093/bja/88.2.241. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz HR, Cortínez LI, Ibacache ME, León PJ. Effect site concentrations of propofol producing hypnosis in children and adults: Comparison using the bispectral index. Acta Anaesthesiol Scand. 2006;50:882–7. doi: 10.1111/j.1399-6576.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 7.Coda BA. Opioids. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, editors. Clinical Anaesthesia. 6th ed. Philadelphia: Wolters Kluwer Health; 2009. pp. 465–94. [Google Scholar]
- 8.Fayaz MK, Abel RJ, Pugh SC, Hall JE, Djaiani G, Mecklenburgh JS. Opioid-sparing effects of diclofenac and paracetamol lead to improved outcomes after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:742–7. doi: 10.1053/j.jvca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Legeby M, Sandelin K, Wickman M, Olofsson C. Analgesic efficacy of diclofenac in combination with morphine and paracetamol after mastectomy and immediate breast reconstruction. Acta Anaesthesiol Scand. 2005;49:1360–6. doi: 10.1111/j.1399-6576.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 10.Anwari JS, Anjum S, Al-Khunain S. Placebo controlled comparison of the opioid sparing effect of meloxicam and diclofenac after abdominal hysterectomy. Saudi Med J. 2008;29:379–83. [PubMed] [Google Scholar]
- 11.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- 12.Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, et al. The novel role of the mu opioid receptor in lung cancer progression: A laboratory investigation. Anesth Analg. 2011;112:558–67. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: Role of receptor transactivation. Microvasc Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]


