Abstract
Introduction
Approximately 23% of acute myeloid leukemia (AML) patients younger than 60 years of age carry a mutation in the transmembrane domain of the FMS-like tyrosine kinase-3 (FLT3) gene (FLT3/internal tandem duplications [ITD]). In normal karyotype AML, the presence of a FLT3/ITD mutation is associated with poor prognosis, as mirrored by a high risk of relapse even after allogeneic stem cell transplantation. The poor prognostic impact along with the observation that FLT3 is frequently overexpressed in the majority of AML cases has formed the platform for the development of FLT3-targeted strategies. To date, several FLT3 kinase inhibitors have been investigated in preclinical and clinical studies. However, as of yet, none of the studied FLT3 inhibitors has received FDA approval for routine clinical use in AML. This is in part due to the ‘off target’ effects observed with most inhibitors when administered at concentrations needed to achieve sustained levels of FLT3 inhibition, which are required to exhibit substantial cytotoxic effects against leukemic blasts. Furthermore, the development of resistance mutations has emerged as a clinical issue posing a threat to successful FLT3 inhibitor therapy.
Areas covered
In this review, the authors provide a brief summary of FLT3 inhibitors investigated thus far, and discuss current treatment approaches and strategies how to best incorporate FLT3 tyrosine kinase inhibitors (TKIs) into therapy.
Expert opinion
The combination of a FLT3 inhibitor with conventional chemotherapeutic regimens, epigenetic modifiers or inhibitors of FLT3 downstream and collateral effectors has emerged as a promising strategy to improve treatment outcome. The future of a tailored, molecular-based treatment approach for FLT3-mutated AML demands novel clinical trial concepts based on harmonized and aligned research goals between clinical and research centers and industry.
Keywords: acute myeloid leukemia, drug resistance, FMS-like tyrosine kinase-3/internal tandem duplications mutation, hypomethylation
1. Introduction
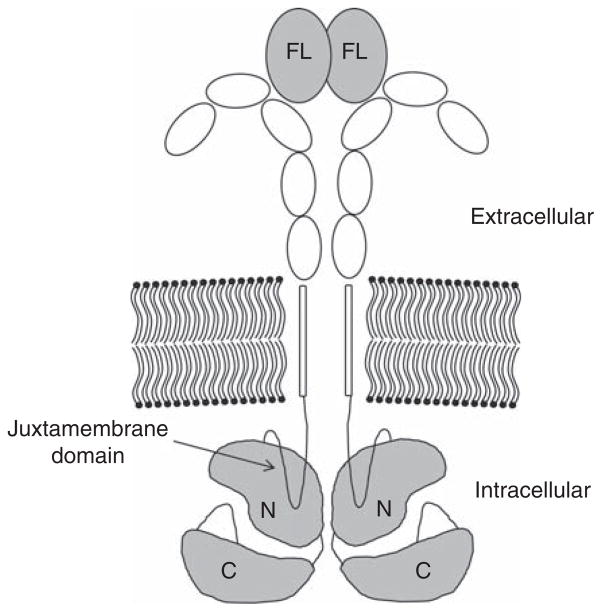
Although the majority of acute myeloid leukemia (AML) cases present with a normal karyotype and therefore fall into the intermediate-risk category, recent advances in gene expression profiling has further honed risk stratification in this subgroup [1]. Herein, internal tandem duplications (ITD) of the juxtamembrane region of the FMS-like tyrosine kinase-3 (FLT3) receptor tyrosine kinase (Figure 1), present in a substantial proportion of AML patients of all age groups, have shown to be an important negative prognostic indicator of disease outcome [2,3], even in older patients [4]. Whereas point mutations in the activation loop of the kinase domain (FLT3/tyrosine kinase domain [TKD]) do not appear to have a significant prognostic impact [5], patients harboring an ITD have a dismal prognosis with a median survival of less than a year [2,3]. Interestingly, early reports could not establish an association between age and the incidence of FLT/ITD mutations. However, most of these studies were either conducted in small patient populations or focused on patients at the age of 60 or younger [3,6]. More recent data suggests that the incidence of FLT3/ITD mutations decreases with age, with an incidence of 16 – 20% in patients older than 60 years as compared to up to 35% in patients between 20 and 59 years [4,7,8].
Figure 1. The FLT3 receptor.
FLT3 ligand (FL) binds in dimeric form to induce receptor dimerization. FLT3/ITD mutations are inserted into the juxtamembrane domain.
C: C-lobe of kinase domain; FLT3: FMS-like tyrosine kinase-3; ITD: Internal tandem duplications; N: N-lobe of kinase domain.
ITD of the FLT3 receptor gene invariably lead to the constitutive activation of the receptor-tyrosine kinase (RTK) and its downstream signaling effectors, such as RAS/RAF/MEK/ERK kinases, STAT5 and PI3-kinase [9]. As a consequence, altered mechanisms of cellular proliferation and apoptosis promote cell survival thereby conferring a substantial growth advantage to leukemic stem and progenitor cells. From a clinical perspective, this frequently translates into a higher percentage of blood and bone marrow blasts, and a worse overall survival primarily due to a high relapse rate. Lines of evidence suggest that the allelic burden plays a pivotal role in predicting the level of ‘FLT3 addiction’ and clinical outcome [10–12]. For example, Pratz et al. showed that low allelic burden FLT3/ITD AML, which appears to more commonly present at the time of initial diagnosis, is less responsive to FLT3 inhibition compared with high allelic burden FLT3/ITD AML, a disease more frequently diagnosed at the time of relapse. Although numerous clinical trials were able to demonstrate that patients with FLT3/ITD AML frequently achieve remission rates similar to other AML patients, the disease usually relapses within a matter months in most cases. Relapsed FLT3/ITD AML represents a portentous clinical situation given the lack of treatment options available to these patients.
In a randomized trial of FLT3-mutated AML patients in first relapse, only 11% of patients with a first remission duration of < 6 months achieved a second remission, and only 29% of patients with a first remission duration of 6 months or longer achieved a second remission when treated with salvage chemotherapy (high-dose cytarabine or mitoxantrone/etoposide/cytarabine) [13] highlighting the urgent need for novel therapeutic strategies to improve the outcome in this patient group. To this end, the role of allogeneic stem cell transplantation (SCT) as a consolidation regimen for FLT3/ITD-mutated patients in first remission has been a topic of controversy among experts in the field [14]. Allogeneic SCT is a treatment modality that offers a potentially higher chance of cure for AML in general, and most studies suggest that allogeneic SCT reduces relapse and improves leukemia-free survival, indicating that this approach may be superior to standard induction and post-remission chemotherapeutic protocols [15]. However, this approach is associated with a considerable morbidity and mortality rate, particularly in older patients. Based on the analyses of two clinical trial populations in the UK involving 1135 patients, Gale et al. did not find good evidence that FLT3 status should guide the decision to proceed with SCT [16]. On the other hand, investigators from a study of 872 cytogenetically normal adult AML patients younger than 60 years from four clinical trial populations in Germany, Austria and Belgium reported that the benefit of SCT is limited to FLT3/ITD+ patients, as well as to patients harboring wild-type (wt) nucleophosmin 1 and CCAAT/enhancer-binding protein α (CEBPA) in the absence of FLT3/ITD [17]. Herein, the authors reported a difference in event free but not overall survival with allogeneic SCT. In line with these findings, an analysis of 206 AML patients (n = 120 [FLT3/ITD+]; n = 86 [FLT3/ITD−]) in first CR (CR1) treated with either HLA-identical sibling or matched unrelated donor SCTs demonstrated an improvement in the 2-year relapse-free survival and leukemia-free survival in the FLT3-mutated group [18]. Differences in outcome with SCT between various trials might be a reflection of the difference in cohorts and subgroups studied. For example, while the study of Gale et al. focused on FLT3/ITD-mutated patients, the study by Schlenk et al. focused on the cohort of cytogenetically normal AML patients with unfavorable genotypes, including, but not limited to, the FLT3/ITD mutation. In the former study, the rate of allo-SCT during the first period of complete response (CR) was 63% in the setting of a treatment related mortality of 30%, whereas in the latter study these parameters were 82 and 21%, respectively. Moreover, AML patients in the study of Schlenk et al. were younger than 60 years, whereas in the study by Gale et al. patients older than 60 years were included in the study population. The combined evidence of multiple smaller studies suggest that FLT3/ITD-mutated AML patients in first remission may derive greater benefit from allogeneic SCT compared to patients treated with consolidation chemotherapy. However, this patient population remains at higher relapse risk than transplanted AML patients who are FLT3/ITD− [19–21].
2. FLT3/ITD+AML – the evolution of the disease from diagnosis to relapse and treatment response at relapse
Whole genome sequencing studies of mutation profiles in AML cells indicate that AML is polyclonal at diagnosis, whereas a dominant clone tends to emerge at relapse. This is largely due to the fact that recurrent disease is frequently driven by distinct clonal evolution patterns consisting of either a founding clone that has acquired additional mutations over time and evolved into a relapsed clone, or a sub-clone of the founding clone that was not eliminated by initial therapy, acquired additional mutations and expanded, or both [22]. Growing evidence suggest that tumor cells remain dependent on the same oncogene signaling pathways as their malignant predecessors [23]. This is particularly evident in FLT3/ITD+AML, in which the cells at relapse are essentially addicted to signaling from the constitutively activated FLT3 RTK [10]. This addiction to FLT3 signaling is demonstrated by the fact that small molecule FLT3 inhibitors selectively kill AML cells harboring FLT3-activating mutations [24]. In vitro studies of these agents predicted that their use would overcome the differentiation block in this AML subtype [25,26]. This was in fact highlighted by several studies with different FLT3 inhibitors in relapsed FLT3/ITD+ AML, which concordantly reported treatment responses that were associated with terminal myeloid differentiation, some of them even with clinical manifestations of differentiation syndrome [27–29].
Primary refractory AML is rapidly fatal in most cases [30], whereas relapsed AML occasionally responds to salvage chemotherapy [31]. Recent data on prognostic factors in relapsed AML indicate that cytogenetics, FLT3/ITD status and duration of first remission are the most important predictors of survival in the relapsed setting [32]. FLT3/ITD+ AML characteristically relapses after a shorter first remission than other AML subtypes [13]. From published data, relapsed/refractory FLT3/ITD+ AML patients can be categorized into two groups: i) patients who are primary refractory or who have relapsed after a remission duration of 6 months or less and ii) patients who have relapsed after a remission duration of > 6 months. With currently approved therapies, the former group has an expected long-term survival of 0 – 3%, whereas the latter would be expected to achieve a second remission in 20% of the time and to have a 5 – 10% long-term survival [30,31,33]. FLT3 inhibition as a therapeutic modality offers the possibility of clinical benefit in these patient populations through the reduction of tumor volume in a relatively non-toxic fashion, resulting in improved survival either directly or by allowing for allogeneic transplant. The criteria for response to such compounds should be a reflection of the efficacy of this tumor reduction (Table 1).
Table 1.
Proposed classification scheme for response to FLT3 inhibitors used as single agents in patients with relapsed/refractory FLT3/ITD acute myeloid leukemia (AML), based on what is known about the clinical characteristics of the study population and mechanism of action of FLT3 inhibitors.
| No response | Persistent blasts in the bone marrow aspirate (Wright–Giemsa stain) and/or core sample, with characterization of leukemia-associated phenotype by multi-parameter flow cytometry, comprising ≥ 20% of total cellularity, corresponding to the 2008 WHO classification of acute myeloid leukemia |
| Minor response | Absence of circulating blasts in the peripheral blood Persistent marrow blasts ≥ 5% but < 20% of total cellularity FLT3-mutant allele detectable by polymerase chain reaction (PCR) from a bone marrow aspirate sample |
| Major response | Absence of circulating blasts in the peripheral blood Minimal residual disease, blasts < 5% of total marrow cellularity FLT3-mutant allele detectable by PCR from a bone marrow aspirate sample |
| Major molecular response | Absence of circulating blasts in the peripheral blood No morphologic evidence of leukemia by morphology or flow cytometry FLT3-mutant allele not detectable by PCR from a bone marrow aspirate sample |
This classification scheme is independent of any platelet or red cell transfusion requirements. The categories of No Response and Minor Response essentially amount to the persistence of disease that is readily discernible by conventional light microscopic techniques. A Major Response is the reduction of the tumor burden to a minimal residual disease (MRD) state, in which the presence of the FLT3/ITD clone is detectable only with multi-parameter flow cytometry and PCR. A Major Molecular Response indicates the absence of any detectable evidence of the FLT3/ITD clone by any conventionally available methodologies. The latter two categories would be expected to be associated with clinical benefit, a hypothesis which could be tested in well-designed randomized trials using overall survival as an end point.
FLT3: FMS-like tyrosine kinase-3; ITD: Internal tandem duplications.
3. FLT3 inhibitors investigated in clinical trials
Midostaurin (PKC412; N-benzoylstaurosporin), a multi-targeted tyrosine kinase inhibitor (TKI) with activity against PKC-α, VEGFR2, KIT, PDGFR and FLT3 tyrosine kinases [34] demonstrated some encouraging anti-leukemic activity in multiple clinical trials. In a Phase IIB trial of 95 patients with either FLT3 wt or mutated AML or myelodysplastic syndrome (MDS), the administration of twice daily 50 mg midostaurin resulted in a greater than 50% reduction of peripheral blood or bone marrow blasts in 42% (wt) and 71% (FLT3 mutant) of patients [35]. When combined with conventional chemotherapy in younger, newly diagnosed AML patients, midostaurin induced high remission and survival rates in both FLT3-mutated and wt patients [36]. The combination of midostaurin with a hypomethylating agent, such as decitabine (DEC), furthermore, demonstrated synergistically enhanced antileukemic activity in a Phase I study in elderly AML patients [37]. Phase III clinical trials of midostaurin are currently ongoing. To this end, the CALGB is currently spearheading an international, randomized, double-blind, placebo-controlled Phase III trial (RATIFY) in treatment-naive FLT3-mutated AML patients younger than 60 years of age (ClinicalTrials.gov Identifier: NCT00651261). This trial encompasses induction chemotherapy (daunorubicin/cytarabine) and four postremission cycles of high-dose cytarabine combined with placebo or midostaurin, followed by midostaurin maintenance or placebo for 1 year. Accrual started in 2008 and completed in 2013 (R. Stone, pers. commun.). The indolocarbazole derivative lestaurtinib (CEP-701), a small molecule inhibitor of JAK2, TrkA, TrkB, TrkC and FLT3, displayed cytotoxic, dose-responsive inhibition of FLT3 in vitro and in vivo. In addition, the administration of lestaurtinib was associated with an improvement in survival in mouse models of FLT3/ITD-mutated leukemia [38], which altogether provided the framework for further evaluation in clinical trials. Data from Phase I/II trials of single-agent lestaurtinib as salvage treatment for partially heavily pretreated patients with refractory, relapsed or poor-risk AML carrying FLT3-activating mutations indicated clinical activity in the context of significant reductions of peripheral blood and bone marrow blasts [39]. These findings also held true in a Phase II trial of elderly, newly diagnosed and treatment-naive AML patients considered unfit for intensive chemotherapy [40]. However, combination studies of salvage chemotherapy followed by lestaurtinib in FLT3-mutated AML in first relapse did not show any significant difference in remission rates or benefit in survival. The authors reported that only a small proportion of patients achieved sustained FLT3 inhibition in vivo and therefore only limited conclusions regarding the efficacy of FLT3 inhibition combined with chemotherapy could be made [13]. Sorafenib (Nexavar), a small molecule multi-targeted kinase inhibitor targeting VEGFR, PDGFR, Raf kinase and FLT3, has been approved by the FDA for the treatment of advanced renal cell cancer and hepatocellular carcinoma. Studies in Ba/F3 AML cell lines demonstrated that sorafenib effectively induces growth arrest and apoptosis in blasts harboring FLT3/ITD mutations. Furthermore, sorafenib exhibited marked anti-leukemic effects by decreasing the leukemic burden and prolonging median survival (36.5 days [sorafenib] vs 16 days [vehicle]) in a mouse leukemia xenograft model [41] indicating therapeutic efficacy in AML. In an open-labeled single-arm study, the majority of relapsed or refractory FLT/ITD+ AML patients treated with sorafenib experienced clearance or near complete clearance of bone marrow blasts along with evidence of myeloid differentiation. However, the initial response was lost in most patients after 72 days [28]. When studying the effects of sorafenib combined with idarubicin and cytarabine in FLT3-mutated patients, Al-Kali et al. reported overall response rates of 100% (CR = 16/18, CR with incomplete platelet recovery = 2/18). However, after a median follow-up of 9 months more than half of the patients relapsed in the absence of any acquired FLT3 resistance mutations. The authors concluded that the combination of sorafenib with chemotherapy is feasible and effective in inducing remissions but that yet unknown mechanisms of resistance may develop over time [42]. In a Phase II study of relapsed or refractory, FLT3/ITD+ AML, the combination of sorafenib and the hypomethylating agent azacitidine yielded response rates of 46%, consisting of CR, CR with incomplete count recovery (CRi) and partial response (PR) rates of 16, 27 and 3%, respectively [43], suggesting that the combination of the two drugs may represent a clinically valuable regimen for relapsed, FLT3/ITD+ AML. Quizartinib (AC220), a second-generation, small molecule inhibitor with activity against FLT3, PDGFR and KIT demonstrated unique selectivity and potency in cell culture assays and animal models [44]. Early phase trials of quizartinib revealed promising results as a single agent in patients with relapsed or refractory FLT3/ITD AML [45,46]. In contrast to other FLT3 inhibitors such as lestaurtinib and midostaurin which inhibit multiple other kinases besides FLT3, quizartinib is much more selective and associated with fewer side effects, less protein bound and therefore up to 50 times more potent in vivo. Furthermore, its half-life of greater than 24 h appears optimal given the fact that sustained FLT3 inhibition is essential to exert meaningful cytotoxicity against leukemic blasts [47,48]. In this context, treatment response is mainly represented by a rapid clearance of peripheral blasts through induction of apoptosis and terminal myeloid differentiation in the bone marrow compartment [27]. Preliminary data from several trials suggest that quizartinib, combined with conventional induction and consolidation chemotherapy in younger and older patients with newly diagnosed AML is feasible and safe based upon which multiple Phase III studies are currently being planned.
4. Resistance mutations
Although FLT3 inhibitors induce clinical remissions in the majority of patients, drug-resistant disease frequently develops within the first 12 months of treatment [28,49]. This in some ways parallels the clinical course of chronic myeloid leukemia (CML) treated with imatinib, where imatinib-resistant clones appear in a number of patients during therapy [50–52]. However, while drug resistance in CML is most commonly conferred by point mutations within the Abl kinase domain of the Bcr-Abl gene, the genetic basis for resistance to FLT3 inhibitors in AML blasts are less well understood. Based on studies on AML cell lines and primary cells, distinct patterns of resistance – FLT3-dependent and FLT3-independent – have been described, such as increased expression of the FLT3 receptor and its ligand, acquired mutations in the TKD of FLT3 or in other kinase genes, development of FLT3-independent pathways of signal transduction and increased expression of antiapoptotic proteins [53–57]. The acquisition of secondary FLT3 kinase domain (FLT3 KD) mutations have been recognized as one of the most common mechanisms of resistance to FLT3 inhibitors [49,58–60]. Moore et al. established FLT3 inhibitor-resistant cells by incubating MOLM-13 cells in the presence of increasing doses of MLN518, a selective FLT3 inhibitor. After an incubation period of ~ 7 weeks, a D835Y TKD mutation conferring high relative resistance to quizartinib and sorafenib was detected by FLT3 mutational analysis. In contrast, MOLM-13 cells cultured in parallel in the absence of MLN518 were negative for the mutation [61]. FLT3 KD mutations most often occur at residues D835 (F/V/Y) and Y842 (‘activation loop residue’) and F691L (‘gatekeeper residue’), leading to a gain of function in the context of constitutive tyrosine phosphorylation and activation of the RTK through stabilization of the activation loop of the open ATP-binding configuration [5,49]. Data from CML patients treated with imatinib have shown that resistance-conferring mutations in the Abl kinase domain occur prior to imatinib treatment in some patients [62]. Similarly, Man et al. demonstrated that FLT3/ITD/TKD clones are in some cases present prior to therapy with a FLT3 inhibitor. In their study of 13 relapsed or chemo-refractory FLT3/ITD-positive AML patients, the authors unveiled potential mechanisms contributing to the nonresponsiveness to sorafenib after an initial response. Utilizing polymerase chain reaction (PCR) assays followed by allele-specific EcoRV digestion Man et al. reported that a D835Y mutation that was previously not detectable in treatment-naive blasts became detectable upon engrafting in NOD/SCID mice in three out of six patients who have developed resistance to sorafenib, suggesting that the mutation was already present in the leukemia-initiating cell population. Furthermore, in one patient who did not show an ITD-D835 mutation in the leukemia-initiating clone in treatment-naive blasts, the double mutation subsequently emerged and became detectable when the engrafted mice were treated with sorafenib [28]. A novel mechanism of resistance to sorafenib, and potentially other FLT3 inhibitors, was recently reported by Man et al. Their work on FLT3/ITD-mutated cell lines demonstrated that tescalcin, a recently discovered protein which is frequently upregulated at leukemia progression, promotes an increase in intracellular pH (pHi) by direct interaction with the Na+/H+ exchanger type 1. The authors postulated that lowering the pHi might abrogate FLT3 activity, induce apoptosis and foster cell cycle progression [63].
5. Patient categories
Emerging data from clinical trials suggest that the incorporation of FLT3 inhibitors into current AML therapy depends on several factors, such as the disease setting (newly diagnosed vs relapsed or refractory) and the intended treatment regimen (single agent vs combined therapy).
6. Newly diagnosed FLT3/ITD+ AML – younger versus older patients
Because the disease consists of multiple leukemic clones at the time of diagnosis and therefore appears to be less FLT3 signaling ‘addicted’ [10], selective FLT3 inhibition by itself is unlikely to achieve clinically significant antileukemic effects. Although conventional induction regimens have yielded similar remission rates in FLT3/ITD+ and FLT3/ITD− AML patients, several studies were conducted with the goal to make the best use of the FLT3 target by incorporating a FLT3 inhibitor into the induction regimen, thus improving treatment response. To this end, a Phase I/II study of sorafenib combined with idarubicin and cytarabine in young adults (age 18 to 65 years) with AML reported that FLT3-mutated patients were more likely to achieve a CR than FLT3 wt patients (p = 0.033). In this study, 14 out 15 patients (93%) with FLT3-mutated AML achieved a CR compared to 24 out of 36 patients (66%) with FLT3 wt disease [64]. In contrast, investigators of a more recent, randomized placebo-controlled trial reported that sorafenib combined with intensive chemotherapy is not beneficial for elderly patients. In this trial, treatment-related mortality and CR rates were indeed higher in the sorafenib arm when compared to the standard induction arm. However, the authors appreciated that the lack of benefit in the sorafenib arm may in part be due to upregulated FLT3 ligand (FL) levels post-chemotherapy and that different scheduling regimens of sorafenib, such as concurrent with chemotherapy, may result in a more favorable outcome [65]. Consequently, whenever a combined approach is used, the involved drugs should be sequenced in accordance to their cell cycle specificity and FL-inducing effects in order to avoid antagonism and optimize synergy [66]. As of yet, the most appropriate treatment regimen for newly diagnosed FLT3/ITD+ AML remains undefined. Given the enormous molecular and genetic diversity of leukemic clones at presentation, the natural aggressiveness of the disease and the historically poor treatment outcome, intensive treatment protocols consisting of induction chemotherapy followed by SCT in CR1 represents a widely accepted treatment approach, if feasible. While the incorporation of FLT3 inhibitors into these regimens appears as an attractive option, additional clinical trials are warranted to expand our experience with these promising agents. Therefore, any newly diagnosed FLT3-mutated patient, irrespective of age, should be guided to a clinical trial whenever possible.
7. Relapsed/refractory FLT3/ITD+ AML – younger versus older patients
Relapsed or refractory FLT3/ITD+ AML constitutes a clinical dilemma, irrespective of the patient’s age group. In theory, being derived from an initially declining, heterogenous leukemic clone, the leukemic blasts presenting at relapse are likely more homogenous in their molecular and genetic architecture, more ‘addicted’ to distinct, survival promoting signaling pathways and therefore expected to be more ‘drugable’. However, virtually, all patients with relapsed/refractory FLT3/ITD+ AML succumb to their disease within a few months, despite intensive treatment regimens. Lines of evidence suggest that the dismal prognosis at relapse may be correlated with a larger initial tumor burden [67], which is consistent with the highly proliferative nature of FLT3/ITD+ AML. In a recent study of quizartinib in patients with relapsed/refractory AML who failed second-line chemotherapy or hematopoietic SCT, selective FLT3 inhibition with quizartinib yielded high response rates. In one cohort of FLT3/ITD+ (n = 99) and FLT3/ITD− (n = 38) AML patients older than 18 years with relapsed/refractory disease, single-agent quizartinib induced composite CR rates (CRc, CRc = CR with incomplete platelet recovery [CRp] + CR with incomplete hematologic recovery [CRi]) of 44% with a median duration of 11.3 weeks and median OS of 23.1 weeks in the FLT3/ITD+ group. The CRc rate in FLT3/ITD− patients was 34% with a median response duration of 5.0 weeks and a median OS of 25.6 weeks. In another cohort of elderly patients (60 years and older) with either FLT3/ITD+ (n = 92) or FLT3/ITD− (n = 41) AML, CRc rates of 54 and 32% were observed, respectively. The median CRc duration was 12.7 weeks with a median OS of 25.3 weeks in the FLT3/ITD+, and 22.1 weeks with a median OS of 19.0 weeks in the FLT3/ITD− group. Combining the cohorts, 79% of FLT3/ITD+ AML patients who did not respond to prior therapy achieved at least a PR. Moreover, a total of 37% of patients were successfully bridged to an allogeneic hematopoietic SCT and achieved a median OS of 33.3 weeks, whereas patients not bridged to transplant showed a median OS of 17.7 weeks [47,68]. Based on these observations, treatment of relapsed/refractory FLT3/ITD+ AML patients of all age groups with a selective FLT3 inhibitor such as quizartinib, followed by hematopoietic SCT, if feasible, has emerged as a promising strategy to improve the outcome of a patient population that is known to be very difficult to treat. The clinical value of FLT3 inhibitors as maintenance therapy in the post-transplant setting, as well as for patients unable to undergo SCT, remains to be further evaluated on a wider scale.
8. Relapsed/refractory FLT3/ITD+ with TKI-resistance mutation, de novo D835 mutation
In the relapsed or refractory setting where previous treatment may have fostered the development of FLT3-mutated clones, selective FLT3 targeting has come forth as a beneficial strategy. However, resistance-conferring mutations in the FLT3 TKD, commonly at residue D835, are increasingly seen following FLT3 inhibitor therapy. Likewise, FLT3/D835 mutations have frequently been described de novo in the absence of ITD mutations, which has furthered the development of second-generation compounds. To this end, ponatinib (AP24534), a multikinase inhibitor initially designed to be effective against TKI-resistant, mutated CML, also demonstrated activity against FLT3/ITD+ AML in preclinical studies. Herein, Zirm et al. reported that ponatinib exerted pro-apoptotic effects against FLT3/ITD and FLT3/TKD (N676D, F691I or G697R) mutated cell lines, which was further mirrored in the potent inhibition of the FLT3/ITD protein as well as its downstream effectors STAT5, AKT and ERK1/2 in western blot assays [69]. Similarly, upon evaluating the in vitro effects of ponatinib against TKI-resistance associated, FLT3/ITD mutant isoforms, another group showed that ponatinib conferred antileukemic activity against F691L-mutated blasts but not against blasts carrying D835 mutations, which exhibited a high level of resistance, suggesting that other treatment strategies are needed for these patients [70].
Crenolanib, a benzamidine quinolone derivative and potent, selective FLT3 inhibitor, has shown promising activity in preclinical and clinical studies [71–73]. In cell culture assays, crenolanib demonstrated substantially higher binding affinity for the dual FLT3/ITD/D835V mutant than quizartinib and conferred pro-apoptotic and anti-proliferative effects in FLT3/ITD+ cell lines in the nanomolar range. In addition, treatment with crenolanib was associated with improved survival in a FLT3/ITD+ leukemia murine bone marrow transplant model [73]. When comparing the antileukemic effects of crenolanib to sorafenib and quizartinib in primary AML samples harboring different FLT3 mutations, including the D835 mutation, only crenolanib demonstrated activity, with an IC50 in FLT3 dephosphorylation of 2 nM in culture medium. Notably, crenolanib is 33-fold less active against c-Kit than against FLT3 and may therefore confer less myelosuppressive effects than quizartinib. This hypothesis was corroborated in colony-forming assays, where 200 nM of crenolanib yielded markedly less inhibition of erythroid colonies than quizartinib at the same dose (abundance of erythroid colonies: 75% [crenolanib] vs 23% [quizartinib]) [72]. Clinical data on crenolanib in 19 heavily pretreated patients with relapsed or refractory, mutated AML (FLT3/ITD, FLT3/D835, FLT3/ITD/D835) were recently reported [74]. In this trial, nanomolar concentrations of crenolanib (median concentration: 473 nM) were well tolerated and associated with a rapid molecular and clinical CR with full count recovery in one patient, a CR with incomplete count recovery in two patients and a PR in four patients. Four patients were bridged to transplant. In sum, crenolanib represents a promising treatment option for FLT3/D835-mutated AML patients. A number of clinical trials to further evaluate the effects of crenolanib in TKI-naive and TKI pretreated FLT3-mutated AML patients are currently under way (NCT01657682, NCT01522469).
9. Preclinical and clinical rationales for combined treatment approaches
AML is a disease of the elderly patient. Unfortunately, this patient population has a generally dismal prognosis secondary to a disease biology that frequently arises out of a preexisting disorder, such as MDS. Furthermore, elderly patients are typically unable to tolerate the intensive induction and consolidation regimens used in younger patients. Although younger patients with FLT3/ITD+ AML seem to benefit from allogeneic transplant, this is not an option for many older patients. An effective treatment regimen for the elderly patient with FLT3/ITD+ AML, therefore, represents a significant unmet need in this field. Many hematologists and oncologists frequently choose not to aggressively treat elderly patients or to guide them to a clinical trial, due to concerns for reduced tolerance as indicated by a report from the Swedish Leukemia Registry [75]. However, taking into consideration that AML patients who achieve a remission often report improved quality of life and require less hospitalizations and supportive care, one may conclude that remission induction may be beneficial not only from a clinical but also from an economical point of view.
10. Hypomethylating agents have proven beneficial in AML, including in elderly patients
Methylation refers to the biochemical process of controlling gene expression without changing the DNA sequence. Deviant methylation of gene promoter CpG islands represent commonly observed epigenetic alterations in cancer cells [76] and may play a critical role in leukemogenesis [77]. Indeed, hypermethylation of tumor suppressor gene promoters, such as ER, P15 and/or P16, have been described in a wide range of human hematopoietic tumors, including AML [78–80]. As a consequence, hypomethylating agents are currently under comprehensive evaluation for their antileukemic potential in AML, either as single agents or in combination with other compounds.
11. FLT3 inhibition confers pro-apoptotic effects on peripheral leukemic blasts but induces differentiation in bone marrow blasts
It has recently been shown that potent and selective inhibition of FLT3 results in rapid clearance of peripheral blasts by induction of apoptotic cell death in most cases of FLT3/ITD-mutated AML. However, rather than inducing apoptosis in bone marrow blasts, selective FLT3 inhibition led to terminal myeloid differentiation occurring during the 4 weeks of treatment followed by a surge of leukemia-derived neutrophils in the peripheral blood during the next 4 weeks, as reported by studies conducted by Sexauer et al. Although these neutrophils resembled normal neutrophils in morphology and lacked FLT3 protein expression, they still carried the FLT3/ITD mutation which indicated that they were derived from the malignant clone. In line with a differentiation process, flow cytometric analysis revealed loss of expression of CD34 and CD117, along with gained expression of CD15 [27]. From a clinical point of view, the reported neutrophil surge into the peripheral blood was often accompanied by a steroid-responsive differentiation syndrome similar to the differentiation syndrome observed in acute promyelocytic leukemia patients treated with all trans-retinoic acid. A recently published case series by Fathi et al. further substantiated this finding by reporting that dermatopathologic evaluation of skin nodules presenting after initiation of FLT3 inhibition for FLT3/ITD-mutated AML revealed neutrophilic infiltrates that were uniformly positive for the FLT3/ITD transcript on PCR analysis [29].
12. FL surge post-chemotherapy impedes effects of FLT3 inhibitors
FL constitutes an early hematopoietic growth factor and ligand for the FLT3 receptor. FL is predominantly synthesized and released by bone marrow microvascular endothelial cells [81], T cells [82] and by leukemic cells in an autocrine fashion [83]. FL stimulates the proliferation and colony formation of human hematopoietic progenitor cells and acts synergistically with other cytokines such as G-CSF, M-CSF, IL-3 and SCF. As such, highly elevated serum levels of FL have been described in stem-cell-deficient conditions in the bone marrow [84,85] as an attempt to restore a depleted hematopoietic stem cell compartment. A rapid and massive increase in FL levels that is frequently sustained for several weeks has also been described in response to induction and consolidation chemotherapy for AML. This poses a major problem for FLT3-targeted therapy, because FL acts directly on the mutated receptor, elicits a proliferative response in leukemic blasts [86] and therefore impedes the effects of the most potent FLT3 inhibitors as demonstrated in several clinical trials. For example, in a randomized trial of lestaurtinib administered in sequence with chemotherapy (mitoxantrone, etoposide and cytarabine) for FLT3-mutated AML patients in first relapse (Cephalon 204 trial), the FLT3 inhibitor failed to improve the clinical outcome. In this trial, in vivo FLT3 inhibition was observed in only 58% of patients which correlated with the remission rate. Quantification of FL baseline levels in the plasma of the lestaurtinibarm patients were consistently < 20 pg/ml but markedly rose to levels greater than 1000 pg/ml in many cases, and partially remained elevated at the outcome assessment [13]. A similar increase in FL levels was observed in the MRC AML 15 trial, a randomized trial of lestaurtinib administered in sequence with chemotherapy (cytarabine, daunorubicin and etoposide) for newly diagnosed AML patients with FLT3-activating mutations. Here, mean FL levels rose higher with each subsequent course of chemotherapy [57]. Results from both trials indicated that FL levels may remain elevated for several weeks after initiation therapy. In vitro studies on Molm14 cells, which harbor the FLT3/ITD mutation, further confirmed the FL induced, obviating effects on FLT3 inhibitors. When incubating Molm14 cells in the presence and absence of exogenous FL, the presence of FL, even at low concentrations (1 ng/ml), was associated with significant autophosphorylation of the FLT3 receptor that cannot be overcome by the most potent FLT3 inhibitors lestaurtinib, midostaurin, sorafenib, quizartinib and KW-2449. Of note, although being constitutively activated in FLT3/ITD, the FLT3 receptor remains highly sensitive to stimulation by FL [87]. It appears therefore likely that successive rounds of chemotherapy promote survival of FLT3/ITD-positive leukemic blasts through upregulation of FL. In line with this hypothesis, many newly diagnosed patients with FLT3/ITD AML achieve remission in response to conventional chemotherapy but eventually relapse during consolidation.
13. DNMTis are less intense and do not induce a surge of FL
A number of clinical trials have clearly demonstrated that hypomethylating agents have single-agent activity, consisting of remission induction and improvement of survival and quality of life, in elderly patients with AML and MDS [88–91]. Based on their potential clinical benefits and favorable toxicity profile, hypomethylating agents have become attractive compounds in the treatment of AML, either as monotherapy or combined with other agents. Furthermore, the finding that these agents do not appear to induce large magnitude increases in FL levels [43] translates into a clear rationale of combining DNA methyltransferase inhibitors (DNMTis), as well as other inhibitors from the same drug class, with a potent FLT3 inhibitor. As the degree of FLT3 inhibition closely correlates with the degree of induced cytotoxicity, a combination of a FLT3 inhibitor with an agent that leaves FL levels unaffected offers the theoretic potential to achieve a higher degree of antileukemic activity.
14. DNMTis and FLT3 TKIs confer apoptotic and differentiation-inducing effects
Several studies on cancer cells, including AML cell lines and primary AML cells, indicate that hypomethylating agents, at clinically relevant doses, confer pro-apoptotic effects. In one study, treatment of Kasumi cells with DEC resulted in apoptotic cell death, expression of TNF-related apoptosis-inducing ligand (TRAIL) and demethylation of the CpG-A element [92]. These findings are in line with the report by Lund et al., who found that DEC induces apoptosis in HRAS (G12V) transformed cells by upregulation of endogenous TRAIL in concert with favorable regulation of co-factors acting in TRAIL-mediated apoptosis [93]. Similarly, stimulation of the MDS/AML derived SKM-1S cells with vidaza resulted in upregulation of the pro-apoptotic protein FOXO3A as well as its targets BIM and PUMA [94]. In a different study, treatment of KG-1a cells with either DEC or 5-azacytidine for 48 h resulted in an increased fraction of cells undergoing apoptosis as detected by flow cytometry. Interestingly, DEC-treated cells demonstrated a more potent upregulation of pro-apoptotic markers, whereas a higher degree of cell kill was observed with 5-azacytidine indicating that 5-azacytidine-induced cell death may be mediated by mechanisms other than apoptosis alone [95].
The regulation of apoptosis in FLT-3-mutated leukemic cells is largely regulated by the constitutively activated FLT3 receptor and its downstream effectors STAT5, PI3K-Akt and ERK which jointly interact with the pro- and anti-apoptotic proteins BAD, and Bcl2, Bcl-XL, and Mcl-1, respectively. As leukemic transformation is mainly mediated by accelerated proliferation and suppressed apoptosis [96,97], inhibition of FLT3 signaling may theoretically result in increased induction of apoptosis and thereby eliminate leukemic cells. Indeed, FLT3 inhibition by bis(1H-indol-2-yl) methanones effectively induced apoptosis in FLT3/ITD transfected murine myeloid cells and in primary FLT3/ITD-positive blasts as indicated by PARP cleavage. Correlative studies demonstrated dose-dependent de-phosphorylation of FLT3/ITD as well as its downstream effectors STAT5, PI3K-Akt and ERK [98]. In a different study, Fl-700, a selective FLT3 inhibitor, potently induced G1 arrest and apoptosis in the FLT3/ITD-expressing AML cell lines Molm13 and MV4-11R cells after a 48 h incubation period. On a molecular level, these effects were mediated by rapid and marked downregulation of the anti-apoptotic protein Mcl-1 at 2 h, whereas other Bcl-2 protein members were largely unaffected [99]. Similarly, 24-h treatment of MV4-11 and M14 cells with quizartinib, to date the most effective and selective FLT3 inhibitor, resulted in induction of apoptosis in a dose-dependent manner along with a rapid decrease in FLT3, STAT5 and Erk1/2 phosphorylation [100].
It has been postulated that reduced expression or loss of function of the CEBPA, a major regulator of hematopoiesis, plays a key role in leukemogenesis by inhibiting myeloid differentiation [101,102]. In this context, functional deprivation of C/EBP-α can occur either through aberrant methylation in the upstream promoter [103] or through ERK1/2-mediated phosphorylation [26], or both. In vitro studies in Kasumi-1 and CD34+ RUNX-ETO expressing cord blood cells demonstrated that clinically achievable concentrations of DEC induced morphologic signs of terminal differentiation, while maintaining normal hematopoietic self-renewal capacity. In this study, DEC-induced hypomethylation was greatest at promoter CpGs that are hypomethylated with myeloid maturation and accompanied by cellular differentiation [104]. An in vitro comparison of the antileukemic effects of 5-azacytidine and DEC in AML cell lines indicated that both drugs share mechanistic, DNA-specific effects but display different effects on protein synthesis, cell cycle regulation and gene expression. Gene expression analysis in KG-1a cells using microarrays demonstrated that 5-azacytidine regulated > 1000 genes and was more potent than DEC at two different time points. Moreover, pathway analysis revealed that 5-azacytidine predominantly downregulated genes involving cell cycle, cell division and apoptosis, whereas DEC, in contrast to 5-azacytidine, more potently upregulated genes involved in cell differentiation (p = 0.00024 [vidaza], p = 4.4E-12 [DEC]) [95]. In vitro studies on FLT3/ITD-expressing 32D cells demonstrated that inhibition of FLT3 by lestaurtinib induced granulocytic differentiation [105]. This release of the differentiation block appears to represent an ‘on-target’ effect of FLT3 inhibition because in vitro differentiation has successfully been induced with several other FLT3 inhibitors [26,27].
To summarize, by virtue of their differentiating effects, their lack of induction of an FL surge, and their relative toleratibility, hypomethylating agents represent an intriguing class of drug to combine with FLT3 inhibitors. A number of such trials are actively accruing patients at his time.
15. Conclusions
ITD and point mutations in the FLT3 gene constitutively activate the cytokine receptor and lead to uncontrolled proliferation of leukemic blasts by upregulated anti-apoptotic and growth signaling pathways. While remissions, although short lived, can in most cases be achieved with initial induction therapy they are much less likely to occur in the relapsed setting which provides a strong rationale for the current approach to consider consolidation therapy with allogeneic SCT in CR1. Consistent with a complex, adaptive system seen in many aggressive malignancies [106], FLT3-mutated AML evolves from a polyclonal state at diagnosis to a dominant, ‘FLT3 addicted’ clone at the time of relapse. To tackle this issue, several FLT3 inhibitors have been developed and evaluated in clinical trials (Table 2). While a rapid clearance of leukemic blasts in the peripheral blood is frequently seen, most FLT3 inhibitors only confer limited effects on bone marrow blasts. Recent reports on more potent, second-generation FLT3 inhibitors, such as quizartinib, suggest that high levels of sustained FLT3 inhibition induces cell cycle arrest and terminal myeloid differentiation rather than apoptotic cell death. The protective effect of the bone marrow microenvironment on leukemic blasts, partially mediated through persistent activation of survival signaling pathways and expression of FL, represents a significant obstacle in successfully eradicating the disease and founded the framework for combined treatment approaches with other agents. To this end, preclinical and clinical studies identified hypomethylating compounds and MEK inhibitors as promising agents [43,107], particularly for elderly patients who are frequently not able to tolerate intensive treatment regimens. Although the identification of a FLT3 mutation has not yet changed remission-induction and consolidation strategies using approved protocols, the incorporation of a FLT3 inhibitor into these treatment paradigms appear inevitable in the near future. Another treatment paradigm for the use of a FLT3 TKI may be derived from the treatment of Philadelphia chromosome-positive acute lymphocytic leukemia (Ph+ ALL) and high-risk CML where TKIs are sometimes offered as maintenance therapy after SCT. Herein, several trials demonstrated that the patients receiving a prophylactic TKI after transplant were more likely to remain minimal residual disease (MRD) negative [108–110]. However, prior to the use of a TKI in the post-transplant setting for remission maintenance therapy several issues need to addressed, such as the timing of the TKI (early after the transplant vs at the time of MRD detection), the duration of therapy, and the willingness of the patient to undergo frequent MRD monitoring. Several advanced phase, pivotal clinical trials will need to be completed to optimize the management of FLT3/ITD-mutated patients in the newly diagnosed, relapsed/refractory and post-transplant setting.
Table 2.
FLT3 inhibitors under active clinical investigation either as single agent or in combination.
| TKI | Trial phase | Patient population | Single agent/ combination | Ref./identifier | Status |
|---|---|---|---|---|---|
| Sunitinib | I | Patients with all AML FAB classification types, except AML M3 (n = 29) | Single agent | [115] | Completed |
| I | Patients with primary or secondary AML; relapsed after at least 1 cycle of conventional chemotherapy; refractory to at least 1 cycle of induction chemotherapy; or not candidates for induction chemotherapy; any AML FAB subtype; at least 18 years of age (n = 16) | Single agent | [116] | Completed | |
| I/II | Patients aged 60 years or higher with AML with activating FLT3 mutations (FLT3/ITD, FLT3/ TKD); fit enough for intensive chemotherapy (n = 22) | Combination with standard induction and consolidation therapy | [117] | Completed | |
| Sorafenib | I | Patients with refractory or relapsed AML; median age 61.5 years; median of 2 prior therapies (n = 16) | Single agent | [41] | Completed |
| I | Relapsed, chemotherapy-refractory or frail FLT3/ITD AML patients ineligible for alternative treatments (n = 65) | Single agent | [118] | Completed | |
| I/II | AML patients younger than 65 years (n = 61) | Combination with conventional cytotoxic chemotherapy (AraC, idarubicin) | [64] | Completed | |
| II | Elderly AML patients (n = 197) | Combination with AraC/daunorubicin induction followed by consolidation and sorafenib maintenance | [119] | Completed | |
| I/II | AML by FAB criteria or high-risk MDS defined as IPSS category of intermediate-2 or greater; patients 60 years and older | Combination with low-dose cytarabine | NCT00516828 | Completed | |
| I | FLT3/ITD AML patients who have undergone allogeneic HSCT; age 18 to 75 years | Single agent | NCT01398501 | Recruiting | |
| I | FLT3/ITD AML patients in CR or PR who plan to undergo bone marrow transplantation; age 18 years or older | Single agent | NCT01578109 | Recruiting | |
| I | Relapsed/refractory FLT3/ITD AML; age 18 years and older | Combination with G-CSF and plerixafor/mozobil | NCT00943943 | Ongoing, not recruiting | |
| I | AML patients age 60 years and older and not candidates/refuses standard induction treatment or who have one of the following: poor-risk cytogenetics, AML following antecedent hematologic disorders, or therapy-related AML; patients with relapsed or refractory AML age 18 years are also eligible | Combination with several regimens, including bortezomib and DEC | NCT01861314 | Recruiting | |
| I/II | Patients with newly diagnosed, refractory or relapsed AML, poor-risk or complex cytogenetics, del5, del7 or FLT3/ITD; age 18 years or older | Combination with vorinostat and bortezomib | NCT01534260 | Recruiting | |
| I | Patients with AML or MDS; patients with APL if refractory to ATRA and arsenic trioxide; age 18 years and older | Combination with vorinostat | NCT00875745 | Ongoing, not recruiting | |
| I | Patients with MDS, CMML or AML who have failed prior therapy | Combination with 5-azacitidine | NCT01254890 | Ongoing, but not recruiting | |
| III | Patients with newly diagnosed AML; age up to 29 years | Combination with bortezomib | NCT01371981 | Recruiting | |
| Midostaurin | I | Previously untreated AML patients; age 18 – 60 years (n = 69) | Combination with conventional cytotoxic chemotherapy (daunoru-bicin/cytarabine induction, Ara-C consolidation) | [36] | Completed |
| II | Patients with FLT3 mutated, relapsed/refractory AML or high-grade MDS; not candidates for chemotherapy; median age of 62 years (n = 20) | Single agent | [120] | Completed | |
| I | Patients with relapsed or refractory AML; Patients with secondary AML; age 18 years and older | Combination with bortezomib and conventional chemotherapy | NCT01174888 | Recruiting | |
| II | Patients with suspected diagnosis of AML or related precursor neoplasm, or acute leukemia of ambiguous lineage; age 18 – 70 years | Combination with conventional chemotherapy | NCT01477606 | Ongoing, not recruiting | |
| II | Patients with FLT/ITD-mutated AML who have undergone allogeneic HSCT in CR1; age 18–60 years | Combination with conventional chemotherapy | NCT01883362 | Recruiting | |
| II | Patients with c-KIT or FLT3/ITD mutated t(8;21) AML; age 18 – 65 years | Combination with conventional chemotherapy | NCT01830361 | Recruiting | |
| II | Patients with newly diagnosed AML; except t(15;17); age 60 years and older | Combination with DEC | NCT01846624 | Recruiting | |
| II | Patients with AML, MDS (RAEB-1, −2) or CMML who are either relapsed or refractory to standard therapy, or are considered inappropriate candidates for standard therapy; age 18 years and older | Combination with RAD001 | NCT00819546 | Ongoing, not recruiting | |
| I/II | Patients with MDS, CMML, AML or biphenotypic or bilineage leukemia who have failed prior therapy; patients must have evidence of FLT3-activating mutations; age 18 years and older | Combination with 5-azacitidine | NCT01202877 | Ongoing, not recruiting | |
| I/II | Elderly AML patients (APL excluded); age 60 years and older | Combination with 5-azacitidine | NCT01093573 | Recruiting | |
| III | AML patients with activating FLT3 mutations; patients with antecedent MDS allowed, provided they received no prior cytotoxic therapy (including azacitidine or DEC); age 18–59 years | Combination with standard cytotoxic chemotherapy (daunoru-bicin/cytarabine induction with cytarabine consolidation) | NCT00651261 | Ongoing, not recruiting | |
| Lestaurtinib | I/II | Patients with refractory, relapsed or poor-risk AML; median age 61 years (n = 14) | Single agent | [39] | Completed |
| III | Patients with AML and activating FLT3 mutation (FLT3/ITD or FLT3/ D835); age 18 years and older (n = 224) | Combination with standard cytotoxic chemotherapy | [13] | Completed | |
| III | British MRC trial, AML patients < 60 years considered suitable for intensive chemotherapy (n = 2800) | Combination with standard induction and consolidation regimens | ISRCTN55675535 | Completed | |
| Crenolanib | II | Patients with relapsed/refractory primary AML or AML secondary to antecedent hematologic disorder; age 18 years and older | Single agent | NCT01522469 | Active, not recruiting |
| Patients with primary AML relapsed or refractory after prior therapy; AML secondary to antecedent chemotherapy or radiation therapy, or AML due to prior MDS/MPN with presence of either FLT3/ITD and/or other FLT3 activating mutation; age 18 years and older | Single agent | NCT01657682 | Recruiting | ||
| PLX3397 | I/II | Patients with primary AML, prior-chemotherapy-related AML or AML secondary to an antecedent hematologic disorder; in at least first relapse or refractory AML; positive for FLT3/ITD; age 18 years and older | Single agent | NCT01349049 | Recruiting |
| Quizartinib | I | Patients with primary or secondary AML (excluding APL), refractory to standard chemotherapy, relapsed after one or more cycles of induction chemotherapy or not candidate for standard chemotherapy; age 18 year or older | Single agent | [45] | Completed |
| II (cohort 1) | Elderly AML patients with relapsed/refractory disease; age 60 years and older (n = 134) | Single agent | [68] | Accrual completed | |
| II (cohort 2) | AML patients with relapsed disease or refractory to second-line salvage chemotherapy or relapsed after HSCT; age 18 years and older (n = 137) | Single agent | [47] | Accrual completed | |
| II | Patients with primary AML or AML secondary to MDS; relapsed or refractory after one second-line salvage regimen or after HSCT; FLT/ ITD positive; age 18 years and older | Single agent | NCT01565668 | Ongoing, not recruiting | |
| I | Patients with newly diagnosed AML; age 18–60 years | Combination with standard 7+3 induction, high-dose cytarabine consolidation, and quizartinib maintenance | NCT01390337 | Ongoing, not recruiting | |
| I | AML patients who have received a high dose or a reduced intensity conditioning allogeneic HSCT during first or second remission; age 18 years and older | Single agent | NCT01468467 | Ongoing, not recruiting | |
| I/II | Patients with AML (excluding APL and CML in blast crisis) or high-risk MDS; age 60 years and older | Combination with standard daunorubicin, ara-C and etoposide | NCT01236144 | Completed | |
| I/II | AML, MDS or CMML patients with relapsed/refractory disease; age 18 years and older | Combination with azacitidine or cytarabine | NCT01892371 | Recruiting |
AML: Acute myeloid leukemia; APL: Acute promyelocytic leukemia; ATRA: All trans-retinoic acid; CML: Chronic myeloid leukemia; CR: Complete response; DEC: Decitabine; FLT3: FMS-like tyrosine kinase-3; ITD: Internal tandem duplications; MDS: Myelodysplastic syndrome; PR: Partial response; TKI: Tyrosine kinase inhibitor.
16. Expert opinion
The most effective management of a patient with FLT3/ITD-mutated AML requires an understanding of the disease biology, including the evolution from diagnosis to relapse and the prognostic impact of the mutations in the context of other genetic lesions. Several compounds that target specific signaling pathways have brought major advances in leukemia therapy over the past decade. However, when administered as single agents development of resistance through upregulation of compensatory, collateral pathways is frequently encountered. In addition, bone marrow stromal cells further leukemic cell survival and mediate drug resistance mainly through activation of critical survival pathways such as Akt, Erk and Stat3 [111,112]. In part due to their undeviating response to FL, FLT3/ITD+ leukemia stem and progenitor cells, furthermore, appear to be better protected by bone marrow stromal cells than their wt counterparts [113]. Lines of evidence suggest that the development of resistance might be delayed or prevented if a combination of different targeted agents is used, irrespective of each agent’s activity in the monotherapeutic setting. Hence, combining a FLT3 inhibitor with conventional chemotherapeutic regimens, epigenetic modifiers or inhibitors of FLT3 downstream and collateral effectors has emerged as a potential strategy to overcome de novo and acquired resistance. Illustrating the potential benefits of a combined, targeted treatment approach, Weisberg et al. reported that the combination of BAG956, a dual PI3K/PDK-1 inhibitor, and midostaurin yielded additive to synergistic antiproliferative effects when tested against FLT3-mutated cell lines and AML primary cells [114]. More recently, Yang et al. showed that persistent activation of downstream ERK signaling mediates resistance to FLT3 inhibitor-induced apoptosis in leukemic blasts co-cultured with FL and stroma. In their study, combined inhibition of FLT3 and MEK signaling resulted in synergistic cytotoxicity in relapsed/refractory, and additive cytotoxicity in newly diagnosed FLT3/ITD+, primary AML samples [107], thereby providing a strong rationale for a potential benefit of combining FLT3 and MEK inhibitors. The hope is that a significant improvement in prognosis for all FLT3/ITD-mutated patients can be achieved by FLT3 TKI-based treatment protocols. Ongoing clinical trials might not only further our understanding of the molecular events leading to FLT3-mutated AML but also deliver more detailed information about mechanisms of resistance, which altogether will hopefully result in the development of novel targeted agents. The future of a molecular-based treatment approach for FLT3-mutated AML faces the challenge to effectively study novel compounds within small subgroups and molecular entities. When compared to solid malignancies such as breast, prostate, lung or colon cancer, AML presents with a relatively low incidence rate. Hence, large randomized trials conducted by a limited number of centers appear impracticable. In order to identify, recruit and enroll sufficient patient numbers, novel concepts of cooperative research must be employed, which demands closer cooperation between clinical and research centers, and industry. Novel clinical trial designs based on harmonized and aligned research goals need to supplant traditional clinical trial concepts, where the enrollment of large patient numbers compensated for the potential risks of neglecting individual molecular patient characteristics, and, ultimately, for obtaining inconclusive data. In the future, clinical trials need to aim for small, well-defined and carefully selected patient collectives.
Article highlights.
FMS-like tyrosine kinase-3 (FLT3)/internal tandem duplications mutated AML evolves from diagnosis to relapse.
FLT3 inhibition induces apoptosis of peripheral blasts but induces differentiation of blasts in the bone marrow.
Although FLT3 inhibitors induce clinical remissions in most patients, drug-resistant disease develops rapidly.
The incorporation of a FTL3 inhibitor into current treatment concepts bears the potential to improve outcome.
DNA methyltransferase inhibitors (DNMTis) do not induce a surge of FLT3 ligand, and they confer apoptotic and differentiation inducing effects and therefore represent an intriguing class of drugs to combine with FLT3 inhibitors.
Owing to the complexity of AML pathogenesis, future clinical trials need to focus on small, well-defined and carefully selected patient collectives demanding closer cooperation between research centers.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 3.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 4.Lazenby M, Gilkes AF, Marrin C, et al. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia. 2014 doi: 10.1038/leu.2014.90. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Bacher U, Haferlach C, Kern W, et al. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters–an analysis of 3082 patients. Blood. 2008;111(5):2527–37. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 6.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 7.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Schneider F, Hoster E, Schneider S, et al. Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML) Ann Hematol. 2012;91(1):9–18. doi: 10.1007/s00277-011-1280-6. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J Hematol Oncol. 2011;4:13. doi: 10.1186/1756-8722-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratz KW, Sato T, Murphy KM, et al. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–32. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos FP, Jones D, Qiao W, et al. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer. 2011;117(10):2145–55. doi: 10.1002/cncr.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Pratcorona M, Brunet S, Nomdedeu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–8. doi: 10.1182/blood-2012-06-431122. The authors report that, in intermediate-risk AML, the effect of FMS-like tyrosine kinase-3 (FLT3) burden is modulated by nucleophosmin 1 mutation, particularly in patients presenting with low allelic ratio. [DOI] [PubMed] [Google Scholar]
- 13.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bornhauser M, Illmer T, Schaich M, et al. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109(5):2264–5. doi: 10.1182/blood-2006-09-047225. author reply 2265. [DOI] [PubMed] [Google Scholar]
- 15.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60–70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17(12):1796–803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–65. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 17.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 18.Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–41. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 19.Dezern AE, Sung A, Kim S, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17(9):1404–9. doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laboure G, Dulucq S, Labopin M, et al. Potent graft-versus-leukemia effect after reduced-intensity allogeneic SCT for intermediate-risk AML with FLT3-ITD or wild-type NPM1 and CEBPA without FLT3-ITD. Biol Blood Marrow Transplant. 2012;18(12):1845–50. doi: 10.1016/j.bbmt.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Lin PH, Lin CC, Yang HI, et al. Prognostic impact of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia patients with internal tandem duplication of FLT3. Leuk Res. 2013;37(3):287–92. doi: 10.1016/j.leukres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–10. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21(24):3214–31. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 24.Levis M, Tse KF, Smith BD, et al. A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98(3):885–7. doi: 10.1182/blood.v98.3.885. [DOI] [PubMed] [Google Scholar]
- 25.Zheng R, Friedman AD, Small D. Targeted inhibition of FLT3 overcomes the block to myeloid differentiation in 32Dcl3 cells caused by expression of FLT3/ITD mutations. Blood. 2002;100(12):4154–61. doi: 10.1182/blood-2002-03-0936. [DOI] [PubMed] [Google Scholar]
- 26.Radomska HS, Basseres DS, Zheng R, et al. Block of C/EBP alpha function by phosphorylation in acute myeloid leukemia with FLT3 activating mutations. J Exp Med. 2006;203(2):371–81. doi: 10.1084/jem.20052242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Sexauer A, Perl A, Yang X, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120(20):4205–14. doi: 10.1182/blood-2012-01-402545. This study demonstrated that inhibition of FLT3 by quizartinib leads to terminal myeloid differentiation of bone marrow blasts associated with a clinical differentiation syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man CH, Fung TK, Ho C, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119(22):5133–43. doi: 10.1182/blood-2011-06-363960. [DOI] [PubMed] [Google Scholar]
- 29.Fathi AT, Le L, Hasserjian RP, et al. FLT3 inhibitor-induced neutrophilic dermatosis. Blood. 2013;122(2):239–42. doi: 10.1182/blood-2013-01-478172. [DOI] [PubMed] [Google Scholar]
- 30.Revesz D, Chelghoum Y, Le QH, et al. Salvage by timed sequential chemotherapy in primary resistant acute myeloid leukemia: analysis of prognostic factors. Ann Hematol. 2003;82(11):684–90. doi: 10.1007/s00277-003-0730-1. [DOI] [PubMed] [Google Scholar]
- 31.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116(17):3147–56. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 32.Chevallier P, Labopin M, Turlure P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25(6):939–44. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- 33.Ravandi F, Kantarjian H, Faderl S, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. 2010;34(6):752–6. doi: 10.1016/j.leukres.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millward MJ, House C, Bowtell D, et al. The multikinase inhibitor midostaurin (PKC412A) lacks activity in metastatic melanoma: a phase IIA clinical and biologic study. Br J Cancer. 2006;95(7):829–34. doi: 10.1038/sj.bjc.6603331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–45. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Stone RM, Fischer T, Paquette R, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26(9):2061–8. doi: 10.1038/leu.2012.115. This study showed that the combination of the FLT3 inhibitor midostaurin and standard chemotherapy is feasible and effective, inducing high response and survival rates in newly diagnosed acute myeloid leukemia (AML) patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams CB, Kambhampati S, Fiskus W, et al. Preclinical and phase I results of decitabine in combination with midostaurin (PKC412) for newly diagnosed elderly or relapsed/refractory adult patients with acute myeloid leukemia. Pharmacotherapy. 2013;33(12):1341–52. doi: 10.1002/phar.1316. [DOI] [PubMed] [Google Scholar]
- 38.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 39.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 40.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–70. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 42.Al-Kali A, Cortes J, Faderl S, et al. Patterns of molecular response to and relapse after combination of sorafenib, idarubicin, and cytarabine in patients with FLT3 mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2011;11(4):361–6. doi: 10.1016/j.clml.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravandi F, Alattar ML, Grunwald MR, et al. Phase II study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–62. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114(14):2984–92. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortes JE, Kantarjian H, Foran JM, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 2013;31(29):3681–7. doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortes J, Tallman M, Schiller G, et al. Results of a phase 2 randomized, open-label, study of lower doses of quizartinib (AC220; ASP2689) in subjects with FLT3-ITD positive relapsed or refractory acute myeloid leukemia (AML) Blood. 2013;122:494. [Google Scholar]
- 47.Levis M, Perl A, Dombret H, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood. 2012;120:673a. [Google Scholar]
- 48.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113(17):3938–46. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99(9):3472–5. doi: 10.1182/blood.v99.9.3472. [DOI] [PubMed] [Google Scholar]
- 51.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102(1):276–83. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 52.Ernst T, Hochhaus A. Chronic myeloid leukemia: clinical impact of BCR-ABL1 mutations and other lesions associated with disease progression. Semin Oncol. 2012;39(1):58–66. doi: 10.1053/j.seminoncol.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12(1–2):8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffith J, Black J, Faerman C, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell. 2004;13(2):169–78. doi: 10.1016/s1097-2765(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 55.Piloto O, Wright M, Brown P, et al. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109(4):1643–52. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–92. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 57.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–93. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bagrintseva K, Geisenhof S, Kern R, et al. FLT3-ITD-TKD dual mutants associated with Acute Myeloid Leukemia (AML) confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bcl-x(L) Blood. 2004;105(9):3679–85. doi: 10.1182/blood-2004-06-2459. [DOI] [PubMed] [Google Scholar]
- 59.Barry EV, Clark JJ, Cools J, et al. Uniform sensitivity of FLT3 activation loop mutants to the tyrosine kinase inhibitor midostaurin. Blood. 2007;110(13):4476–9. doi: 10.1182/blood-2007-07-101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams AB, Nguyen B, Li L, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27(1):48–55. doi: 10.1038/leu.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore AS, Faisal A, Gonzalez de Castro D, et al. Selective FLT3 inhibition of FLT3-ITD+ acute myeloid leukaemia resulting in secondary D835Y mutation: a model for emerging clinical resistance patterns. Leukemia. 2012;26(7):1462–70. doi: 10.1038/leu.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100(3):1014–18. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- 63.Man CH, Lam SS, Sun MK, et al. A novel tescalcin-sodium/hydrogen exchange axis underlying sorafenib resistance in FLT3-ITD+ AML. Blood. 2014;123(16):2530–9. doi: 10.1182/blood-2013-07-512194. [DOI] [PubMed] [Google Scholar]
- 64.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28(11):1856–62. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–18. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 66.Levis M, Pham R, Smith BD, et al. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104(4):1145–50. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Sung C, Dartois C, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86(2):167–74. doi: 10.1038/clpt.2009.64. [DOI] [PubMed] [Google Scholar]
- 68.Cortes J, Perl A, Dombret H, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients 60 years of age with FLT3 ITD positive or negative relapsed/refractory acute myeloid leukemia. Blood. 2012;120:48a. [Google Scholar]
- 69.Zirm E, Spies-Weisshart B, Heidel F, et al. Ponatinib may overcome resistance of FLT3-ITD harbouring additional point mutations, notably the previously refractory F691I mutation. Br J Haematol. 2012;157(4):483–92. doi: 10.1111/j.1365-2141.2012.09085.x. [DOI] [PubMed] [Google Scholar]
- 70•.Smith CC, Lasater EA, Zhu X, et al. Activity of ponatinib against clinically-relevant AC220-resistant kinase domain mutants of FLT3-ITD. Blood. 2013;121(16):3165–71. doi: 10.1182/blood-2012-07-442871. Ponatinib, due to its encouraging activity in the preclinical and early clinical setting, and its favorable toxicity profile, may represent a useful second line tyrosine kinase inhibitor in AML patients with acquired quizartinib or sorafenib resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmerman EI, Turner DC, Buaboonnam J, et al. Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood. 2013;122(22):3607–15. doi: 10.1182/blood-2013-07-513044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Galanis A, Ma H, Rajkhowa T, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123(1):94–100. doi: 10.1182/blood-2013-10-529313. Herein, the authors report that crenolanib displays a broader range of activity against FLT3-mutated leukemic cells when compared to other agents in development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith CC, Lasater EA, Lin KC, et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci USA. 2014;111(14):5319–24. doi: 10.1073/pnas.1320661111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collins R, Kantarjian H, Levis M, et al. Clinical activity of Crenolanib in patients with D835 mutant FLT3-positive relapsed/refractory acute myeloid leukemia (AML) J Clin Oncol. 2014;325s:7027. [Google Scholar]
- 75.Juliusson G, Billstrom R, Gruber A, et al. Attitude towards remission induction for elderly patients with acute myeloid leukemia influences survival. Leukemia. 2006;20(1):42–7. doi: 10.1038/sj.leu.2404004. [DOI] [PubMed] [Google Scholar]
- 76.Baylin SB, Herman JG, Graff JR, et al. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 77.Claus R, Plass C, Armstrong SA, et al. DNA methylation profiling in acute myeloid leukemia: from recent technological advances to biological and clinical insights. Future Oncol. 2010;6(9):1415–31. doi: 10.2217/fon.10.110. [DOI] [PubMed] [Google Scholar]
- 78.Issa JP, Baylin SB, Herman JG. DNA methylation changes in hematologic malignancies: biologic and clinical implications. Leukemia. 1997;11(Suppl 1):S7–11. [PubMed] [Google Scholar]
- 79.Teofili L, Morosetti R, Martini M, et al. Expression of cyclin-dependent kinase inhibitor p15(INK4B) during normal and leukemic myeloid differentiation. Exp Hematol. 2000;28(5):519–26. doi: 10.1016/s0301-472x(00)00139-9. [DOI] [PubMed] [Google Scholar]
- 80.Chim CS, Liang R, Kwong YL. Hypermethylation of gene promoters in hematological neoplasia. Hematol Oncol. 2002;20(4):167–76. doi: 10.1002/hon.694. [DOI] [PubMed] [Google Scholar]
- 81.Solanilla A, Grosset C, Lemercier C, et al. Expression of Flt3-ligand by the endothelial cell. Leukemia. 2000;14(1):153–62. doi: 10.1038/sj.leu.2401635. [DOI] [PubMed] [Google Scholar]
- 82.Chklovskaia E, Nissen C, Landmann L, et al. Cell-surface trafficking and release of flt3 ligand from T lymphocytes is induced by common cytokine receptor gamma-chain signaling and inhibited by cyclosporin A. Blood. 2001;97(4):1027–34. doi: 10.1182/blood.v97.4.1027. [DOI] [PubMed] [Google Scholar]
- 83.Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103(1):267–74. doi: 10.1182/blood-2003-06-1969. [DOI] [PubMed] [Google Scholar]
- 84.Lyman SD, Seaberg M, Hanna R, et al. Plasma/serum levels of flt3 ligand are low in normal individuals and highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood. 1995;86(11):4091–6. [PubMed] [Google Scholar]
- 85.Wodnar-Filipowicz A, Lyman SD, Gratwohl A, et al. Flt3 ligand level reflects hematopoietic progenitor cell function in aplastic anemia and chemotherapy-induced bone marrow aplasia. Blood. 1996;88(12):4493–9. [PubMed] [Google Scholar]
- 86.Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10(4):588–99. [PubMed] [Google Scholar]
- 87.Zheng R, Bailey E, Nguyen B, et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene. 2011;30(38):4004–14. doi: 10.1038/onc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 89.Cashen AF, Schiller GJ, O’Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–61. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 90.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 91•.Maurillo L, Venditti A, Spagnoli A, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer. 2012;118(4):1014–22. doi: 10.1002/cncr.26354. The authors report that azacitidine promises to be an effective therapy for elderly patients with untreated AML. [DOI] [PubMed] [Google Scholar]
- 92.Soncini M, Santoro F, Gutierrez A, et al. The DNA demethylating agent decitabine activates the TRAIL pathway and induces apoptosis in acute myeloid leukemia. Biochim Biophys Acta. 2013;1832(1):114–20. doi: 10.1016/j.bbadis.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Lund P, Kotova I, Kedinger V, et al. Transformation-dependent silencing of tumor-selective apoptosis-inducing TRAIL by DNA hypermethylation is antagonized by decitabine. Mol Cancer Ther. 2011;10(9):1611–23. doi: 10.1158/1535-7163.MCT-11-0140. [DOI] [PubMed] [Google Scholar]
- 94.Thepot S, Lainey E, Cluzeau T, et al. Hypomethylating agents reactivate FOXO3A in acute myeloid leukemia. Cell Cycle. 2011;10(14):2323–30. doi: 10.4161/cc.10.14.16399. [DOI] [PubMed] [Google Scholar]
- 95.Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5(2):e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Markovic A, MacKenzie KL, Lock RB. FLT-3: a new focus in the understanding of acute leukemia. Int J Biochem Cell Biol. 2005;37(6):1168–72. doi: 10.1016/j.biocel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Kikushige Y, Yoshimoto G, Miyamoto T, et al. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J Immunol. 2008;180(11):7358–67. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
- 98.Heidel F, Lipka DB, Mirea FK, et al. Bis (1H-indol-2-yl)methanones are effective inhibitors of FLT3-ITD tyrosine kinase and partially overcome resistance to PKC412A in vitro. Br J Haematol. 2009;144(6):865–74. doi: 10.1111/j.1365-2141.2008.07567.x. [DOI] [PubMed] [Google Scholar]
- 99.Kojima K, Konopleva M, Tsao T, et al. Selective FLT3 inhibitor FI-700 neutralizes Mcl-1 and enhances p53-mediated apoptosis in AML cells with activating mutations of FLT3 through Mcl-1/Noxa axis. Leukemia. 2010;24(1):33–43. doi: 10.1038/leu.2009.212. [DOI] [PubMed] [Google Scholar]
- 100•.Gunawardane RN, Nepomuceno RR, Rooks AM, et al. Transient exposure to quizartinib mediates sustained inhibition of FLT3 signaling while specifically inducing apoptosis in FLT3-activated leukemia cells. Mol Cancer Ther. 2013;12(4):438–47. doi: 10.1158/1535-7163.MCT-12-0305. This study showed that transient exposure to quizartinib, both in vitro and in vivo, leads to prolonged inhibition of FLT3 signaling, induction of apoptosis and reductions in tumor burden and pharmacodynamic endpoints. [DOI] [PubMed] [Google Scholar]
- 101.Radomska HS, Huettner CS, Zhang P, et al. CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol. 1998;18(7):4301–14. doi: 10.1128/mcb.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nerlov C. C/EBPalpha mutations in acute myeloid leukaemias. Nat Rev Cancer. 2004;4(5):394–400. doi: 10.1038/nrc1363. [DOI] [PubMed] [Google Scholar]
- 103.Hackanson B, Bennett KL, Brena RM, et al. Epigenetic modification of CCAAT/enhancer binding protein alpha expression in acute myeloid leukemia. Cancer Res. 2008;68(9):3142–51. doi: 10.1158/0008-5472.CAN-08-0483. [DOI] [PubMed] [Google Scholar]
- 104.Negrotto S, Ng KP, Jankowska AM, et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors. Leukemia. 2012;26(2):244–54. doi: 10.1038/leu.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng R, Friedman AD, Levis M, et al. Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBP{alpha} expression. Blood. 2004;103(5):1883–90. doi: 10.1182/blood-2003-06-1978. [DOI] [PubMed] [Google Scholar]
- 106.Schwab ED, Pienta KJ. Cancer as a complex adaptive system. Med Hypotheses. 1996;47(3):235–41. doi: 10.1016/s0306-9877(96)90086-9. [DOI] [PubMed] [Google Scholar]
- 107.Yang X, Sexauer A, Levis M. Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. Br J Haematol. 2014;164(1):61–72. doi: 10.1111/bjh.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254–62. doi: 10.1038/leu.2012.352. [DOI] [PubMed] [Google Scholar]
- 109.Wassmann B, Pfeifer H, Stadler M, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2005;106(2):458–63. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- 110.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109(7):2791–3. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tabe Y, Jin L, Tsutsumi-Ishii Y, et al. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67(2):684–94. doi: 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- 112.Civini S, Jin P, Ren J, et al. Leukemia cells induce changes in human bone marrow stromal cells. J Transl Med. 2013;11:298. doi: 10.1186/1479-5876-11-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parmar A, Marz S, Rushton S, et al. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against first-generation FLT3 tyrosine kinase inhibitors. Cancer Res. 2011;71(13):4696–706. doi: 10.1158/0008-5472.CAN-10-4136. [DOI] [PubMed] [Google Scholar]
- 114.Weisberg E, Wright RD, McMillin DW, et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther. 2008;7(5):1121–9. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Farrell AM, Foran JM, Fiedler W, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465–76. [PubMed] [Google Scholar]
- 116.Fiedler W, Serve H, Döhner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 117.Fiedler W, Kayser S, Kebenko M, et al. Sunitinib and intensive chemotherapy in patients with acute myeloid leukemia and activating FLT3 mutations: results of the AMLSG 10–07 study (ClinicalTrials.gov No. NCT00783653) Blood (ASH Annual Meeting Abstracts) 2012;120:abstract 1483. [Google Scholar]
- 118.Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353–9. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 119.Serve H, Wagner R, Sauerland C, et al. Sorafenib in combination with standard induction and consolidation therapy in elderly AML patients: Results from a randomized, placebo-controlled phase II trial. Blood (ASH Annual Meeting Abstracts) 2010;116:abstract 333. [Google Scholar]
- 120.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]