Abstract
Cell-to-cell signaling molecules such as the Wnt proteins that directly influence the expression of cell-type specific transcriptional programs are essential for tissue generation in metazoans. The mechanisms supporting cellular responses to these molecules represent potential points of intervention for directing cell fate outcomes in therapeutic contexts. Small molecules that modulate Wnt-mediated cellular responses have proven to be powerful probes for Wnt protein function in diverse biological settings including cancer, development, and regeneration. Whereas efforts to develop these chemicals as therapeutic agents have dominated conversation, the unprecedented modes-of-action associated with these molecules and their implications for drug development deserve greater examination. In this review, we will discuss how medicinal chemistry efforts focused on first in class small molecules targeting two Wnt pathway components – the polytopic Porcupine (Porcn) acyltransferase and the cytoplasmic Tankyrase (Tnks) poly-ADP-ribosylases – have contributed to our understanding of the druggable genome and expanded the armamentarium of chemicals that can be used to influence cell fate decision-making.
Keywords: cancer, membrane bound O-acyl transferases, Tankyrase, poly ADP-ribosylation, Porcupine, regenerative medicine, tissue homeostasis, Wnt signaling
1. INTRODUCTION
Adult stem cells and persistent cancer cells have in common robust mechanisms in place for resisting depletion [1]. On the one hand this property is a barrier to age-dependent tissue erosion and on the other it promotes cancer re-emergence and metastasis. In this regard, the ability to achieve therapeutic goals in regenerative medicine and anti-cancer research are both dependent upon an understanding of self-renewal properties of adult cells.
Much effort has been devoted to delineating the molecular nature of the intercellular signals that enforce the hierarchy of stem cells and their progeny with the hopes of controlling both normal and cancer stem cell outcomes [2]. The foundation of our current understanding of these signals stems from decades of research that have identified conserved genetic programs that relay communal cell organization into cell-specific decisions throughout the metazoan life cycle. This knowledgebase has provided an experimentally tractable framework for identifying agents that could be leveraged for co-opting signals that dictate cell fate outcome such as those targeting the Wnt, Notch, and Hedgehog (Hh) pathways.
Whereas the reliability of these agents have been measured by their ability to disrupt specific markers in cancerous cells and oftentimes their ability to induce predicted effects on tissue homeostasis or development, the recent advent of induced pluripotent stem cells (iPSCs) has afforded new metrics of chemical specificity based on their ability to direct cell fate outcome in vitro. Indeed, in addition to their potential anti-cancer utilities, these agents hold promise for improving cancer therapy by restoring tissue ravaged by disease.
In this review, we discuss recent progress in the development of small molecules targeting signaling mechanisms engaged by the secreted Wnt signaling molecules. Whereas the driving force for investing in these molecules is the nearly universal involvement of Wnt signaling in colorectal cancer, the participation of Wnt signaling in tissue homeostasis and development suggests additional utilities for such agents in both research and clinical settings. Here we provide an overview of how chemically disabling Wnt signaling could be used in anti-cancer and regenerative medicine programs with a focus on two druggable components of Wnt signaling - Porcn and Tnks.
2. CONTROLLING CELL FATE OUTCOMES BY CHEMICAL MODULATION OF WNT SIGNALING
2.1. Normal tissues
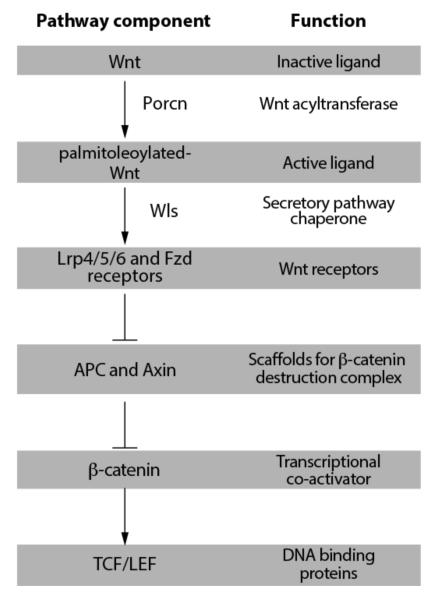
The cellular interactions that promote tissue emergence during development are frequently employed in the maintenance of adult tissues. In adult metazoans, the sensitivity of various tissues to altered Wnt/β-catenin signaling has been recorded using targeted deletion of pathway components in model organisms as well as genetic observations in humans (Figure 1). These studies have in turn provided a framework for prioritizing various tissues for evaluating the therapeutic utility of Wnt chemical modulators in regenerative medicine and cancer. At the same time, these sensitivities reveal possible unwanted tissue toxicities. Indeed, the reliance of gut epithelial regeneration on Wnt signaling will likely impose dose limitations for small molecules targeting Wnt pathway components used in clinical settings.
Fig. (1). Overview of the Wnt/β-catenin signal transduction pathway.
Reviews of Wnt-mediated cellular responses that do not utilize β-catenin can be found in [12, 27].
The gut epithelium completes a cycle of regeneration every 4-5 days and thus similar to the rapidly regenerating hematopoietic system frequently forms the basis of dose limitations for chemotherapies targeting mechanisms supporting rapid cell division. The source of differentiated gut cells is stem cells and progenitors found at the base of intestinal crypts – glandular structures that delve into the mucosa of the intestines (reviewed in [3]). Genetic insults that compromise the activity of Wnt signaling components disrupt epithelial regeneration thus providing strong evidence that Wnt signaling controls stem cell self renewal in the gut [4]. Indeed, the reliability of the Wnt/β-catenin transcriptional target gene LGR5 as a biomarker of multipotent cells and its own role in promoting Wnt signaling provides additional support for the importance of Wnt signaling in gut stem cell renewal [5]. At the same time, stem cells marked by expression of the BMI1 protein constitute another source of epithelial cells in the gut [6, 7]. The insensitivity of BMI1 cells to loss of Wnt pathway activity suggest that these cells may provide a mechanism for buttressing the integrity the gut epithelium exposed to various chemical inhibitors of Wnt signaling.
Similarly, a number of genetic observations in humans and model organisms support a role for Wnt signaling in regulating bone homeostasis [8]. Wnt signaling controls multiple aspects of bone catabolism, most notably the suppression of osteoclastogenesis [9]. Thus, loss of Wnt/β-catenin signaling induced by loss of function mutations in Wnt receptors belonging to the LRP family generally promotes bone reabsorption. At the same time, mutations in Lrp5 that disrupt its binding to Wnt inhibitory molecules such as sclerostin, for example, give rise to high bone mass [10]. Therefore, augmenting Wnt activity either with recombinant Wnt protein, antibodies targeting sclerostin, or GSK3β inhibitors that disrupt phosphorylation-dependent β-catenin destruction hold promise for improving bone repair [8, 10].
Genetic observations such as those described above in the gut and bone provide the rationale for investing in the development of agents that modulate Wnt signaling for anti-cancer and regenerative medicine goals. Indeed, the loss of APC in nearly 90% of colorectal cancer cases is the primary focus of Wnt-associated anti-cancer programs. The result of these efforts so far is a large collection of small molecules that target various Wnt signaling components (reviewed in [11, 12]. Two classes of molecules targeting the Wnt acyltransferase Porcn and the cytoplasmic regulator Tnks (Figure 2) are discussed here in more depth given their extensive use in tissue engineering and in testing the promise of Wnt targeted cancer therapies. The vulnerability of Wnt signaling to chemicals targeting these proteins was identified from high throughput chemical library screens [13-16]. Porcn is an ER-localized multi-spanning membrane protein belonging to a family of membrane bound O-acyltransferases (MBOATs) that acylate lipids and proteins [17] that is essential to fatty acylation of presumably all Wnt molecules. On the other hand the two Tnks proteins form a subfamily of poly ADP ribose polymerase (PARPs) that regulate β-catenin abundance and thus Wnt cellular responses that engage the TCF/LEF transcriptional regulators (see Figure 2).
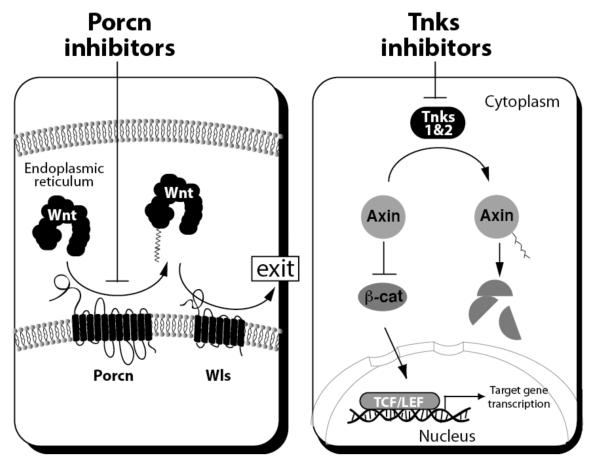
Fig. (2). Mechanism of action for Porcn and Tnks inhibitors.
Left: Inhibition of endoplasmic reticulum-localized Porcn results in loss of Wnt fatty acylation. Wnt proteins devoid of their lipid moiety are not recognized by the Wntless (Wls) chaperone resulting in their sequestration in the secretory pathway. Wnt molecules in addition to regulating β-catenin/TCF activity control other cellular responses not depicted here. Right: Disruption of Tnks1 & 2 activity with chemicals results in loss of Axin protein PARylation, a biochemical change that promotes Axin destruction by ubiquitinylation. Thus in cells treated with Tnks inhibitors, Axin accumulates and accelerates the rate of β-catenin turnover. Without a sufficient abundance of β-catenin, the TCF/LEF proteins are unable to elicit a meaningful transcriptional response. The turnover rate of other proteins in addition to β-catenin that are regulated by Tnks and Axin are not depicted here but discussed in Section 3.
Despite the frequent employment of genetic strategies for modulating β-catenin as a surrogate approach to disrupting TCF/LEF activity, the shared role of β-catenin in both cell-cell adhesion and transcription compromises the ability to use evidence derived from such approaches for anticipating the effects of Tnks inhibitors which primarily target β-catenin transcriptional activity [18]. Some evidence that chemical disruption of β-catenin transcriptional activity will differ in phenotypic outcome from studies using engineered animals that express a β-catenin lacking signaling activity but retains cell-cell adhesion functions [19, 20]. When also considered with the essential roles of Tnks enzymes in development and the often time overlapping function of the two homologous enzymes [21], Tnks inhibitors should be valuable probes for understanding β-catenin in adult tissues that bypasses several limitations of genetic approaches.
Similarly, understanding the anticipated effects of Porcn inhibitors on adult tissues has been complicated by the essential role of Porcn in developing tissues and [22]. Cell-type specific deletion of the Wntless (WLS) chaperone or Porcn (see Figure 1) has provided a strategy for evaluating the contribution of Wnt ligands to tissue homeostasis (examples in [23-26]). Yet the interpretation of results stemming from the use of either of these genetic strategies are complicated by the multiple sources of Wnt ligands that can likely provide compensation when one source has been disrupted. Indeed, targeted deletion of Porcn in the gut epithelium has little effect on tissue homeostasis presumably due to stromal contribution of Wnt molecules in the stem cell niche [24]. An additional challenge to understanding the consequences of Porcn inhibition is the phenotype could be a consequence of disrupting the interplay of up to 19 Wnt molecules. Indeed, many Wnt molecules do not directly control β-catenin activity but regulate other cellular processes such as cell polarity and calcium signaling (see[12, 27]).
Despite the limitations of these genetic approaches and the strong evidence supporting the importance of Wnt/β-catenin signaling in gut epithelium regeneration, the gut epithelium nevertheless exhibits surprising robustness with a Porcn inhibitor reaching concentrations sufficient levels to block the expression of Wnt/β-catenin target genes such as the LGR5 stem cell marker and to inhibit tumor growth without apparent deleterious effects on animal health [28]. On the other hand, in vivo studies using two similar Tnks inhibitors show activity against mouse models of colorectal cancer but that differ with respect to the level of toxicity induced in normal gut tissue [14, 16]. These observations may be influenced by differences in mouse strains utilized in each study or possibly differences in chemical bioavailability. Yet in the end the tolerance to these chemicals seen in rodents suggests a larger than anticipated therapeutic window may exist in humans. The effects of these inhibitors in bone tissue upon prolonged exposure remain untested.
2.2. Cancerous tissues
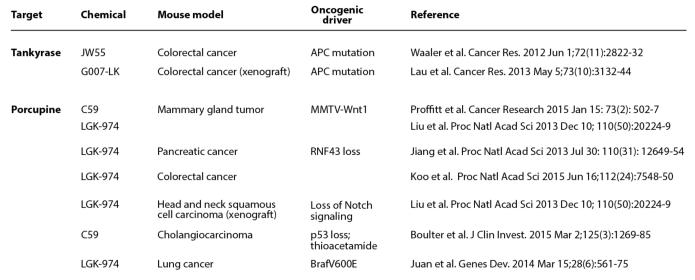
The reliance of certain cancer types on Wnt pathways for sustaining growth likely represents an exploitation of normal tissue maintenance cues provided by Wnt proteins. For example, loss in activity of the tumor suppressor and β-catenin inhibitor Apc heightens Wnt signaling in a tissue that is well established to rely on Wnt signals for homeostatic renewal. The prevalence of APC mutations suggests that these lesions arise early in the course of disease progression [29, 30]. At the same time, deregulated growth control in the gut epithelium is observed when APC is mutated in putative stem cells but not in differentiated cells thus providing compelling evidence that the cell of origin in gut cancers are likely stem cells [31]. Similarly, β-catenin mutations are frequently found in cancers of the liver, a tissue that relies on Wnt signaling for regeneration [32] (see Figure 1). Given the frequency of mutations that give rise to a truncated Apc protein observed in colorectal cancer (~90%), the development of agents targeting the β-catenin transcriptional apparatus such as Tnks inhibitors are routinely tested in preclinical models of CRC (Figure 3).
Fig. (3). Pre-clinical cancer therapeutic studies of Porcn and Tnks inhibitors in mice.
C59 and JW55 are predecessor molecules of LGK-974 and G007-LK, respectively. MMTV-Wnt1 transgenic mice develop ductal hyperplasia in mammary gland tissue due to introduction of a viral transcriptional enhancer that induces Wnt1 expression. Liu et al has shown that loss of Notch signaling in head and neck squamous cell carcinoma cell lines induce cell growth-promoting Wnt/β-catenin signaling. Thioacetamide is a chemical that induces dysregulated growth in the biliary tract of animals that are compromised for the p53 tumor suppressor. Juan et al. has shown that lung cancers initiated by a mutation in the protooncogenic Braf protein (V600E) are dependent upon β-catenin signaling.
Significant effort has been devoted to defining chemical strategies for normalizing β-catenin activity as a means to counter the induction of deviant transcriptional response by Apc loss of function. The transcriptional activity of the four TCF/LEF family members (TCF7/TCF1, TCF7L1/TCF3, TCF7L2/TCF4, and LEF1) is regulated by β-catenin and its accessory factors such as the histone acetyltransferase CBP (Creb-binding protein; also known as CREBBP) [33]. Three major chemical strategies have emerged for targeting β-catenin transcriptional activity: a) breaking β-catenin interaction with TCF/LEF family members, b) disrupting β-catenin interaction with CBP [34, 35], or c) inducing β-catenin destruction by inhibiting the Tnks enzymes [13]. These strategies that disable the Wnt transcriptional machinery are likely to be additionally useful in other cancers with well-defined mutations that result in deviant β-catenin activation including those found in the β-catenin binding adhesion molecule FAT1 (FAT atypical cadherin) [36] and the transcriptional repressor form of Tcf7l2 that counters the positively activating TCF/LEF family members [29, 37]. Amongst these strategies, a peptidomimic that disrupts CBP binding to β-catenin (PRI-724) has entered clinical testing whereas several Tnks inhibitors in various stages of pre-clinical development [35, 38]. The role of TCF7L1/TCF3 and certain splice variants of TCF/LEF family members as transcriptional repressors may contribute to tissue-specific differences in responses to inhibitors of β-catenin activity [33].
The anti-cancer goals underlying efforts that uncovered the chemical tractability of Porcn are not as obvious as those driving efforts to disable the Wnt transcriptional apparatus. As such, a greater number of pre-clinical studies that incorporate Porcn inhibitors have been executed by comparison to those associated with Tnks inhibitors (see Figure 3). Indeed, the contribution of Wnt ligands and thus the value of Porcn inhibitors to cells with APC mutations in vivo remains unclear [39]. On the other hand, the essential and unique role of Porcn in the production of functional Wnt ligands has made these chemicals powerful probes for Wnt signaling and has galvanized efforts to identify other cancer types that may rely on Wnt ligands and that may be sensitive to Porcn inhibitors. For example, growth-promoting mutations in the transmembrane ubiquitin ligases Znrf3 or Rnf43 that result in increased levels of Wnt receptors in pancreatic ductal adenocarcinoma and colorectal cancer can be countered with Porcn inhibitors [40-43]. Although much less is known about the Wnt responses that are independent of β-catenin activity including those governing cell polarity [44], conceivably cancerous cells may also depend on such cellular responses for growth or metastasis.
Whereas cancer genome sequencing has assigned Wnt signaling to various cancers based on the prevalence of mutations in Wnt pathway components, other cancers such as acute myeloid leukemia (AML) that have shown sensitivity to Wnt pathway modulation in animal models are devoid of such mutations [45]. Given our understanding of Wnt signaling in cancer has been predominantly informed by studies in the gut, the existence of these other cancers either reflects our partial inventory of Wnt signaling components in other cell types or the limitations of genomic sequencing for revealing epigenetic changes in Wnt signaling such as altered RNA splicing or histone methylation. Either Porcn or β-catenin targeting agents or both could be useful in these disease contexts.
2.3. Embryonic and induced pluripotent stem cells
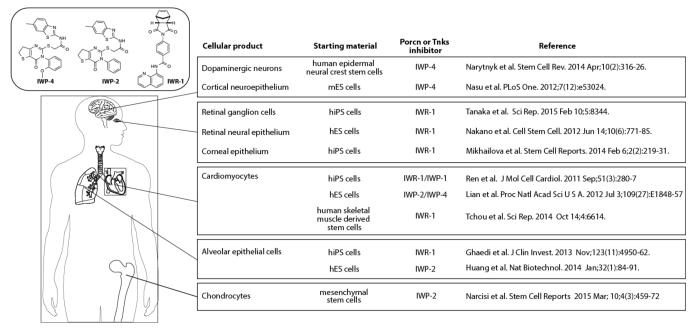
The potential applications of Wnt inhibitors as anti-cancer agents has in many ways overshadowed the tremendous progress made in the use of such molecules for influencing cell differentiation programs in vitro. Indeed, two classes of Porcn and Tnks inhibitors have been widely adopted for use in the production of medically useful cell types from various precursor cells including induced pluripotent stem cells (iPSCs) [46] (Figure 4). When used in combination with other small molecules that influence cell fate outcome and in different cell culture conditions, nearly homogenous cell products can be achieved in some cases with these Wnt pathway antagonists [47, 48]. For example, a chemically based strategy for cardiomyocyte production from iPSCs entails the use of two chemicals – one compound to activate Wnt signaling (GSK3β inhibitor) under embryoid body formation conditions and a Porcn inhibitor (IWP-2 and IWP-4) to inactivate Wnt signaling under monolayer growth conditions [47]. In another protocol a Tnks inhibitor could substitute for a Porcn inhibitor thus demonstrating the necessity of on-target effects of these Wnt inhibitors for robust cardiomyocyte induction [49].
Fig. (4). Summary of cell engineering achievements using the IWP and IWR classes of Porcn and Tnks inhibitors, respectively.
IWP-1, -2, and -4 inhibit Porcn whereas IWR-1 compound inhibits Tnks1 & 2. mES cells=mouse embryonic stem cells; hES cells=human embryonic stem cells; hiPS cells= human induced pluripotent stem cells.
The production of cardiomyocytes from iPSCs using only chemical reagents targeting the Wnt/β-catenin pathway has not only confirmed the well-recognized prowess of Wnt signaling in cell fate determination processes but also galvanized efforts to deploy Wnt pathway modulators in other tissue engineering agendas (see Figure 4). Other successes include the production of dopaminergic neurons and retinal pigmented epithelial cells which could be used for in vitro screening for molecules with biological activity in these cell types or for the replacement of prematurely degenerated cells [48, 50]. The availability of these agents and the ease with which they can be applied to cultured cells has helped fuel the rapid growth in their use for tissue engineering. With the successful production of therapeutically relevant cell types, a challenge in the future will be to improve the integration of these cells into the host, a process that could be facilitated by stemming fibrotic responses in injured or aged tissues with the use of Wnt inhibitors with favorable pharmacokinetic properties [51-53].
3. EXPANDING THE DRUGGABLE GENOME WITH PORCN AND TNKS INHIBITORS
Chemically based studies focused on Wnt signaling have expanded our understanding of the druggable genome with the identification of the first inhibitors of an MBOAT gene family member (Porcn) and a novel approach for selective disablement of PARP family members as revealed by efforts to selectively target the Tnks enzymes. Here we discuss recent advances and potential challenges in generating selective agents targeting Porcn and Tnks with drug-like properties.
3.1. An unanticipated druggable pocket in Tnks enzymes
Members of the seventeen-gene PARP family play diverse cellular roles including those in DNA damage repair, a process that is essential to survival for some cancers when challenged with DNA damaging chemotherapeutic agents [54]. Whereas Parp-1 is the target of several anti-cancer agents in late stage clinical testing [55], the value of disabling Tnks enzymes in disease contexts remain mostly unexplored. Initially identified as regulators of Trf1/Terf1, a protein essential to the replication fidelity of telomeric repeats [56, 57], the potential of Tnks enzymes as targets for inducing telomeric shortening has been largely short-changed by the focus on agents that directly target telomerase, the enzyme that extends telomere ends [58]. In addition to their roles in Wnt/β-catenin signaling and telomere length maintenance, Tnks enzymes may be valuable therapeutic targets in the context of their other cellular functions including the regulation of the PTEN tumor suppressor [59] and the glucose transporter Glut4 [60, 61]. At the same time, the ankyrin repeats in Tnks enzymes suggest that in addition to their enzymatic activities, Tnks 1&2 also perform protein scaffolding functions. Indeed, they form macro-structures in the cytoplasm that likely facilitate protein turnover [62, 63]. Thus, Tnks inhibitors may not always phenocopy the effects of genetically based efforts to disrupt Tnks activity. For example, the anti-mitotic effects of RNAi-mediated mitigation of Tnks expression [64] are not observed with chemical inhibitors of Tnks inhibitors [13] suggesting Tnks function in this context may be independent of its PARylation activity.
The identification of Tnks as a key regulator of Wnt signaling from a chemical library screen elegantly demonstrates the utility of small molecules for identifying genes with redundant roles that would otherwise be difficult to detect using single gene interrogation strategies [13]. The compound identified in this screen (XAV-939), is reminiscent of other PARP inhibitors that target the nicotinamide-binding pocket (Figure 5). Indeed, in addition to its anti-Tnks activity, XAV-939 exhibits low micromolar activity against other PARPs (PARP1-3) [13, 65].
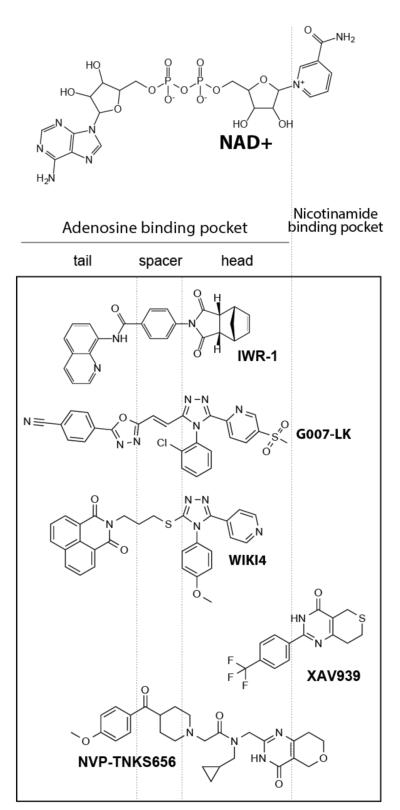
Fig. (5). Summary of selected classes of Tnks inhibitors and their mode of binding to Tnks enzymes.
NAD+ is the substrate for Tnks enzymes. Chemical components of small molecules that occupy the adenosine-binding pocket are aggregated into head, spacer, and tail for discussion purposes. Although representative molecules targeting various active site pockets are discussed here, a more extensive overview that includes references to additional Tnks inhibitors can be found in these excellent reviews [68, 89, 90].
A similar screen executed in parallel with the one described above identified compounds that induced Axin accumulation and β-catenin loss [18] and that were subsequently shown to be Tnks inhibitors [13]. Remarkably these compounds (represented here by IWR-1) showed little resemblance to XAV-939 or other PARP inhibitors (see Figure 5). A co-crystal structure of IWR-1 with either Tnks 1 or 2 proteins revealed that it occupies an induced pocket that houses the adenosine portion of NAD+, the substrate for PARPs [66, 67]. The basis for this mode of engagement is the presence of stacking interactions between the quinolone group found in the “tail” region of IWR-1 (see Figure 5) and a histidine uniquely found in the D-loop sequence (that forms the “roof” of the adenosine-binding pocket) of the two Tnks proteins. Thus, IWR-1 exhibits a greater than 100 fold specificity for Tnks enzymes over other PARP family members given it exploits structural features unique to the adenosine-binding pocket of Tnks enzymes [68]. Two other Tnks inhibitors (G007-LK and WIKI4) also employ stacking interactions with the D-loop histidine [69, 70] (see Figure 5). The largest of these molecules NVP-TNKS656 occupies both pockets [71]. A take away observation from these collective medicinal chemistry efforts is that agents that target the adenosine-binding pocket exhibit greater specificity for Tnks molecules than nicotinamide mimetics. Given that other PARPs harbor a phenylalanine or tyrosine that may provide stacking interactions at a similar position as the histidine in the D-loop sequence, agents with selectivity for other PARP family members could be achieved by engineering additional adenosine-binding pocket inhibitors.
3.2. Porcupine antagonists – first in class inhibitors of an endoplasmic reticulum-localized acyltransferase
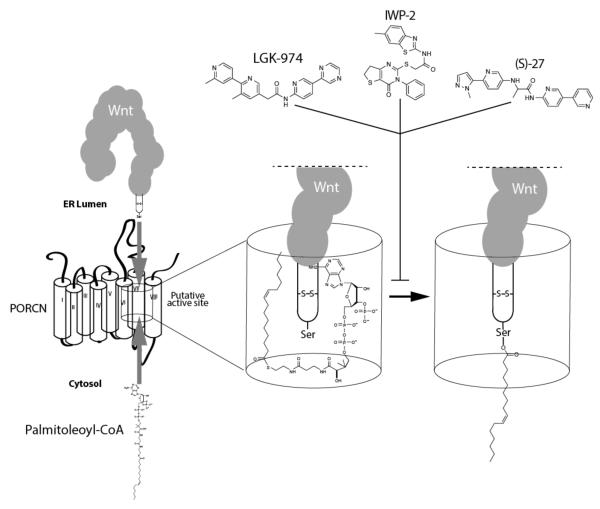
Whereas medicinal chemistry efforts focused on Tnks inhibitors have been greatly helped by a structural understanding of the Tnks active site, a similarly detailed map of the Porcn site of catalysis is likely not in the offing. In addition to the polytopic nature of Porcn that renders traditional structural approaches mostly inaccessible, the hydrophobicity contributed by the fatty acyl motifs found in Porcn further decrease prospects for achieving Porcn crystals [72]. Thus, an understanding of how Porcn inhibitors such as IWP-2 or LGK-974 achieve their specificity and potency will have to be derived from other experimental approaches. Moving forward, the availability of chemically diverse Porcn inhibitors that appear to target the same Porcn binding pocket [73, 74] will certainly help in mapping the chemical-protein determines in presumably the active site which includes a highly conserved histidine residue found in a long hydrophobic region [17]. Indeed a systematic mutagenesis study of Porcn supports an active site embedded within the lipid bilayer that is likely assembled from multiple membrane spanning regions of Porcn [75] (Figure 6).
Fig. (6). The Porcn active site brings together cytoplasmic palmitoleoyl-CoA and ER luminal Wnt proteins to produce fatty acylated Wnt proteins.
Various Porcn inhibitors presumably target the Porcn active site to prevent the formation of a functionally meaningful tri-partite complex.
The collective Porcn inhibitor program has already seen the discovery of compounds with picomolar activity in the form of LGK-974 and a derivative of IWP-2 (IWP-L6) [15, 76]. Thus, future medicinal chemistry efforts will likely focus on improving chemical specificity and limiting unwanted chemical off-target effects as exemplified in more a recent study that describes a high-throughput screen that netted a novel Porcn inhibitor [77]. The active site of MBOAT proteins with secreted protein substrates such as Porcn must bring into close proximity a fatty acyl-CoA molecule found in the cytoplasm with the target protein found in the ER lumen. In the case of Wnts, the serine residue that supports acylation is found in a peptide loop in Wnt molecules [78, 79] that enters the Porcn active site. Superimposed on this simplistic mechanistic perspective are likely unique protein determinants that support selectivity for acyl donors found in each MBOAT protein. For example, the Hhat enzyme that modifies the three Hh protein family members is able to utilize diverse fatty acyl donors in reactions that are not sensitive to saturation status of the fatty acyl donor [80, 81]. At the same time, given the greater abundance of palmitate relative to palmitoleate in cells the Porcn active site must harbor determinants that support selectivity for monounsaturated fatty acids. How preference of these enzymes for fatty acids with a certain carbon lengths is attained at the molecular level also remains unclear but could involve cell type-specific abundance of fatty acids [81] or possibly the rapid turnover of proteins modified with fatty acids of a certain length. Presumably then compounds that exhibit selectivity for Porcn such as LGK-974, IWP-2, or (S)-27 [77] would incorporate chemical features that exploit the presence of residues that compromise the unsaturated bond recognition moiety (see Figure 6). Similarly, there must exist features that enable specific substrate recognition. For example, MBOATs that possess lysophospholipid acyltransferase (LPAT) activity likely have distinct features that enable them to recognize lysosphospholipids [82]. Similarly, Porcn and Hhat must respectively harbor determinants for recognizing Wnt and Hh proteins. Elucidating the mechanistic basis for acyl donor and substrate specificity with further studies will be essential to establishing MBOAT gene family members as viable therapeutic targets.
4. CLOSING REMARKS
Ongoing and now defunct drug development programs focused on targeting protein acylation and poly-ADP ribosylation could offer some glimpses into the future that await for Porcn and Tnks inhibitors in clinical settings. The development of Porcn inhibitors comes at the heels of high profile failures to target the lipidation of Ras proteins as a means to disable Ras driven tumors. Farnesylation and palmitoylation influence the subcellular distribution and thus the activity of Ras proteins [83]. Attempts to inhibit farnesyltransferase (FTase) saw compensatory Ras lipidation by geranylgeranylation that restores Ras oncogenic activity [84]. At the moment, there is no evidence that cells when confronted with a Porcn inhibitor have the means for compensatory Wnt acylation [85]. However, resistance to Porcn inhibitors could arise with mutations in Porcn itself, or in pathway suppressor molecules that are frequently mutated in cancer such as Apc or β-catenin. Given that Tnks induce telomeric shortening as well as Wnt pathway suppression in cells with prolonged chemical exposure [86], Tnks inhibitors could provide a single agent combinatorial therapeutic strategy for targeting cancerous cells that frequently harbor mutations that affect telomeres and Wnt signaling such as liver cancer [87].
Although inhibitors of PARP-1 have fared well in clinical development (candidates are now in late stage clinical testing), difficulties in the selective targeting of PARP family members in general have hindered efforts to test the value of targeting other PARP proteins in therapeutic contexts [65]. Studies focused on Tnks inhibitors have revealed potentially a novel chemical approach for achieving PARP-specific inhibition by targeting their adenosine-binding space. Although this strategy may be restricted to PARPs with sufficiently flexible D-loop sequences that form part of adenosine-binding pocket, it may nevertheless uncover agents for targeting PARPs that are not currently matched with synthetic ligands. At the same time, how chemicals target the protein could induce unique cellular consequences relevant to unwanted toxicities or drug resistance that are not necessarily based on loss of catalytic activity alone but also on structural changes. For example, MAPK/ERK Kinase (MEK) inhibitors that target different activation states of MEK exhibit differential ability to block feedback phosphorylation by the proto-oncogene BRAF thereby providing a rationale for favoring the use of a specific drug for BRAF-driven tumors [88].
Anti-cancer agendas have traditionally absorbed the bulk of the resources available for the development of agents targeting developmentally important pathways such as those governed by the Hh and Wnt molecules. However, recent advances in the production or isolation of multipotent precursor cells that can be programmed into a variety of therapeutically relevant cell types coupled with improved 3-D printing processes suggest that a re-allocation of such resources with increased investment in these pathways as part of tissue engineering programs may be timely. The evaluation of drug candidates in the production of certain cell types could afford robust specificity and toxicity testing platforms that are complementary to current approaches for assessing such parameters. At the same time, the seamless integration of newly engineered tissues into the host remains a daunting challenge despite the recent advances in cellular engineering. Potentially, molecules targeting development pathways such as the ones described here could be used to improve cell transplantation for regenerative needs in patients with cancer-ravaged tissues or in the elderly that are in need of tissue replacement therapy.
ACKNOWLEDGEMENTS
We thank Rubina Tuladhar for useful discussions. This work was funded by the National Institutes of Health Grant R01CA168761 and P50-CA70907 (L.L.), the Welch Foundation Grants I-1665 (L.L.), and the Cancer Prevention and Research Institute of Texas Grant RP130212 (L.L. and C.C.).
Footnotes
CONFLICT OF INTEREST
LL and CC are named inventors on several patents relating to the IWR and IWP classes of Wnt pathway inhibitors.
REFERENCES
- [1].Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell stem cell. 2013;12(2):152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13(7):497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- [4].Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19(4):379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- [5].Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011 doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109(2):466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nature reviews. Endocrinology. 2013;9(10):575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- [9].Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harbor perspectives in biology. 2012;4(12) doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocrine reviews. 2012;33(5):747–783. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- [11].Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- [12].Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt pathways in disease. Cold Spring Harbor perspectives in biology. 2012;4(11) doi: 10.1101/cshperspect.a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- [14].Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, Machon O, Korinek V, Choo E, Diaz D, Merchant M, Polakis P, Holsworth DD, Krauss S, Costa M. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73(10):3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- [15].Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110(50):20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, Gradl D, Voronkov A, von Kries JP, Krauss S. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72(11):2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- [17].Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25(3):111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- [18].Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gay MH, Valenta T, Herr P, Paratore-Hari L, Basler K, Sommer L. Distinct adhesion-independent functions of beta-catenin control stage-specific sensory neurogenesis and proliferation. BMC biology. 2015;13:24. doi: 10.1186/s12915-015-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Valenta T, Gay M, Steiner S, Draganova K, Zemke M, Hoffmans R, Cinelli P, Aguet M, Sommer L, Basler K. Probing transcription-specific outputs of beta-catenin in vivo. Genes Dev. 2011;25(24):2631–2643. doi: 10.1101/gad.181289.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chiang YJ, Hsiao SJ, Yver D, Cushman SW, Tessarollo L, Smith S, Hodes RJ. Tankyrase 1 and tankyrase 2 are essential but redundant for mouse embryonic development. PLoS ONE. 2008;3(7):e2639. doi: 10.1371/journal.pone.0002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355(2):275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- [23].Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108(31):12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141(11):2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- [25].Kabiri Z, Numata A, Kawasaki A, Blank E, Tenen DG, Virshup DM. Wnts are dispensable for differentiation and self-renewal of adult murine hematopoietic stem cells. Blood. 2015 doi: 10.1182/blood-2014-09-598540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, Lang RA, Williams BO. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109(33):E2197–2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131(7):1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- [28].Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- [29].Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- [31].Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- [32].Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. beta-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60(3):964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harbor perspectives in biology. 2012;4(11) doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci U S A. 2011;108(15):5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer science. 2014;105(9):1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morris LG, Kaufman AM, Gong Y, Ramaswami D, Walsh LA, Turcan S, Eng S, Kannan K, Zou Y, Peng L, Banuchi VE, Paty P, Zeng Z, Vakiani E, Solit D, Singh B, Ganly I, Liau L, Cloughesy TC, Mischel PS, Mellinghoff IK, Chan TA. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet. 2013;45(3):253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tang W, Dodge M, Gundapaneni D, Michnoff C, Roth M, Lum L. A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc Natl Acad Sci U S A. 2008;105(28):9697–9702. doi: 10.1073/pnas.0804709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lehtio L, Chi NW, Krauss S. Tankyrases as drug targets. FEBS J. 2013;280(15):3576–3593. doi: 10.1111/febs.12320. [DOI] [PubMed] [Google Scholar]
- [39].Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T, Anchang B, Demir K, Boehm C, Leible S, Ball CR, Glimm H, Spang R, Boutros M. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nature communications. 2013;4:2610. doi: 10.1038/ncomms3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- [41].Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, Smith TR, Avello M, Charlat O, Xie Y, Porter JA, Pan S, Liu J, McLaughlin ME, Cong F. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110(31):12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- [43].Koo BK, van Es JH, van den Born M, Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci U S A. 2015;112(24):7548–7550. doi: 10.1073/pnas.1508113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annual review of genetics. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Blauwkamp TA, Nigam S, Ardehali R, Weissman IL, Nusse R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nature communications. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nature protocols. 2013;8(1):162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Narytnyk A, Verdon B, Loughney A, Sweeney M, Clewes O, Taggart MJ, Sieber-Blum M. Differentiation of human epidermal neural crest stem cells (hEPI-NCSC) into virtually homogenous populations of dopaminergic neurons. Stem cell reviews. 2014;10(2):316–326. doi: 10.1007/s12015-013-9493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ren Y, Lee MY, Schliffke S, Paavola J, Amos PJ, Ge X, Ye M, Zhu S, Senyei G, Lum L, Ehrlich BE, Qyang Y. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol. 2011;51(3):280–287. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell stem cell. 2012;10(6):771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- [51].Distler A, Deloch L, Huang J, Dees C, Lin NY, Palumbo-Zerr K, Beyer C, Weidemann A, Distler O, Schett G, Distler JH. Inactivation of tankyrases reduces experimental fibrosis by inhibiting canonical Wnt signalling. Annals of the rheumatic diseases. 2013;72(9):1575–1580. doi: 10.1136/annrheumdis-2012-202275. [DOI] [PubMed] [Google Scholar]
- [52].Henderson WR, Jr., Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107(32):14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang C, Zhu H, Sun Z, Xiang Z, Ge Y, Ni C, Luo Z, Qian W, Han X. Inhibition of Wnt/beta-catenin signaling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. American journal of physiology. Cell physiology. 2014;307(3):C234–244. doi: 10.1152/ajpcell.00366.2013. [DOI] [PubMed] [Google Scholar]
- [54].Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Curtin N. PARP inhibitors for anticancer therapy. Biochemical Society transactions. 2014;42(1):82–88. doi: 10.1042/BST20130187. [DOI] [PubMed] [Google Scholar]
- [56].Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23(17):2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138(1):90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Palm W, de Lange T. How shelterin protects mammalian telomeres. Annual review of genetics. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- [59].Li N, Zhang Y, Han X, Liang K, Wang J, Feng L, Wang W, Songyang Z, Lin C, Yang L, Yu Y, Chen J. Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth. Genes Dev. 2015;29(2):157–170. doi: 10.1101/gad.251785.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chi NW, Lodish HF. Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. The Journal of biological chemistry. 2000;275(49):38437–38444. doi: 10.1074/jbc.M007635200. [DOI] [PubMed] [Google Scholar]
- [61].Guo HL, Zhang C, Liu Q, Li Q, Lian G, Wu D, Li X, Zhang W, Shen Y, Ye Z, Lin SY, Lin SC. The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation. Cell Res. 2012;22(8):1246–1257. doi: 10.1038/cr.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thorvaldsen TE, Pedersen NM, Wenzel EM, Schultz SW, Brech A, Liestol K, Waaler J, Krauss S, Stenmark H. Structure, Dynamics and Functionality of Tankyrase Inhibitor-induced Degradasomes. Mol Cancer Res. 2015 doi: 10.1158/1541-7786.MCR-15-0125. [DOI] [PubMed] [Google Scholar]
- [63].Vyas S, Chesarone-Cataldo M, Todorova T, Huang YH, Chang P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nature communications. 2013;4:2240. doi: 10.1038/ncomms3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chang P, Coughlin M, Mitchison TJ. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat Cell Biol. 2005;7(11):1133–1139. doi: 10.1038/ncb1322. [DOI] [PubMed] [Google Scholar]
- [65].Wahlberg E, Karlberg T, Kouznetsova E, Markova N, Macchiarulo A, Thorsell AG, Pol E, Frostell A, Ekblad T, Oncu D, Kull B, Robertson GM, Pellicciari R, Schuler H, Weigelt J. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nature biotechnology. 2012;30(3):283–288. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- [66].Gunaydin H, Gu Y, Huang X. Novel binding mode of a potent and selective tankyrase inhibitor. PLoS ONE. 2012;7(3):e33740. doi: 10.1371/journal.pone.0033740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Narwal M, Venkannagari H, Lehtio L. Structural basis of selective inhibition of human tankyrases. Journal of medicinal chemistry. 2012;55(3):1360–1367. doi: 10.1021/jm201510p. [DOI] [PubMed] [Google Scholar]
- [68].Haikarainen T, Krauss S, Lehtio L. Tankyrases: structure, function and therapeutic implications in cancer. Current pharmaceutical design. 2014;20(41):6472–6488. doi: 10.2174/1381612820666140630101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Haikarainen T, Venkannagari H, Narwal M, Obaji E, Lee HW, Nkizinkiko Y, Lehtio L. Structural basis and selectivity of tankyrase inhibition by a Wnt signaling inhibitor WIKI4. PLoS ONE. 2013;8(6):e65404. doi: 10.1371/journal.pone.0065404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Voronkov A, Holsworth DD, Waaler J, Wilson SR, Ekblad B, Perdreau-Dahl H, Dinh H, Drewes G, Hopf C, Morth JP, Krauss S. Structural basis and SAR for G007-LK, a lead stage 1,2,4-triazole based specific tankyrase 1/2 inhibitor. Journal of medicinal chemistry. 2013;56(7):3012–3023. doi: 10.1021/jm4000566. [DOI] [PubMed] [Google Scholar]
- [71].Shultz MD, Cheung AK, Kirby CA, Firestone B, Fan J, Chen CH, Chen Z, Chin DN, Dipietro L, Fazal A, Feng Y, Fortin PD, Gould T, Lagu B, Lei H, Lenoir F, Majumdar D, Ochala E, Palermo MG, Pham L, Pu M, Smith T, Stams T, Tomlinson RC, Toure BB, Visser M, Wang RM, Waters NJ, Shao W. Identification of NVP-TNKS656: the use of structure-efficiency relationships to generate a highly potent, selective, and orally active tankyrase inhibitor. Journal of medicinal chemistry. 2013;56(16):6495–6511. doi: 10.1021/jm400807n. [DOI] [PubMed] [Google Scholar]
- [72].Gao X, Hannoush RN. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat Chem Biol. 2014;10(1):61–68. doi: 10.1038/nchembio.1392. [DOI] [PubMed] [Google Scholar]
- [73].Dodge ME, Lum L. Drugging the cancer stem cell compartment: lessons learned from the hedgehog and Wnt signal transduction pathways. Annu Rev Pharmacol Toxicol. 2011;51:289–310. doi: 10.1146/annurev-pharmtox-010510-100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dodge ME, Moon J, Tuladhar R, Lu J, Jacob LS, Zhang LS, Shi H, Wang X, Moro E, Mongera A, Argenton F, Karner CM, Carroll TJ, Chen C, Amatruda JF, Lum L. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. The Journal of biological chemistry. 2012;287(27):23246–23254. doi: 10.1074/jbc.M112.372029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rios-Esteves J, Haugen B, Resh MD. Identification of key residues and regions important for porcupine-mediated Wnt acylation. The Journal of biological chemistry. 2014;289(24):17009–17019. doi: 10.1074/jbc.M114.561209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang X, Moon J, Dodge ME, Pan X, Zhang L, Hanson JM, Tuladhar R, Ma Z, Shi H, Williams NS, Amatruda JF, Carroll TJ, Lum L, Chen C. The development of highly potent inhibitors for porcupine. Journal of medicinal chemistry. 2013;56(6):2700–2704. doi: 10.1021/jm400159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Duraiswamy AJ, Lee MA, Madan B, Ang SH, Tan ES, Cheong WW, Ke Z, Pendharkar V, Ding LJ, Chew YS, Manoharan V, Sangthongpitag K, Alam J, Poulsen A, Ho SY, Virshup DM, Keller TH. Discovery and Optimization of a Porcupine Inhibitor. Journal of medicinal chemistry. 2015 doi: 10.1021/acs.jmedchem.5b00507. [DOI] [PubMed] [Google Scholar]
- [78].Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337(6090):59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Miranda M, Galli LM, Enriquez M, Szabo LA, Gao X, Hannoush RN, Burrus LW. Identification of the WNT1 residues required for palmitoylation by Porcupine. FEBS Lett. 2014;588(24):4815–4824. doi: 10.1016/j.febslet.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. The Journal of biological chemistry. 2008;283(32):22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Long J, Tokhunts R, Old WM, Houel S, Rodgriguez-Blanco J, Singh S, Schilling N, Capobianco AJ, Ahn NG, Robbins DJ. Identification of a family of Fatty-Acid-speciated sonic hedgehog proteins, whose members display differential biological properties. Cell reports. 2015;10(8):1280–1287. doi: 10.1016/j.celrep.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shindou H, Eto M, Morimoto R, Shimizu T. Identification of membrane O-acyltransferase family motifs. Biochemical and biophysical research communications. 2009;383(3):320–325. doi: 10.1016/j.bbrc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- [83].Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: post-translational modification of RAS. Nature reviews. 2012;13(1):39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. The Journal of biological chemistry. 1997;272(22):14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- [85].Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. The Journal of biological chemistry. 2012;287(41):34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kulak O, Chen H, Holohan B, Wu X, He H, Borek D, Otwinowski Z, Yamaguchi K, Garofalo LA, Ma Z, Wright W, Chen C, Shay JW, Zhang X, Lum L. Disruption of Wnt/beta-Catenin Signaling and Telomeric Shortening Are Inextricable Consequences of Tankyrase Inhibition in Human Cells. Mol Cell Biol. 2015;35(14):2425–2435. doi: 10.1128/MCB.00392-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R, Hewitt JF, Zak M, Peck A, Orr C, Merchant M, Hoeflich KP, Chan J, Luoh SM, Anderson DJ, Ludlam MJ, Wiesmann C, Ultsch M, Friedman LS, Malek S, Belvin M. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501(7466):232–236. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- [89].Riffell JL, Lord CJ, Ashworth A. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov. 2012;11(12):923–936. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- [90].Zhan P, Song Y, Itoh Y, Suzuki T, Liu X. Recent advances in the structure-based rational design of TNKSIs. Molecular bioSystems. 2014;10(11):2783–2799. doi: 10.1039/c4mb00385c. [DOI] [PubMed] [Google Scholar]