Abstract
Background
Up to 90 % of people with scleroderma have gastrointestinal (GI) problems such as constipation, bloating, diarrhea, and malabsorption. These problems significantly impair quality of life. Our objective was to determine the risk factors for gastrointestinal issues in people with scleroderma.
Methods
We conducted a systematic review of observational studies that report GI problems in patients with scleroderma along with the associated risk factors. We were interested in any GI problem and any risk factor as long as the study included patients diagnosed with scleroderma according to the 1980 or 2013 American College of Rheumatology guideline. We searched the following databases: CINAHL, EMBASE, LILACS, MEDLINE, and Web of Science for relevant articles from June 1884 to May 2014. Two authors independently screened citations and full text articles and extracted data. Discrepancies were resolved by consensus or by consulting a third author. Methodological quality of included studies was assessed using the Newcastle-Ottawa Scale.
Results
After removing duplicates, 645 unique citations were identified. A total of three studies, three cross-sectional (n = 64, n = 42, n = 606), were included in this systematic review. Collectively, these three studies explored Helicobacter pylori and smoking status as risk factors. We found conflicting evidence on the role of H. pylori with two studies showing opposite yet statistically significant results. One moderate quality study showed smoking as a risk factor. Key limitations include the small sample sizes of two studies and poor study designs to draw causal links.
Conclusions
There is insufficient evidence to describe the risk factors for GI problems in patients with scleroderma. Longitudinal observational studies are warranted in patients with scleroderma.
Systematic review registration
PROSPERO CRD42014010707
Keywords: Systematic review, Scleroderma, Gastrointestinal, Risk factors
Background
Scleroderma, otherwise known as systemic sclerosis, is a disease manifested by collagen overproduction affecting various organs including the gastrointestinal (GI) tract [1]. Patients with scleroderma commonly present with inflammation and fibrosis of the skin, vascular abnormalities, organ damage, and autoantibody production [2]. Diagnosis is made when these signs and corresponding symptoms are present [3]. Scleroderma is a debilitating disease that is estimated to affect approximately 16,000 Canadians—with four to five times more females than males [1]. The prevalence of the disease is higher among people of African origin and varies across countries, appearing more in North America and Australia than in Europe and Japan [4]. Scleroderma is associated with debilitating morbidity such as reduction in mobility and depression [5]. Patients with this disorder have a 30.8 % mortality rate, although this number can vary depending on gender and which visceral organs are involved [6]. Currently, there are no effective treatments to combat scleroderma, partly because its pathogenesis remains unclear [7]. Consequently, much attention has shifted towards predicting the complications of scleroderma and managing them appropriately [8]. The underlying rationale is that it is easier to manage the disease before these complications arise [8].
In around 90 % of all patients with scleroderma, the GI tract is involved. This contributes considerably to impairment in quality of life [9–11]. Malabsorption, gastroesophageal reflux, nausea, vomiting, diarrhea, and constipation are some of the GI complications that can occur [9]. Although environmental risk factors are clearly linked to increased risk of developing scleroderma [12], it is unclear whether the GI involvement secondary to scleroderma is influenced by these environmental factors [12]. This study reviews the association between GI involvement and environmental and other exposures in patients with scleroderma. These findings will help patients with scleroderma and healthcare professionals in preventing GI morbidity. In Canada, the average annual economic burden of scleroderma is estimated to be $18,453 per patient [13]. Knowing that the cost of care for patients with scleroderma increases with more organ involvement, the findings from this study can inform policy developers to identify ways to curb healthcare costs [13]. The objective of this systematic review was to determine the factors associated with development of GI problems in patients with scleroderma.
Methods
Criteria for inclusion of studies
Design and time frame
Observational studies from June 1884 to May 2014 were considered in this review. June 1884 was chosen because it is the earliest entry of scleroderma in available databases.
Exposure
The exposures of interest were silica, silicone implantation and rupture, antacid, solvents, epoxy resins, welding fumes, anorexigens, pentazocine, bromocriptine, l-tryptophan, pesticides, constant hand/arm vibration, alcohol, intestinal microbiota, and food along with any other reported risk factors [14–19].
Participants
The population of interest was patients with a formal diagnosis of systemic sclerosis according to the 1980 and 2013 American College of Rheumatology guidelines [20, 21].
Outcome
Outcomes of interest included any reported GI problems such as nausea, vomiting, and diarrhea.
Search methods for identification of studies
Search strategy
The lead author (BYH) with the help of a hospital librarian (LC) developed the search strategy. We designed a comprehensive and exhaustive search strategy of five electronic databases to identify observational studies that reported on risk factors for GI problems in scleroderma. Our search strategy for MEDLINE via Ovid is reported in Table 1.
Table 1.
Search strategy of MEDLINE via Ovid
| Database: Ovid MEDLINE(R) | |
|---|---|
| Search strategy: | |
| 1. | scleroderma.mp. |
| 2. | systemic sclerosis.mp. |
| 3. | exp scleroderma, systemic/ |
| 4. | exp scleroderma, localized/ |
| 5. | or/1-4 |
| 6 | risk.mp. |
| 7. | exp risk/ |
| 8. | ((factor$ or feature$ or aspect$ or characteristic$) adj4 (risk)).mp. |
| 9. | exp silicon dioxide/ |
| 10. | exp silicones/ |
| 11. | exp antacids/ |
| 12. | exp solvents/ |
| 13. | organic solvent$.mp. |
| 14. | exp epoxy resins/ |
| 15. | welding fume$ or welding gas*.mp. |
| 16. | exp anorexigenic drugs/ |
| 17. | exp pentazocine/ |
| 18. | exp bromocriptine/ |
| 19. | exp tryptophan/ |
| 20. | exp pesticides/ |
| 21. | exp vibration/ |
| 22. | hand vibration or arm vibration.mp. |
| 23. | exp alcohol drinking/ |
| 24. | or/6-23 |
| 25. | exp gastrointestinal tract/ |
| 26. | exp gastrointestinal diseases/ |
| 27. | ((problem$ or issue$ or concern$ or complication$) adj3 (alimentary canal or alimentary tract or digestive tube or digestive tract or GI tract)).mp. |
| 28. | exp weight loss/ |
| 29. | uninten** weight loss.mp. |
| 30. | ((uninten**) adj3 (weight loss)).mp. |
| 31. | exp appetite/ |
| 32. | poor appetite or limited appetite or reduced appetite or sparse appetite.mp. 34. ((poor or limited or reduced or sparse) adj3 (appetite)).mp. |
| 35. | exp deglutition disorders/ |
| 36. | dysphagia or swallowing disorders.mp. |
| 37 | exp gastroesophageal reflux/ |
| 38. | acid reflux.mp. |
| 39. | exp colonic pseudo-obstruction/ |
| 40. | exp duodenal obstruction/ |
| 41. | exp intestinal obstruction/ |
| 42. | exp intestinal pseudo-obstruction/ |
| 43. | chok*.mp. |
| 44. | early satiety.mp. |
| 45. | bloat* or disten* or bulg* or enlarg* or expan*.mp. |
| 46. | exp nausea/ |
| 47. | exp vomiting/ |
| 48. | exp constipation/ |
| 49. | exp diarrhea/ |
| 50. | ((use or prescri** or administ**) adj2 (antibiotic$ or antibacterial$ or antimicrobial$)).mp. |
| 51. | exp steatorrhea/ |
| 52. | exp fecal incontinence/ |
| 53. | exp parenteral nutrition/ |
| 54. | or/25-53 |
| 55. | 5 and 24 and 54 |
Databases searched
Cumulative Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica database (EMBASE), Latin American and Caribbean Health Sciences Literature (LILACS), Medical Literature Analysis and Retrieval System Online (MEDLINE), and Web of Science were searched to identify relevant citations for this systematic review.
Other sources searched
We manually checked the reference lists of studies identified in an attempt to identify additional publications. Conference websites were not separately searched because EMBASE catalogues abstracts from several notable rheumatology conferences. A practicing rheumatologist and also an author of this review (ML) was queried to identify additional studies. We searched the Scleroderma Society of Canada, the Scleroderma Foundation, and the Scleroderma Research Foundation websites.
Study screening, selection, and assessment of methodological quality
Two authors (BYH and RJ), working independently, reviewed all citations and abstracts resulting from the search strategy to identify eligible papers. The full text of eligible articles were obtained and assessed independently using a pre-designed eligibility form based on the inclusion criteria. Eligible studies were included in the review. Disagreements were resolved by discussion or by seeking the opinion of a third party (LT/ML). The two authors (BYH and RJ) independently assessed the methodological quality of included studies using the Newcastle-Ottawa Scale and extracted data using a pre-established data extraction form [22].
Data analysis
We planned to pool the results using the DerSimmonian and Laird random effects method if there was a measure of relative risk (risk ratio, odds ratio, hazard ratio) and precision (95 % confidence intervals, standard error, variance) and if there was sufficient clinical and statistical homogeneity [23], and present the results as an odds ratios with 95 % confidence interval and p values. Between-study heterogeneity was also to be tested using Cochran’s Q and the I2 statistic. However, given the considerable clinical and design heterogeneity in the included studies, we adopted a narrative approach to summarize our findings.
Results
Results of search
Our electronic literature search identified a total of 645 unique citations after duplicates were removed. One study was identified by searching the reference lists of included studies, but was excluded from this review. Title and abstract screening identified 235 articles that were potentially eligible for inclusion. During the full text screening, 232 citations were removed resulting in a total of three studies being included [24–26]. Figure 1 provides a flow diagram of the study selection process and the reasons for exclusion.
Fig. 1.
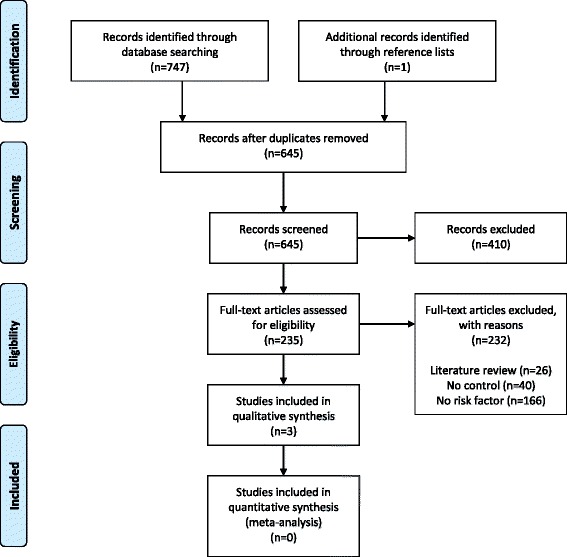
Flow diagram of study selection
Overview of study characteristics and summary of study findings
There were three studies included in this systematic review, conducted in Japan, Canada, and Croatia in 2008, 2011, and 2013, respectively [24–26]. The number of enrolled patients ranged from 42 to 606 with the total of 712 patients included in this systematic review [24–26]. All three citations were cross-sectional studies [24–26]. Although all three studies examined GI problems as the outcome of interest, only one used endoscopic examination to diagnose reflux esophagitis [24], while the other two relied on self-reported severity of GI problems. Two studies examined Helicobacter pylori as a risk factor for GI problems in patients with scleroderma but gave contrasting results [24, 26]. The three included studies are summarised in Table 3.
Table 3.
Summary of included studies
| Author, year | Country | Time | Study design | Sample size | Age (years) | Male:female | Patient characteristics | Exposure | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yamaguchi et al. [24] | Japan | Oct 1998–June 2005 | Cross-sectional | n = 64 | Mean, range = 60.7, 24–85 | 12:52 | Disease duration (<1 year, <5, <10, >10): n = 19, n = 17, n = 18, n = 10 | H. pylori: positive (n = 37), negative (n = 27) | H. pylori positive | |
| % reflux esophagitis 27 | ||||||||||
| % diffuse disease 17 | H. pylori negative | |||||||||
| % smoking 30 | % reflux esophagitis 70 | |||||||||
| % alcohol 34 | ||||||||||
| % corticosteroid use 72 | ||||||||||
| Hudson et al. [25] | Canada | Aug 2004–Nov 2009 | Cross-sectional | n = 606 | Mean, SD = 55, 12 | 79:527 | % Caucasian: 87.0 | Smoking status: never (n = 255), past (n = 252; mean pack-years of smoking = 17, SD = 18), current (n = 99; mean pack-years of smoking = 25, SD = 17) | Never | Current |
| Disease duration (mean, SD): 11, 9 | Mean severity of GI symptoms: 1.61 out of 10 | Mean severity of GI symptoms: 2.18 out of 10 | ||||||||
| % diffuse disease 36.0 | Mean number of GI symptoms: 3.99 out of 14a | Mean number of GI symptoms: 4.91 out of 14a | ||||||||
| % poor appetite 28.6a | % poor appetite 47.5a | |||||||||
| % difficulty swallowing 54.1 | % difficulty swallowing 59.6 | |||||||||
| % acid reflux 60.8 | % acid reflux 68.7 | |||||||||
| % nocturnal choking 28.6 | % nocturnal choking 29.3 | |||||||||
| % heartburn 41.6 | % heartburn 54.5 | |||||||||
| % early satiety 38.8 | % early satiety 51.5 | |||||||||
| % abdominal bloating 34.9 | % abdominal bloating 43.4 | |||||||||
| % nausea and vomiting 14.1 | % nausea and vomiting 23.2 | |||||||||
| % chronic constipation 25.5 | % chronic constipation 32.3 | |||||||||
| % chronic diarrhea 23.9 | % chronic diarrhea 26.3 | |||||||||
| % antibiotics for bacterial overgrowth 7.5 | % antibiotics for bacterial overgrowth 6.1 | |||||||||
| % greasy stools 18.8 | % greasy stools 24.2 | |||||||||
| % fecal incontinence 19.2 | % fecal incontinence 22.2 | |||||||||
| % parenteral nutrition 2.4 | % parenteral nutrition 2.0 | |||||||||
| % taking medications for GI symptoms 73.7 | % taking medications for GI symptoms 59.6 | |||||||||
| % taking medications for GI symptoms 67.1 | ||||||||||
| Radic et al. [26] | Croatia | Jan 2009–Dec 2010 | Cross-sectional | n = 42 | Mean, SD = 54.3, 13.6 | 4:38 | Disease duration (median, range): 6, 1–43 years | H. pylori: positive (n = 26), negative (n = 16) | H. pylori positive | |
| % GI problems 85 | ||||||||||
| % diffuse disease 95.3 | H. pylori negative | |||||||||
| % GI problems 31 | ||||||||||
aDenotes p value less than 0.05
Yamaguchi et al. [24]
The study by Yamaguchi et al. was a cross-sectional study that examined whether or not the presence of H. pylori infection was associated with reflux esophagitis in patients with scleroderma. Of the 138 patients with scleroderma, 74 were excluded because they were taking medications for their GI issues or had laparotomy. Of the remaining 64 patients (mean age = 60.7 years, % female 81.3, % diffuse disease 17), 37 were H. pylori positive. Without adjusting for covariates, it was found that being H. pylori positive is protective against reflux esophagitis in patients with scleroderma (OR = 0.16, 95 % CI = 0.05–0.47).
Hudson et al. [25]
The study by Hudson et al. was also a cross-sectional study, which examined whether smoking (never, past, or current) was associated with GI problems in patients with scleroderma. It examined the presence of 14 different GI problems and the severity of GI symptoms. Six hundred and six patients (mean age = 55.3 years, % female 87, % diffuse disease 36) were included in the analysis. Adjusted for covariates (age, gender, ethnicity, disease duration, diffuse disease), the regression model showed that only the number of GI symptoms and poor appetite showed statistical significant worsening with smoking.
Radic et al. [26]
The study by Radic et al. was another cross-sectional study that examined whether or not the presence of H. pylori infection was associated with self-reported GI problems in patients with scleroderma. Of the 42 patients (mean age = 54.3 years, % female 90.5, % diffuse disease 95.3), 26 were H. pylori positive. An adjusted analysis revealed that being H. pylori positive is correlated with high prevalence of GI issues.
Methodological quality of included studies
Methodological quality of included studies was assessed using the Newcastle-Ottawa Scale and graded according to the guidelines [22]. Being that all three studies were cross-sectional, two criteria regarding follow-up in the Newcastle-Ottawa Scale did not apply. Thus, of the possible 6 points, Yamaguchi et al. scored 4, Radic et al. scored 5, and Hudson et al. scored 6 points. Overall, the quality of included studies were generally low (see Table 2).
Table 2.
Methodological quality of included studies (Newcastle-Ottawa Scale)
| Author, year | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Assessment of outcome | Adequacy of duration of follow-up | Adequacy of completeness of follow-up | ||
| Yamaguchi et al. [24] | √ | √ | √ | ‡ | ‡ | √ | N/A | N/A |
| Hudson et al. [25] | √ | √ | ‡ | √ | √ | ‡ | N/A | N/A |
| Radic et al. [26] | √ | √ | √ | ‡ | √ | √ | N/A | N/A |
√ = yes; ‡ = no
Discussion
Summary of main findings
A total of three cross-sectional studies generally of low quality were included in this systematic review (Table 3). Two studies explored H. pylori infection as a risk factor for GI problems in patients with scleroderma but showed opposing results. Although Yamaguchi et al. [24] had a slightly bigger sample size, only Radic et al. [26] controlled for confounding variables in the analysis. The difference between these two studies may also be attributable to the difference in the patient demographics and outcome adjudication. Only 17 % of the patients had diffuse scleroderma in Yamaguchi et al.’s study, while 95 % had diffuse scleroderma in Radic et al.’s study [26]. Furthermore, Yamaguchi et al. [24] employed endoscopic examination to diagnose reflux esophagitis, while Radic et al. [26] used patient-reported GI problems. Hudson et al.’s study examines the effect of smoking on GI problems in patients with scleroderma and showed that higher proportion of patients with scleroderma who smoke had GI problems [25]. Yamaguchi et al., Hudson et al., and Radic et al. [24–26] looked at reflux esophagitis, poor appetite, and GI symptom severity as the outcome, respectively. None of the papers reviewed discussed other environmental exposures such as silica and epoxy.
Limitations
There are limitations to be noted in our systematic review. First, while our search did not include the gray literature, we undertook a thorough and rigorous search of CINAHL, EMBASE, GI, LILACS, and MEDLINE. Second, the extent of available data with regards to covariates was limited and the length of follow-up was often not specified, which may not be adequate for the observation of GI problems in patients with scleroderma. Third, all three studies are cross-sectional, which are not useful in determining predictors of outcome. These deficiencies highlight the need for further research to address the current knowledge gap exploring the risk factors associated with GI problems in patients with scleroderma.
Implications for practice
There is not enough evidence to inform clinical practice. This paper included three studies that report H. pylori infection (two studies) and smoking (one study) as potential risk factors for GI problems in patients with scleroderma. Given that the evidence is sparse and generally of low quality and that the two included studies on H. pylori contradict each other. It is unclear how to incorporate these findings into clinical practice. Smoking, however, seems to be associated with a wide range of GI problems.
Implications for research
In the field of scleroderma, longitudinal observational studies are warranted to investigate the risk factors for GI problems. For rare exposures like silica and pesticides, case-control designs may be more appropriate. A wealth of useful information can also be obtained from well-kept scleroderma registries. The studies should include patients with scleroderma followed up prior to and after the development of GI problems. Data on all potential risk factors including H. pylori infection, smoking, exposure to environmental toxins, and others should be collected. All possible GI problems should be explored, including data on morbidity and mortality.
Conclusions
There is a lack of evidence to conclusively suggest or refute whether H. pylori infection or smoking is a definitive risk factor for developing GI problems in patients with scleroderma.
Acknowledgements
We thank Lois Cottrell for her assistance in developing and conducting the literature search.
Funding
None.
Abbreviations
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- EMBASE
Excerpta Medica Database
- GI
gastrointestinal
- LILACS
Latin American and Caribbean Health Sciences Literature
- MEDLINE
Medical Literature Analysis and Retrieval System Online
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BYH conceived of the study, revised the research question, provided content to the design, and conducted the study. RG revised the research question, provided content to the design, and conducted the study. LM revised the research question and provided content to the design. ML conceived of the study, revised the research question, and provided content to the design. LT conceived of the study, revised the research question, and provided content to the design. All authors read and approved the final version of the manuscript.
Contributor Information
Brian Younho Hong, Email: brian.youn.hong@learnlink.mcmaster.ca.
Raymond Giang, Email: rgiang@uwaterloo.ca.
Lawrence Mbuagbaw, Email: mbuagblc@mcmaster.ca.
Maggie Larche, Email: mlarche@mcmaster.ca.
Lehana Thabane, Email: thabanl@mcmaster.ca.
References
- 1.Hansi N, Thoua N, Carulli M, Chakravarty K, Lal S, Smyth A, et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin Exp Rheumatol 2014;32:S-214-21. [PubMed]
- 2.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 2010;39:269–77. doi: 10.1016/j.semarthrit.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40:78–83. doi: 10.1007/s12016-010-8198-y. [DOI] [PubMed] [Google Scholar]
- 4.Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev. 2010;9:A311–8. doi: 10.1016/j.autrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Malcarne VL, Hansdottir I, McKinney A, Upchurch R, Greenbergs HL, Henstorf GH, et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. J Rheumatol. 2007;34:359–67. [PubMed] [Google Scholar]
- 6.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine. 2002;81:139–53. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Smith V, Riccieri V, Pizzorni C, Decuman S, Deschepper E, Bonroy C, et al. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J Rheumatol. 2014;40:2023–8. doi: 10.3899/jrheum.130528. [DOI] [PubMed] [Google Scholar]
- 8.Smith V, Decuman S, Sulli A, Bonroy C, Piettte Y, Deschepper E, et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann Rheum Dis. 2012;71:1636–9. doi: 10.1136/annrheumdis-2011-200780. [DOI] [PubMed] [Google Scholar]
- 9.Baron M, Bernier P, Cote F, Delegge MH, Falovitch G, Friedman G et al. Screening and management of malnutrition and related gastro-intestinal disorders in systemic sclerosis: recommendations of a North American expert panel. Clinical and Experimental Rheumatology. 2010; (Suppl. 58): S42-6. [PubMed]
- 10.Khanna D, Nagaraja V, Gladue H, Chey W, Pimentel M, Frech T. Measuring response in the gastrointestinal tract in systemic sclerosis. Curr Opin Rheumatol. 2013;25:700–6. doi: 10.1097/01.bor.0000434668.32150.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37:223–35. doi: 10.1016/j.semarthrit.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Nietert PJ, Richard S. Systemic sclerosis: environmental and occupational risk factors. Current Opinion in Rhuematology. 2000;12:520–6. doi: 10.1097/00002281-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bernatsky S, Hudson M, Panopalis P, Clarke AE, Pope J, Leclercq S, et al. The cost of systemic sclerosis. Arthritis Care Res. 2009;61:119–23. doi: 10.1002/art.24086. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal SK, Tan FK, Arnett FC. Genetics and genomic studies in scleroderma (systemic sclerosis) Rheum Dis Clin N Am. 2008;34:17–40. doi: 10.1016/j.rdc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Lidar M, Agmon-Levin N, Langevitz P, Shoenfeld Y. Silicone and scleroderma revisited. Lupus. 2012;21:121–7. doi: 10.1177/0961203311430703. [DOI] [PubMed] [Google Scholar]
- 16.Wielosz E, Borys O, Zychowska I, Wielowieyska-Szybińska D, Zabińska-Płazak E, Kiełtyka A. Gastrointestinal involvement in patients with systemic sclerosis. Polish Archives Int Med. 2010;120:132–6. [PubMed] [Google Scholar]
- 17.Diot E, Lesire V, Guilmot JL, Metzger MD, Pilore R, Rogier S. Systemic sclerosis and occupational risk factors: a case-control study. Occup Environ Med. 2002;59:545–9. doi: 10.1136/oem.59.8.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie I, Gehanno JF, Bubenheim M, Duval-Modeste AB, Joly P, Dominique S. Prospective study to evaluate the association between systemic sclerosis and occupational exposure and review of the literature. Autoimmun Rev. 2014;13:151–6. doi: 10.1016/j.autrev.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Silman AJ. Epidemiology of scleroderma. Annals of Rheumatic Diseases. 1991;50:846–53. doi: 10.1136/ard.50.Suppl_4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-90. [DOI] [PubMed]
- 21.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis. Ann Rheum Dis. 2013;72:1747–55. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care. 2. London: BMJ Publishing; 2001. pp. 285–312. [Google Scholar]
- 24.Yamaguchi K, Iwakiri R, Hara M, Kikkawa A, Fujise T, Ootani H. Reflux esophagitis and Helicobacter pylori infection in patients with scleroderma. Intern Med. 2008;47:1555–9. doi: 10.2169/internalmedicine.47.1128. [DOI] [PubMed] [Google Scholar]
- 25.Hudson M, Steele R, Lu Y, Hercz D, Baron M, Steele R, et al. Cigarette smoking in patients with systemic sclerosis. Arthritis Rheum. 2011;63:230–8. doi: 10.1002/art.30071. [DOI] [PubMed] [Google Scholar]
- 26.Radic M, Kaliterna DM, Bonacin D, Vergles JM, Radić J, Fabijanić D, et al. Is Helicobacter pylori infection a risk factor for disease severity in systemic sclerosis? Rheumatol Int. 2013;33:2943–8. doi: 10.1007/s00296-012-2585-z. [DOI] [PubMed] [Google Scholar]


