Abstract
Background
The indirect basophil activation test using flow cytometry is a promising tool for autoimmune urticaria diagnosis. We aimed to identify better donor basophils (from atopic vs. non-atopic donors and interleukin-3 primed vs. unprimed basophils) and improve basophil identification and activation markers (eotaxin CC chemokine receptor-3 [CCR3] vs. CD123 and CD63 vs. CD203c).
Methods
Donor basophils were obtained from non-atopic and atopic group O donors. Positive control sera were artificially prepared to simulate autoimmune urticaria patients' sera. Patient sera were obtained from nine children with chronic urticaria. Assay sensitivity was compared among each variation by using positive control sera (n=21), applying cutoff values defined from negative control sera (n=20).
Results
For basophil identification, a combination of CCR3 and CD123 markers revealed a higher correlation with automated complete blood count (r=0.530) compared with that observed using CD123 (r=0.498) or CCR3 alone (r=0.195). Three activation markers on the atopic donor basophils attained 100% assay sensitivity: CD203c on unprimed basophils, CD63+CD203+ or CD63 alone on primed basophils; however, these markers on the non-atopic donor basophils attained lower assay sensitivity.
Conclusions
For basophil identification markers, a combination of CD123 and CCR3 is recommended, while CD123 alone may be used as an alternative. Donor basophils should be obtained from an atopic donor. For basophil activation markers, either CD203c alone on unprimed basophils or CD203c and CD63 on primed basophils are recommended, while CD63 alone on primed basophils may be used as an alternative.
Keywords: Chronic urticaria, Autoimmune urticaria, Basophil, Markers, Donor, Flow cytometry
INTRODUCTION
Urticaria has been traditionally classified into two subtypes, acute and chronic, on the basis of a symptom duration cutoff of six weeks [1]. The etiology of chronic urticaria is heterogeneous; physical urticaria (PU) accounts for an estimated 35% of the cases, while autoimmune urticaria (AIU) accounts for approximately 25% or more. The remaining cases (~35%), which have no known etiological factors despite extensive investigations, are diagnosed as chronic idiopathic urticaria (CIU). In patients with the AIU subtype, autoantibodies react with the α-chain of IgE receptor (FcεRIα) or with IgE itself, thereby directly cross-linking the IgE receptor and inducing histamine release [2].
The AIU subtype represents the most severe form of chronic urticaria. In addition, the effect of antihistamine therapy on this subtype is lower than that on the PU or CIU subtypes [3]. These clinical manifestations emphasize the necessity of differentiating AIU from other subtypes. Identification of the disease etiology is also beneficial to AIU patients, as it would help avoid extensive allergy testing and aggressive elimination of potentially allergenic food.
Conventionally, the basophil activation test (BAT) is performed to identify allergens that stimulate a patient's basophils in vitro (direct BAT). In the basophil histamine release assay (BHRA), supernatant histamine released from activated basophils is measured by immunoassay (e.g., enzyme-linked immunosorbent assay). The discovery of CD63 enabled BAT via flow cytometry (flow BAT) on a single cell level [4]. Flow BAT determines whether various allergens activate a patient's basophils in vitro (direct flow BAT) and can be used to diagnose atopic allergy or anaphylaxis.
Direct BAT cannot be used for AIU diagnosis, as the circulating basophils in these patients display reduced histamine releasability owing to their in vivo desensitization or downregulation [5,6]. In such cases, the activation of donor basophils in patient serum is tested in vitro (indirect BAT) [4]. Indirect BHRA is currently regarded as the gold standard for AIU diagnosis [7]. The autologous serum skin test (ASST) is a relatively simple in vivo screening test, in which the patient's serum is injected into his/her own skin intradermally to observe a wheal and flare reaction caused by activated mast cells or basophils in the skin. Unfortunately, this technique has both low assay sensitivity (70%) and specificity (80%) when compared with those of BHRA as a reference [8].
The basophils from highly sensitized atopic donors suffering from extrinsic atopic dermatitis or allergic rhinitis are good targets for demonstrating functional autoantibodies to FcεRIα [4,9]. Such atopic donors have a higher number of circulating basophils in an already primed state that express a larger number of surface FcεRIα and increased levels of serum IgE [10]. Furthermore, if the assay protocol uses whole blood as the basophil source and a red cell lysis step instead of a basophil enrichment step, the atopic donor should be blood group O to avoid agglutination of donor red cells by ABO antibodies in test serum. Therefore, if indirect flow BAT is performed as a routine test in the clinical laboratory, a suitable voluntary atopic donor with blood group O will be required, which may not always be possible.
In CD63-based direct flow BAT, a patient's basophils should be primed with interleukin (IL)-3 to increase assay sensitivity [11,12], except in the case of potent proteinaceous allergens [13]. When indirect flow BAT is used to diagnose AIU, Gyimesi et al. [10] have proposed that priming of donor basophils might not be crucial, even when the assay is based on CD63.
Indirect flow BAT is a promising tool for AIU diagnosis [4]; however, its diagnostic performance is highly dependent on the method used to prepare donor basophils and on the selection of basophil markers. Therefore, in this study, we searched for better donor basophils (from atopic vs. non-atopic donors and primed vs. unprimed basophils) and better basophil markers (eotaxin CC chemokine receptor-3 [CCR3] vs. CD123 as an identification marker and CD63 vs. CD203c as an activation marker).
METHODS
1. Donor basophils
Whole blood anticoagulated with EDTA was obtained daily from non-atopic and atopic blood group O donors. Non-atopic donors (DNA) were healthy individuals, without atopy history, that visited the Health Promotion Center at Kyungpook National University Hospital (Daegu, Korea) for routine health checkups (n=23). A male patient with blood group O that displayed a higher level of atopy compared with that of the other patients was selected as an atopic donor (DA). His total serum IgE level was 156 IU/mL. Allergen-specific IgE was identified for yeast (0.35 IU/mL), Alternaria (0.49 IU/mL), and Candida (0.88 IU/mL). Blood was obtained from this atopic donor repeatedly throughout the study. Informed consent was obtained from all patients involved in the study. Indirect flow BAT was performed within four hrs of donor blood sampling. The study protocol was approved by the Kyungpook National University Hospital Institutional Review Board.
2. Negative and positive control sera
1) Negative control sera
These samples were obtained from healthy male individuals (n=20) visiting the Health Promotion Center. These individuals were selected to avoid the presence of preformed HLA alloantibodies.
2) Positive control sera
These samples (n=21) were artificially prepared to simulate the serum of AIU patient: 100 µL of negative control serum was spiked with various volumes of stimulation control reagent (3.0 µL [n=1], 6.0 µL [n=13], 8.0 µL [n=1], 10.0 µL [n=1], 13.0 µL [n=3], 25.0 µL [n=1], and 50.0 µL [n=1]).
3) Stimulation control reagent
This reagent was prepared according to the manufacturer's instructions by reconstituting lyophilized monoclonal anti-FcεRIα antibody with stimulation buffer, both of which were obtained from the Flow2 CAST kit (Bühlmann Laboratories AG, Schönenbuch, Switzerland). The stimulation buffer contained calcium, heparin, and IL-3.
4) Patient sera
These samples were obtained from nine children with chronic urticaria of unknown etiology who consecutively visited the outpatient pediatric clinic of Kyungpook National University Hospital and displayed negative ASST.
3. Indirect flow BAT
1) Basophil activation and fluorescent staining
Based on a four-color flow cytometry strategy, CCR3 phycoerythrin (PE) (Bühlmann Laboratories AG) and CD123 PE-cyanine 5 (PECy5) (BD Biosciences, San Jose, CA, USA) were used as basophil identification markers. CD63 fluorescein isothiocyanate (FITC) (Bühlmann Laboratories AG) and CD203c allophycocyanine (APC) (BD Biosciences) were used as basophil activation markers [4].
When primed basophils were required, donor whole blood (100 µL) was incubated with an equal volume of stimulation buffer (Bühlmann Laboratories AG) for 10 min at 37℃ [14]. Test serum was incubated with primed or unprimed whole blood for 30 min at 37℃. The volume of the test serum added was equal to that of the primed (200 µL) or unprimed (100 µL) whole blood. Negative control serum for each variation was run simultaneously. The mixture was then washed once with 3 mL EDTA-PBS (2mM EDTA in phosphate-buffered saline) at 4℃. After washing, the pellet was resuspended and stained with 20 µL of CD123 PECy5, 5 µL of CD203c APC, and 20 µL of a mixture of CD63 FITC and CCR3 PE for 30 min at 4℃. Following red blood cell lysis with 3 mL lysing solution (Bühlmann Laboratories AG) and a washing step, the remaining pellet was resuspended in PBS for data acquisition using flow cytometry.
2) Flow cytometry
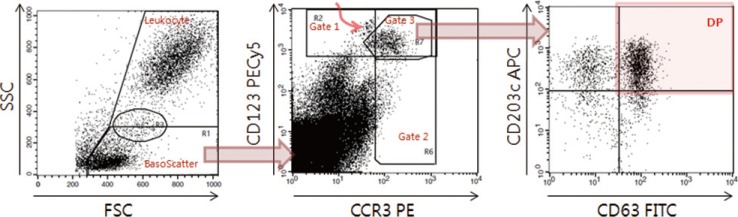
A FACSCalibur flow cytometer with CellQuest Pro software was calibrated daily by using CaliBRITE beads and FACSComp software (all from BD Biosciences). All the available cellular events were acquired with an unlimited target event count (Fig. 1). For the data analysis, leukocyte and potential basophil events were defined by using "Leukocyte" and "BasoScatter" gates, respectively, on the forward/side scatter (FSC/SSC) plot (Fig. 1, left plot). All potential basophil events defined by the BasoScatter gate were purified further by three variations of basophil gates set on the CCR3/CD123 plot (Fig. 1, middle plot). Finally, the double-gated basophils were analyzed on the CD63/CD203c plot. The expression percentage of each activation marker (CD203c+, double positive DP [CD203c+CD63+], or CD63+) was determined by setting previously defined quadrants (Fig. 1, right plot) such that CD63 or CD203c positive events would be less than 1.0% in the negative control tube. To measure CD203c expression (an ectoenzyme expressed constitutively even at a resting state), the stimulation index was calculated as the ratio of mean fluorescence intensity (MFI) of total gated basophils to MFI of sample/MFI of negative control (cutoff=1.38) [11]. To compare diagnostic performance among basophil markers, the signal to noise ratio (SNR) was calculated as the ratio of its expression percentage to the average of expression percentages/corresponding cutoff.
Fig. 1. Data acquisition and analysis of indirect flow BAT for autoimmune urticaria diagnosis. On the FSC/SSC plot (left), the basophil scatter gate (BasoScatter) and leukocyte gate are defined to calculate the basophil percentage among total leukocytes. On the CCR3/CD123 plot (middle), three basophil gates, gates 1, 2, and 3, are defined. The upper left subset, indicated by a curved red arrow within gate 1 and just outside gate 3, appears to be monocyte doublets, an assumption based on their back-gated location on the FSC/SSC plot. On the CD63/CD203c plot (right), a quadrant is set to obtain the expression percentage of CD203c (two upper quadrants), DP (red-shaded quadrant, CD203c+CD63+), or CD63 (two right quadrants).
Abbreviations: FITC, fluorescein isothiocyanate; PE, phycoerythrin; PECy5, PE-cyanine 5; APC, allophycocyanine; FSC, forward scatter; SSC, side scatter; DP, double positivity; CCR3, eotaxin CC chemokine receptor-3.
The complete blood count (CBC) was performed using Advia 2120i (Siemens, Erlangen, Germany), and the ASST was performed as described previously [8].
The basophil preparation method and the measured activation marker are described throughout this report as basophil donor "D"+(atopic "A" or non-atopic "NA")+pretreatment (primed "P" or unprimed "UP")+activation marker ("CD63" or "CD203c"). For example, "DAP CD63" denotes atopic donor primed basophil CD63.
4. Statistical analysis
Statistical analyses were performed by using Excel 2007 (Microsoft Corporation, Redmond, WA, USA) and SPSS v. 11.5 (IBM Corp., Armonk, NY, USA). Comparisons between the two groups were performed by using a correlation coefficient, t-test, Z-test, McNemar's test, χ2 test, or Fisher's exact test. P value <0.05 was considered statistically significant. Results are expressed as mean±SD with range.
RESULTS
1. Test sample volume
We investigated whether the whole blood aliquot volume (100 µL) was adequate for indirect flow BAT. For samples obtained from non-atopic donors, the total acquired basophil event count per tube was 2,949±1,376 (231-7,328, n=87) with 95.4% (83/87) of the counts ≥1,000. For samples obtained from the atopic donor, the total acquired basophil event count was 1,905±522 (378-3,125, n=82) with 96.3% (79/82) of the counts ≥1,000. The total acquired basophil event count per tube was ≥1,000 in over 95% of the cases in which a 100 µL aliquot volume was used.
2. Definition of the cutoff value in negative control sera
We set cutoff values for the expression percentage of each activation marker as the average+(3×SD) in negative control sera (n=20, Table 1). The cutoff values for primed basophils were higher than those for unprimed basophils. The cutoff value for primed basophil CD203c was always over 50%. DAP CD203c (50.0%±13.7%) tended to display a higher expression percentage than that of DNAP CD203c (43.7%±22.3%). This result was consistent with that of another report on atopic allergy [15], although the difference was not significant in our study (P=0.287, t-test, Table 1).
Table 1. Definition of the cutoff values* for basophil activation markers according to four basophil preparation methods and three basophil gating strategies.
| DNAUP basophils | DNAP basophils | DAUP basophils | DAP basophils | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD203c | DP | CD63 | CD203c | DP | CD63 | CD203c | DP | CD63 | CD203c | DP | CD63 | |
| Gate 1 | 1.8 | 0.6 | 5.6 | 76.4 | 3.8 | 8.8 | 5.1 | 0.3 | 4.5 | 70.0 | 6.2 | 10.9 |
| Gate 2 | 5.2 | 3.5 | 10.4 | 70.8 | 4.9 | 13.9 | 3.7 | 0.8 | 10.0 | 56.6 | 6.1 | 14.8 |
| Gate 3 | 2.0 | 0.6 | 2.9 | 110.5 | 4.6 | 8.4 | 1.7 | 0.3 | 4.3 | 91.3 | 7.8 | 10.9 |
Data are presented as percentages.
*The cutoff value for an activation marker was defined as the average of its expression percentages+(3×SD) in negative control sera (n=20), which were measured on the CD63/CD203c plot of gated basophils (Fig. 1, right plot).
Abbreviations: DNAUP, non-atopic donor unprimed; DNAP, non-atopic donor primed; DAUP, atopic donor unprimed; DAP, atopic donor primed basophils; DP, double positive (CD63+CD203c+).
3. Correlation of basophil percentage between flow cytometry and CBC
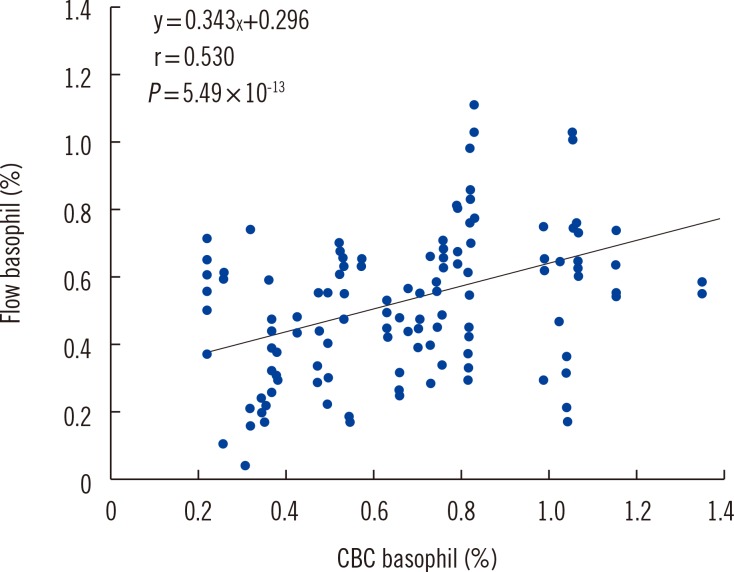
Using flow cytometry, we identified basophils on the basis of three basophil gating strategies (gate 1, 2, or 3; Fig. 1, middle plot). The basophil percentage of total leukocytes was calculated by using the gate event counts (basophil/leukocyte, %). The leukocyte gate was set on the FSC/SSC plot. The correlation coefficients between flow cytometry and CBC were 0.498, 0.195, and 0.530 for gate 1, 2, and 3, respectively (n=142) (Fig. 2). This indicated that gate 3 is the optimal gate because of the benefit of combining CCR3 and CD123. The difference between basophil gate 2 and 3 was significant (P=0.001), whereas that between basophil gate 1 and 3 was not (P=0.717, Z-test, Fig. 1, 2).
Fig. 2. Correlation of the basophil percentage between flow cytometry using basophil gate 3 and automated CBC using Advia 2120i. CBC was more highly correlated with basophil counts using gate 3 (r=0.530) than with gates 1 (r=0.498) or 2 (r=0.195).
Abbreviation: CBC, complete blood count.
Back-gating of CCR3+CD123+ events on the FSC/SSC plot demonstrated that activated basophils had much higher FSC values than did resting basophils (data not shown). Therefore, we drew the basophil scatter gate (BasoScatter) as a polygon, the upper border of which extended to the mid-line of the elliptical monocyte gate, while the right border extended maximally (Fig. 1, left plot), unlike that used in a previous report [15].
4. Assay sensitivity for each variation in positive control sera
As the cutoff value for activation marker expression percentages was defined as the average of the negative control sera+(3×SD), the assay specificity using this cutoff value might be assumed to be 99%. When this cutoff value was applied to positive control sera measurements (n=21), the markers that attained 100% assay sensitivity were all found to be associated with basophil gate 3 or the atopic donor primed basophils (Table 2). The primed basophil CD203c, which had a high cutoff value irrespective of donor type, did not appear to be a valuable marker to discriminate between AIU and non-AIU owing to its low assay sensitivity (Table 2).
Table 2. Comparison of assay sensitivity (%) among basophil activation markers according to four basophil preparation methods and three basophil gating strategies in positive control sera (n=21-24)*.
| DNAUP basophils | DNAP basophils | DAUP basophils | DAP basophils | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD203c | DP | CD63 | CD203c | DP | CD63 | CD203c | DP | CD63 | CD203c | DP | CD63 | |
| Gate 1 | 95.2 | 9.5 | 0.0 | 8.3 | 79.2 | 79.2 | 95.5 | 45.5 | 13.6 | 57.1 | 100 | 100 |
| Gate 2 | 76.2 | 9.5 | 4.8 | 0.0 | 75.0 | 66.7 | 95.5 | 27.3 | 13.6 | 52.4 | 100 | 100 |
| Gate 3 | 95.2 | 4.8 | 4.8 | 0.0 | 75.0 | 75.0 | 100 | 50.0 | 13.6 | 9.5 | 100 | 100 |
Data are presented as percentages.
*See Table 1 for the cutoff values of each marker, which were used to discriminate the expression percentages as positive or negative. The assay specificity using such a cutoff value can be regarded as 99%.
Abbreviations: DNAUP, non-atopic donor unprimed; DNAP, non-atopic donor primed; DAUP, atopic donor unprimed; DAP, atopic donor primed; DP, double positive (CD63+CD203c+).
1) Comparison of basophil gating strategies
The assay sensitivity of the three gating strategies was compared (Table 2). No single activation marker displayed a significant difference between basophil gate 1 and 3 or between basophil gate 2 and 3. However, when the determinations of all four markers (DNAUP CD203c, DNAP CD63, DAUP CD203c, and DAUP DP, which showed relatively large differences among the three gating strategies) were summated, the sensitivity of gate 3 (79.8%) was significantly higher than that of gate 2 (66.3%; P=0.043; n=178). Therefore, we used gate 3 for all further investigations.
2) Comparison of basophil activation markers according to the basophil preparation method
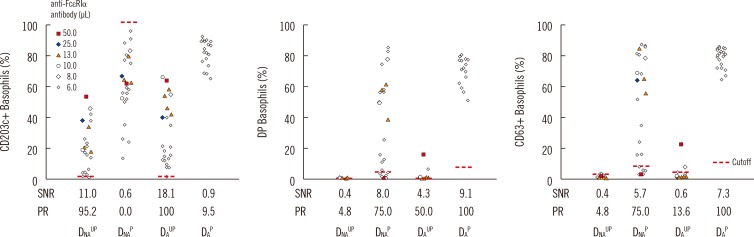
The three activation markers, all originating from the atopic donor, that attained 100% assay sensitivity were DAUP CD203c, DAP DP, and DAP CD63 (Table 2, Fig. 3). In the unprimed basophil CD203c, DNAUP CD203c (95.2%, 20/21) was not different from DAUP CD203c (100%, 21/21; P=1.000). A single false-negative DNAUP CD203c case displayed an expression percentage of 0.74%, and a stimulation index of 1.05. DNAP CD63 (76.2%, 16/21) was significantly higher than DAUP CD63 (14.3%, 3/21; P=0.001), while DAP CD63 (100%, 21/21) was significantly higher than DNAP CD63 (75%, 18/24; P=0.023).
Fig. 3. Comparison of basophil activation markers according to basophil variables. Indirect flow BAT was performed in positive control sera. The expression percentage for each activation marker (CD203c, DP, and CD63) is plotted according to the four basophil preparation methods (DNAUP, DNAP, DAUP, and DAP). To prepare artificial positive control serum, negative control serum was spiked with reconstituted monoclonal anti-FcεRIα antibody. The spiking volume (µL) per 100 µL of negative control serum is indicated by colored markings (see legend). All four types of basophils were tested in parallel by changing the volume of the monoclonal anti-FcεRIα antibody in series of batches. DAP basophils, however, were tested only with the minimum volume of 6 µL. Nevertheless, DAP basophils displayed the highest SNR and positivity rate (PR, assay sensitivity). Expression percentages of each activation marker were measured by using basophil gate 3.
Abbreviations: FcεRIα, α-chain of IgE receptor; DP, double positive (CD63+CD203c+); SNR, signal to noise ratio; PR, positivity rate, DNAUP, non-atopic donor unprimed; DNAP, non-atopic donor primed; DAUP, atopic donor unprimed; DAP, atopic donor primed.
The basophil activation markers in order of SNR (>7.0) were DAUP CD203c (18.1), DNAUP CD203c (11.0), DAP DP (9.1), DNAP DP (8.0), and DAP CD63 (7.3) (Fig. 3). The two markers displaying the highest SNR originated from unprimed basophil CD203c, while the others originated from primed basophil CD63.
5. Pilot study in nine children with chronic urticaria
We attempted to validate the adopted assay protocol for indirect flow BAT in nine consecutive children with chronic urticaria of unknown etiology (undetermined between autoimmune and idiopathic) and negative ASST. A positive indirect flow BAT result in any of these cases would be evidence in support of our in vitro findings. One child was indeed determined as positive. In this child, only two activation markers were positive (DAUP CD203c [2.3%; stimulation index of 1.56] and DAP DP [8.7%]), whereas all other activation markers were negative (DNAUP CD203c [0.3%; stimulation index of 0.64]; DNAP CD63 [2.0%]; DNAP DP [0.6%]; and DAP CD63 [10.4%]).
DISCUSSION
Our study revealed four findings. First, we determined that CD203c should be measured on unprimed basophils. Second, in cases where basophils were primed, CD63 must be included among the activation markers (i.e., CD63+ or CD203c+CD63+). Third, for the unprimed basophil CD203c assay, the difference between atopic and non-atopic donors was not statistically significant, suggesting that in the case of group O atopic donor unavailability, non-atopic donors may be used if potential false negativity can be accepted. Fourth, the assays based on CD63 alone (i.e., CD63+ irrespective of combining with CD203c) in order of sensitivity were DAP CD63 (100%) >DNAP CD63 (75.0%) >DAUP CD63 (13.6%). Therefore, the basophils of non-atopic donors, even after being primed, are not comparable to the primed basophils of atopic donors. Furthermore, unprimed basophils of atopic donors are not comparable to primed basophils of non-atopic donors.
On the basis of these observations, we recommend the following activation markers from atopic donor basophils: DAUP CD203c, DAP DP, and DAP CD63. In the pilot study involving nine children with chronic urticaria and negative ASST, one patient displayed a positive result in indirect flow BAT. In this sample, only the atopic donor basophil markers (DAUP CD203c and DAP DP) scored positive expression percentages, confirming that basophil donors should be atopic irrespective of whether the basophils are primed or unprimed.
Some of our results contradict previous knowledge about basophil identification markers. We found that using a combination of CD123 and CCR3 to identify basophils obviated the need for CD14 and CD3 to facilitate exclusion of CD123+ monocytes and CCR3+ T lymphocytes, respectively. Furthermore, gate 3 displayed higher sensitivity than that of gate 2 and similar sensitivity to that of gate 1, suggesting that CD123 alone might be sufficient to identify basophils. However, in a comparison of basophil identification between CBC and flow cytometry, CBC displayed a higher correlation with gate 3 than with gate 1 or 2. Furthermore, CD203c expression percentages based on gates 1, 2, and 3 in the positive control sera were 23.7%, 16.7%, and 30.2%, and the SNRs were 10.5, 5.3, and 11.8, respectively. Taken together, these findings suggest that gate 3, combining CCR3 and CD123, might be superior to gate 1 or 2 for assay sensitivity.
Unfortunately, the artificially prepared positive control serum (n=21) contained traces of IL-3. This was because lyophilized anti-FcεRIα antibody was reconstituted in a stimulating buffer containing IL-3, according to the manufacturer's instructions. IL-3 upregulates CD203c expression on basophils even in the absence of anti-FcεRIα antibody. Indeed, we found that sera spiked with 6 µL and 13 µL of pure stimulation buffer without anti-FcεRIα antibody displayed DAUP CD203c expression percentages of 2.3% (SNR 1.4) and 3.9% (SNR 2.3), respectively. Therefore, the weak positive expression percentage of CD203c might be due to potential false positivity induced by IL-3 when the positive control sera were used. We investigated an incidence of weak positive CD203c expression with an SNR below 3.0 (1.7-5.0% for DAUP CD203c and 2.0-5.9% for DNAUP CD203c). Only one case fell within this range for DAUP CD203c, whereas three such cases fell within this range for DNAUP CD203c. In addition, the expression percentage SNR for DAUP CD203c (18.1±12.4) was significantly higher than that for DNAUP CD203c (11.0±7.9; P=0.004), which suggests that DAUP CD203c should have lower susceptibility to false positivity than DNAUP CD203c. These findings indicate that the sensitivity of DNAUP CD203c might be overestimated owing to its higher susceptibility to false positive results under the influence of IL-3 compared with that observed with DAUP CD203c.
We tried to reconstitute the lyophilized anti-FcεRIα antibody in distilled water instead of the stimulation buffer. However, we found that sera spiked with the reagent prepared by using distilled water failed to activate basophils (data not shown). The cytokines involved in basophil and mast cell activation are present in the sera of patients with chronic urticaria [22]. Indeed, the IL-3 level in patients with chronic urticaria was higher than that in healthy control subjects, although the difference did not reach statistical significance [14]. Therefore, we propose that the issue pertaining to the authenticity of IL-3-induced "false" positivity warrants further discussion.
The kinetics of CD203c upregulation are rapid and peak after 5-15 min, whereas those of CD63 are slow and peak after 20-40 min [23]. In our study, donor basophils in a test serum sample were incubated for 30 min at 37℃. The 30 min-incubation period was adopted in consideration of the time required for CD63 upregulation. Fortunately, CD63 expression on basophils appeared simultaneously with CD203c in all but a few cases. When determining positive or negative CD203c expression, we found that the stimulation index did not enhance assay sensitivity above that of the expression percentage (data not shown). Our study revealed that CD203c alone (DAUP CD203c) or in combination with CD63 (DAP DP) is a necessary activation marker, a finding that is consistent with those of previous reports that favor CD203c over CD63 [11,16,24].
Finally, the establishment of a mast cell line, such as a stable humanized rat basophil leukemia cell line, is highly desirable as a basophil source for indirect flow BAT [14,25,26]. However, Gentinetta et al. [14] reported low assay sensitivity of a basophil cell line (RBL 7003/21), making it less ideal for usage. Nevertheless, establishing such a cell line would circumvent the need to procure a highly sensitized atopic donor with blood group O or to prime basophils with IL-3, making this test more robust and reliable.
In conclusion, the use of indirect flow BAT to diagnose autoimmune urticaria should be performed with basophils from atopic donors by using an optimized protocol. A combination of CD123 and CCR3 markers is recommended for basophil identification, while CD123 alone may also be used as an alternative. To detect basophil activation, either CD203c alone on unprimed basophils or CD203c and CD63 on primed basophils are recommended, while CD63 alone on primed basophils may also be used as an alternative.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Marrouche N, Grattan C. Childhood urticaria. Curr Opin Allergy Clin Immunol. 2012;12:485–490. doi: 10.1097/ACI.0b013e3283574cb3. [DOI] [PubMed] [Google Scholar]
- 2.Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest. 1998;101:243–251. doi: 10.1172/JCI511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irinyi B, Széles G, Gyimesi E, Tumpek J, Herédi E, Dimitrios G, et al. Clinical and laboratory examinations in the subgroups of chronic urticaria. Int Arch Allergy Immunol. 2007;144:217–225. doi: 10.1159/000103995. [DOI] [PubMed] [Google Scholar]
- 4.Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008;74:201–210. doi: 10.1002/cyto.b.20419. [DOI] [PubMed] [Google Scholar]
- 5.Marone G, Spadaro G, Patella V, Genovese A. The clinical relevance of basophil releasability. J Allergy Clin Immunol. 1994;94:1293–1303. doi: 10.1016/0091-6749(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 6.Zuberbier T, Schwarz S, Hartmann K, Pfrommer C, Czarnetzki BM. Histamine releasability of basophils and skin mast cells in chronic urticaria. Allergy. 1996;51:24–28. doi: 10.1111/j.1398-9995.1996.tb04545.x. [DOI] [PubMed] [Google Scholar]
- 7.Szegedi A, Irinyi B, Gál M, Hunyadi J, Dankó K, Kiss E, et al. Significant correlation between the CD63 assay and the histamine release assay in chronic urticaria. Br J Dermatol. 2006;155:67–75. doi: 10.1111/j.1365-2133.2006.07205.x. [DOI] [PubMed] [Google Scholar]
- 8.Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999;140:446–452. doi: 10.1046/j.1365-2133.1999.02707.x. [DOI] [PubMed] [Google Scholar]
- 9.Frezzolini A, Provini A, Teofoli P, Pomponi D, De Pità O. Serum-induced basophil CD63 expression by means of a tricolour flow cytometric method for the in vitro diagnosis of chronic urticaria. Allergy. 2006;61:1071–1077. doi: 10.1111/j.1398-9995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 10.Gyimesi E, Sipka S, Dankó K, Kiss E, Hidvégi B, Gál M, et al. Basophil CD63 expression assay on highly sensitized atopic donor leucocytes-a useful method in chronic autoimmune urticaria. Br J Dermatol. 2004;151:388–396. doi: 10.1111/j.1365-2133.2004.06042.x. [DOI] [PubMed] [Google Scholar]
- 11.Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandené L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320:40–48. doi: 10.1016/j.jim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Cozon G, Ferrándiz J, Peyramond D, Brunet J. Detection of activated basophils using flow cytometry for diagnosis in atopic patients. Allergol Immunopathol (Madr) 1999;27:182–187. [PubMed] [Google Scholar]
- 13.Ebo DG, Lechkar B, Schuerwegh AJ, Bridts CH, De Clerck LS, Stevens WJ. Comments regarding 'Marked improvement of the basophil activation test by detecting CD203c instead of CD63' by Boumiza et al. Clin Exp Allergy. 2003;33:849, author reply 852-3. [PubMed] [Google Scholar]
- 14.Gentinetta T, Pecaric-Petkovic T, Wan D, Falcone FH, Dahinden CA, Pichler WJ, et al. Individual IL-3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol. 2011;128:1227–1234.e5. doi: 10.1016/j.jaci.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Marone G, Giugliano R, Lembo G, Ayala F. Human basophil releasability. II. Changes in basophil releasability in patients with atopic dermatitis. J Invest Dermatol. 1986;87:19–23. doi: 10.1111/1523-1747.ep12523520. [DOI] [PubMed] [Google Scholar]
- 16.Sturm EM, Kranzelbinder B, Heinemann A, Groselj-Strele A, Aberer W, Sturm GJ. CD203c-based basophil activation test in allergy diagnosis: characteristics and differences to CD63 upregulation. Cytometry B Clin Cytom. 2010;78:308–318. doi: 10.1002/cyto.b.20526. [DOI] [PubMed] [Google Scholar]
- 17.Wedi B, Novacovic V, Koerner M, Kapp A. Chronic urticaria serum induces histamine release, leukotriene production, and basophil CD63 surface expression--inhibitory effects of anti-inflammatory drugs. J Allergy Clin Immunol. 2000;105:552–560. doi: 10.1067/mai.2000.104939. [DOI] [PubMed] [Google Scholar]
- 18.Chirumbolo S. The use of IL-3 in basophil activation tests is the real pitfall. Cytometry B Clin Cytom. 2011;80:137–138. author reply 139. doi: 10.1002/cyto.b.20570. [DOI] [PubMed] [Google Scholar]
- 19.Hausmann OV, Gentinetta T, Fux M, Ducrest S, Pichler WJ, Dahinden CA. Robust expression of CCR3 as a single basophil selection marker in flow cytometry. Allergy. 2011;66:85–91. doi: 10.1111/j.1398-9995.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 20.Ducrest S, Meier F, Tschopp C, Pavlovic R, Dahinden CA. Flowcytometric analysis of basophil counts in human blood and inaccuracy of hematology analyzers. Allergy. 2005;60:1446–1450. doi: 10.1111/j.1398-9995.2005.00910.x. [DOI] [PubMed] [Google Scholar]
- 21.Chirumbolo S, Ortolani R, Vella A. CCR3 as a single selection marker compared to CD123/HLADR to isolate basophils in flow cytometry: some comments. Cytometry A. 2011;79:102–106. doi: 10.1002/cyto.a.21008. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer M, Luquin E, Sanchez-Ibarrola A, Moreno C, Sanz ML, Kaplan AP. Secretion of cytokines, histamine and leukotrienes in chronic urticaria. Int Arch Allergy Immunol. 2002;129:254–260. doi: 10.1159/000066772. [DOI] [PubMed] [Google Scholar]
- 23.Hennersdorf F, Florian S, Jakob A, Baumgärtner K, Sonneck K, Nordheim A, et al. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–335. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 24.Yasnowsky KM, Dreskin SC, Efaw B, Schoen D, Vedanthan PK, Alam R, et al. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006;117:1430–1434. doi: 10.1016/j.jaci.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Laidlaw TM, Steinke JW, Tiñana AM, Feng C, Xing W, Lam BK, et al. Characterization of a novel human mast cell line that responds to stem cell factor and expresses functional FcεRI. J Allergy Clin Immunol. 2011;127:815–822.e1-5. doi: 10.1016/j.jaci.2010.12.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashid A, Sadroddiny E, Ye HT, Vratimos A, Sabban S, Carey E, et al. Review: diagnostic and therapeutic applications of rat basophilic leukemia cells. Mol Immunol. 2012;52:224–228. doi: 10.1016/j.molimm.2012.05.019. [DOI] [PubMed] [Google Scholar]