Abstract
Background
An association has been reported between CYP2C19 polymorphism and the altered antiplatelet activity of clopidogrel. We investigated this association using the newly introduced platelet function analyzer (PFA)-200 (INNOVANCE PFA-200 System; Siemens Healthcare, Germany) P2Y test.
Methods
Polymorphisms of CYP2C19*2, *3, *17 and the degree of inhibition of platelet function were determined in 83 patients. Three different platelet function tests were used to evaluate the degree of platelet inhibition and to check the association with genotype.
Results
The post-procedure PFA-200 values of extensive metabolizers (EM) patients (285.3±38.8) were higher than those of intermediate metabolizers (IM) and poor metabolizers (PM) patients (227.7±98.3 and 133.7±99.2, respectively; P=0.024). Light transmittance aggregometry (LTA) and the VerifyNow system showed that the post-procedure values for EM patients were lower than those of IM and PM patients (LTA: 24.4±15.7, 34.1±17.6, and 42.2±16.9, respectively, P<0.001; VerifyNow: 133.2±60.5, 171.5±42.6, and 218.7±59.3, respectively, P<0.001). The high residual platelet reactivity (HPR) rates were significantly different among the EM, IM, and PM groups using PFA-200 (PM:IM:EM=82.4:40.6:11.8, P<0.001).
Conclusions
Approximately, 59.0% of Korean patients with cardiovascular disease receiving clopidogrel had CYP2C19 loss-of-function genotypes classified as IM or PM, and the frequency was similar to the data from Asian people. The PFA-200, LTA, and VerifyNow platelet function tests revealed evidence of a significant association between the efficacy of clopidogrel and CYP2C19 genotypes.
Keywords: CYP2C19, Clopidogrel, PFA-200, LTA, VerifyNow
INTRODUCTION
The popular P2Y12 receptor inhibitor, clopidogrel plays an important role in preventing recurrent ischemic events in patients with acute coronary syndrome (ACS) [1]. However, clinical studies have shown that inter-individual variability in the response to clopidogrel may be associated with high residual platelet reactivity (HPR) in patients undergoing percutaneous coronary intervention (PCI) and increases the risk of stent thrombosis [1,2,3]. Among several factors, genetic polymorphisms affecting the metabolism of clopidogrel play the most important role during the entire metabolic process [4]. On the genetic level, loss-of-function (LOF) variants of the CYP2C19 gene have been identified as the most prominent contributor to platelet activity after clopidogrel treatment [5].
Recently, various systems for platelet function test have been used to measure platelet reactivity. Current laboratory techniques, such as ADP-induced light transmittance aggregometry (LTA), the VerifyNow P2Y12 assay, multiple electrode platelet aggregometry (MEA), and flow cytometry are all based on different principles and measure different properties to evaluate platelet activation [6,7]. Another platelet function analyzer (PFA) (INNOVANCE PFA-200 System; Siemens Healthcare, Marburg, Germany) equipped with the new P2Y assay is also being used in clinical trials [7,8]. As an improved version of PFA-100, the PFA-200 has now become more usable because it overcame the lack of sensitivity to the effects of clopidogrel and has shown promising results in a few clinical studies [9,10]. However, few studies have investigated the sensitivity and specificity of PFA-200 assays for detecting inhibition of platelet function in patients taking oral clopidogrel, based on their CYP2C19 polymorphism status.
The aim of this study was to evaluate the impact of CYP2C19 polymorphism on various platelet function tests and the ability to predict the efficacy of antiplatelet treatment, focusing on the newly introduced PFA-200 P2Y test. We also investigated the applicability of CYP2C19 genotyping to decisions on the direction of treatment, as well as predictions regarding the efficacy of treatment options.
METHODS
1. Study population and patient characteristics
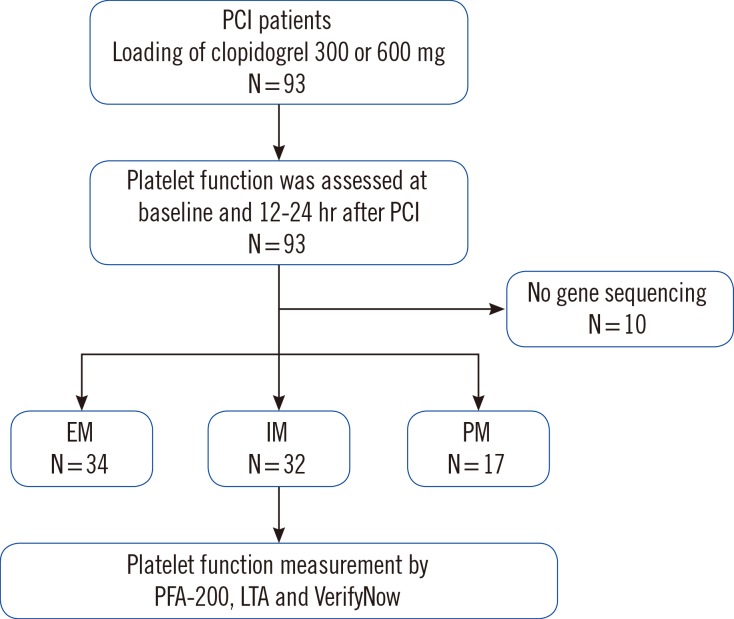
We enrolled 93 patients who underwent PCI with implantation of drug-eluting stents for the evaluation of platelet reactivity from July 2013 to November 2014. However, 10 patients prematurely discontinued the study owing to early revocation of consent (n=3) or an unwillingness to continue (n=7). Therefore, we could perform CYP2C19 genotyping on 83 patients. A flow chart of the study design is shown in Fig. 1. Inclusion criteria identified patients between 18 and 80 yr of age with symptomatic ACS including unstable angina (UA) or non-ST elevation myocardial infarction (NSTEMI). Those with severe renal failure (creatinine level >2.5 mg/dL), active internal bleeding or bleeding diathesis, hemodynamic instability, malignancies, contraindications for antiplatelet agents, concomitant use of warfarin or a glycoprotein (GP) IIb/IIIa receptor blocker, thrombocytopenia (platelet count<0.1×106/L) or anemia (Hb<8.0 g/dL) were all excluded. The baseline clinical characteristics of the patients are shown in Table 1. There were no significant clinical differences among the genotyping subgroups.
Fig. 1. Study flow diagram.
Abbreviations: PCI, percutaneous coronary intervention; EM, extensive metabolizers; IM, intermediate metabolizers; PM, poor metabolizers; LTA, light transmittance aggregometry.
Table 1. Demographics of the study population.
| Variable | Overall | EM | IM | PM | P value |
|---|---|---|---|---|---|
| (N=83) | (N=34) | (N=32) | (N=17) | ||
| Age (yr), mean±SD | 63.76±10.05 | 63.56±9.74 | 64.22±9.26 | 63.29±12.45 | 0.945 |
| Male gender, N (%) | 58 (69.9) | 27 (79.4) | 23 (71.9) | 8 (47.1) | 0.057 |
| Riskfactors | |||||
| Hypertension, N (%) | 47 (56.6) | 22 (64.7) | 15 (46.9) | 10 (58.8) | 0.337 |
| Diabetic mellitus, N (%) | 29 (34.9) | 13 (38.2) | 10 (31.2) | 6 (35.3) | 0.837 |
| Dyslipidemia, N (%) | 13 (15.7) | 7 (20.6) | 4 (12.5) | 2 (11.8) | 0.588 |
| Chronic kidney disease, N (%) | 8 (9.6) | 4 (11.8) | 2 (6.2) | 2 (11.8) | 0.709 |
| Current smoking, N (%) | 9 (10.8) | 2 (5.9) | 5 (15.6) | 2 (11.8) | 0.441 |
| BMI (kg/m2) | 24.84±3.0 | 24.24±3.08 | 25.52±2.47 | 24.77±3.61 | 0.222 |
| Past history | |||||
| Previous PCI, N (%) | 33 (39.8) | 14 (41.2) | 13 (40.6) | 6 (35.3) | 0.914 |
| Previous MI, N (%) | 12 (14.5) | 3 (8.8) | 6 (18.8) | 3 (17.6) | 0.475 |
| Previous stroke, N (%) | 3 (3.6) | 1 (2.9) | 1 (3.1) | 1 (5.9) | 0.853 |
| Previous CABG, N (%) | 1 (1.2) | 0 (0.0) | 1 (3.1) | 0 (0.0) | 0.446 |
| Medication | |||||
| Statin, N (%) | 49 (59.0) | 22 (64.7) | 18 (56.2) | 9 (52.9) | 0.665 |
| Calcium channel blocker, N (%) | 38 (45.8) | 11 (32.4) | 19 (59.4) | 8 (47.1) | 0.088 |
| Beta-blocker, N (%) | 46 (55.4) | 18 (52.9) | 17 (53.1) | 11 (64.7) | 0.689 |
| ACEI, N (%) | 9 (10.8) | 2 (5.9) | 4 (12.5) | 3 (17.6) | 0.413 |
| ARB, N (%) | 10 (12.0) | 3 (8.8) | 4 (12.5) | 3 (17.6) | 0.656 |
| Nitrate, N (%) | 54 (65.1) | 19 (55.9) | 24 (75.0) | 11 (64.7) | 0.266 |
| Diuretics, N (%) | 11 (13.3) | 4 (11.8) | 3 (9.4) | 4 (23.5) | 0.360 |
| LVEF % | 57.26±8.75 | 56.83±9.66 | 57.50±8.60 | 57.67±7.53 | 0.943 |
| Hemoglobin (g/dL) | 13.27±2.41 | 13.19±2.97 | 13.62±1.96 | 12.77±1.92 | 0.501 |
| Creatinine (mg/dL) | 1.42±1.74 | 1.44±2.12 | 1.39±1.62 | 1.43±1.10 | 0.993 |
| Platelet count (109/L) | 209.95±45.42 | 208.09±48.18 | 211.47±42.48 | 210.81±48.06 | 0.954 |
Continuous data are shown as mean±SD, Dichotomous data are shown as N (%).
Abbreviations: EM, extensive metabolizers; IM, intermediate metabolizers; PM, poor metabolizers; LVEF, left ventricular ejection fraction; BMI, body mass index; PCI, percutaneous coronary intervention; MI, myocardial infarction; CABG, coronary artery bypass grafting; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Clopidogrel 300-mg loading dose (LD) was administered at least four hours before PCI for patients who had received maintenance antiplatelet therapy comprising aspirin 100 mg and clopidogrel 75 mg for longer than five days. For those who had not received aspirin and clopidogrel antiplatelet maintenance therapy, an LD of clopidogrel 600 mg and 300 mg aspirin was administered at least four hours before PCI. The patients were then administered a maintenance dose of clopidogrel and aspirin (75 mg and 100 mg once daily). Diagnostic procedures and PCI were performed in line with standard practices. The patients underwent CYP2C19 genotyping once and platelet-function testing before and 24 hr post PCI. The study design was approved by the Institutional Review Board of Dong-A University Hospital, Busan, Korea. Written informed consent was obtained from every patient before enrollment.
2. Platelet function testing
Blood samples for platelet function tests were taken from patients in the catheterization room at pre-loading and were defined as the time between the arrival of the patient at the cardiac catheterization laboratory and the beginning of coronary angiography using a standardized technique through an indwelling arterial sheath into tubes containing 3.2% sodium citrate (Dynabyte, Munich, Germany). Secondary blood sampling from the antecubital vein using a 21-gauge needle was performed the next morning, 24 hr after PCI.
Platelet reactivity was measured by using the INNOVANCE PFA-200 system (Siemens Healthcare), LTA Chrono-log model 560 (Chrono-log Corp, Havertown, PA, USA), and VerifyNow (Accumetrics, San Diego, CA, USA). These assays were performed according to the manufacturers' instructions. The numerical results of PFA-200 assays were expressed as closure time (sec), LTA as a percentage, and the VerifyNow assay as P2Y12 reaction units (PRU).
We also compared the percentage of HPR in each group. We defined HPR as having a PFA-200 assay result of<193 sec [11], a 10 µmol/L ADP maximum platelet aggregation (MPA) ≥ 55% [12], and VerifyNow P2Y12 result >208 PRU [13].
3. CYP2C19 genotyping
CYP2C19 was genotyped by using the Spartan RX CYP2C19 system (Spartan Bioscience Inc, Ottawa, Canada) according to the manufacturer's instructions, as previously described [14]. In brief, we acquired buccal swabs and inserted the samples into the test cartridges of the Spartan RX CYP2C19 system. The results were categorized into three subgroups: extensive metabolizers (EM, including the *1/*1 and *1/*17 diplotypes), intermediate metabolizers (IM, including the *1/*2 and *1/*3 diplotypes), and poor metabolizers (PM, including the *2/*2, *3/*3, and *2/*3 diplotypes).
4. Statistics analysis
The findings for the CYP2C19 subgroups were compared by using the Chi-square and Kruskal-Wallis tests. Continuous variables were presented as means±SD and compared by using the unpaired t-test. Categorical variables were presented as numbers or percentages and were compared by using Chi-square or Fisher's exact tests when the expected frequency was <5. Pearson's correlation coefficient was used to evaluate associations between the results of the PFA-200, LTA, and VerifyNow assay systems. The correlation coefficient was classified into three grades: high correlation (0.5 to 1.0), medium correlation (0.3 to 0.5), and low correlation (0.1 to 0.3) [15]. P values <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS for Windows software package, version 14.0K (SPSS Inc, Chicago, IL, USA).
RESULTS
1. Frequencies of CYP2C19 genotypes
The distribution of CYP2C19 polymorphisms was 34 (40.9%) for EM, 32 (38.6%) for IM, and 17 (20.5%) for PM. In total, 49 patients (59.0%) were carriers of CYP2C19*2 or CYP2C9*3. The percentage genotype distribution for each allele was consistent with that predicted by Hardy-Weinberg equilibrium for polymorphisms (P>0.05).
2. Platelet function tests in EM, IM, and PM patients
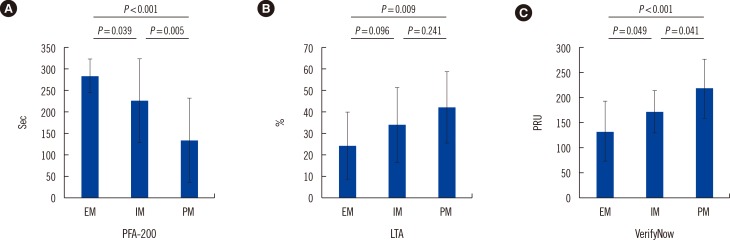
The post-procedure PFA-200 values for EM patients (285.3±38.8) were higher than those of IM and PM patients (227.7±98.3 and 133.7±99.2, respectively; P=0.024). The post-procedure LTA values for EM patients (24.4±15.7) were lower than those of IM and PM patients (34.1±17.6 and 42.2±16.9, respectively; P<0.001). Using the VerifyNow assay, we also observed lower platelet activity in EM patients than in IM and PM groups (post-procedure; EM: 133.2±60.5, IM: 171.5±42.6, PM: 218.7±59.3, P<0.001; Fig. 2).
Fig. 2. The platelet function test values for three different devices, measured post-percutaneous coronary intervention (PCI). (A) PFA-200 (sec); (B) Light transmittance aggregometry (LTA, %); (C) VerifyNow (PRU). Error bars indicate SD.
Abbreviations: EM, extensive metabolizers; IM, intermediate metabolizers; PM, poor metabolizers; PRU, P2Y12 reaction unit.
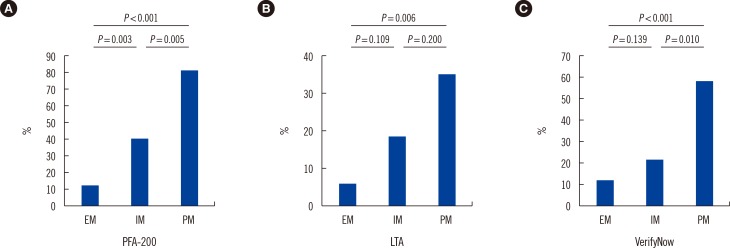
Using the criteria reported in the literature [11,12,13], we also compared the post-procedure HPR rate among different CYP2C19 phenotypes and platelet function results (Fig. 3). PFA-200 assays showed that the HPR rate significantly differed among the EM, IM, and PM groups (PM:IM:EM=82.4:40.6: 11.8; P<0.001). Similar results were observed for LTA, and we observed a higher HPR rate in the IM and PM groups than that in the EM group (PM:IM:EM=35.3:18.8:5.9; P=0.048). We also observed a higher HPR rate in the PM group than the IM and EM groups for the VerifyNow results. However, although HPR rate was higher in the IM group, there was no statistically significant difference compared with the EM group (PM:IM: EM=58.8:21.9:11.8; P=0.001).
Fig. 3. The percentage of high platelet reactivity (HPR) measured post-percutaneous coronary intervention (PCI) in different groups. (A) PFA-200; (B) Light transmittance aggregometry (LTA); (C) VerifyNow.
We also observed a significant correlation between the results for PFA-200 and the other two platelet-function testing systems: r=-0.379 (P<0.001) for PFA-200 and LTA; r=-0.319 (P<0.001) for PFA-200 and VerifyNow assays (Fig. 4).
Fig. 4. Relationships between the results obtained by using PFA-200 (sec) and light transmittance aggregometry (LTA, %) or VerifyNow (PRU) assay systems post-percutaneous coronary intervention (PCI). Correlation coefficient (r) was calculated by using Pearson's method.
Abbreviation: PRU, P2Y12 reaction unit.
DISCUSSION
In the present study, we used three platelet function devices including a new generation PFA-200 to evaluate the impact of CYP2C19 polymorphisms on the clopidogrel response. We observed significantly higher platelet reactivity in certain CYP2C19 (*2 and *3) allele carriers than that in non-carriers among patients with coronary artery disease (CAD) (Fig. 2, 3). We also observed medium correlations between the results for PFA-200 and other conventional platelet function test devices (Fig. 4).
Clopidogrel is a thienopyridine prodrug that requires processing by two sequential oxidative steps that are mediated by several CYP450 isoenzymes, and only 15% is available for transformation to the active metabolite [16]. Among several CYP enzymes, CYP2C19 contributes most to its metabolism (being responsible for approximately 45% of the first step and approximately 20% of the final step) and plays an important role in the clinically relevant effects of clopidogrel [4,16]. CYP2C19 variants, particularly CYP2C19*2 and *3, are reproducibly associated with variability in the bioavailability of the active metabolite and the antiplatelet effects of clopidogrel [17].
In the TRITON-TIMI 38 study, patients with the CYP2C19*2 polymorphism had a 53% increased relative risk of major adverse cardiovascular events [18]. Moreover, the number of the CYP2C19 LOF alleles in the Korean acute myocardial infarction (AMI) patients has been associated with an increased risk of ischemic events including cardiovascular death, nonfatal MI, and ischemic stroke [5]. In addition, previous studies have shown that CYP2C19 LOF allele frequencies for *2 and *3 are higher in Asian populations (approximately 60%) than in the Caucasian population (approximately 30%) [3,19]. Considering the prevalence of the CYP2C19 LOF allele, the Asian ethnic group may increase the level of platelet reactivity and the prevalence of HPR than Caucasian groups when given the same oral dose of clopidogrel. These findings suggest that testing for CYP2C19 polymorphisms should be applicable to CAD patients when using clopidogrel antiplatelet therapy, particularly in East Asian patients.
Various platelet function tests have been used to evaluate platelet reactivity after the administration of aspirin and clopidogrel in large trials. Among those techniques, LTA, VerifyNow, flow cytometry, and MEA are the most commonly used platelet function tests. LTA is the traditional technique used to evaluate platelet activity and has been regarded as the classical method for many years. As a turbidimetric-based, optical detection point-of-care device, the VerifyNow system uses cartridges that contain different agonists to activate the platelets. The test is very easy to perform, and results are reproducible and quickly available. All the above characteristics have led to its use in many clinical trials, and it showed a very good correlation with LTA results [20,21,22]. In our study, we used LTA and VerifyNow platelet function assessment methods and could find evidence for a significant association between the efficacy of clopidogrel and the CYP2C19 genotypes. In addition, we observed a higher HPR rate in the IM and PM groups (Fig. 3).
We also evaluated the novel platelet function evaluation device, PFA-200 in the Korean population. PFA-200 has several advantages: it is easy to use, automated, and rapid, and mimics several characteristics of physiologic platelet function [10]. In a previous study, use of the PFA-100 collagen/ADP assay did not reveal a significant correlation between the HPR (cut-off value of 236 sec) and clinical outcomes including all-cause death, nonfatal AMI, stent thrombosis, and ischemic stroke [23]. However, another clinical study has demonstrated that platelet reactivity above a threshold of 193 sec using PFA-100 is associated with a higher risk of adverse ischemic events [8,11]. A few studies have demonstrated that the PFA-200 system has good sensitivity and specificity for testing platelet function compared with the previous generation of PFA-100 [8]. In the present study, compared to the other platelet function tests, PFA-200 system showed notable differences in the platelet functions at post-PCI among the EM, IM, and PM groups (Fig. 2). We also observed that results of PFA-200 medium correlated with those of other conventional platelet-function test devices. By defining cut-off values for HPR (<193) using PFA-200, we observed statistically significant differences in the HPR rate among the EM, IM, and PM groups (PM:IM:EM=82.4:40.6:11.8; P<0.001) (Fig. 3). However, because PFA-200 still has a maximum value of 300 sec, any correlation with other tests lacked smoothness. To the best of our knowledge, the use of the PFA-200 device for measuring platelet function has still been limited to small-scale trials with few patients. Further evaluation of the sensitivity, specificity, and practicality of the platelet function assay using PFA-200 requires more clinical trials with greater number of patients.
Finally, we need further follow-up of patients to determine correlations between clinical events and HPR defined using PFA-200 assays.
The study limitations were that the active metabolites of clopidogrel were not analyzed according to the presence of CYP2C19 polymorphisms and we did not verify correlations between the CYP2C19 polymorphisms and clinical outcomes.
In the present study, about 59.0% of Korean patients with cardiovascular disease receiving clopidogrel had CYP2C19 polymorphisms classified as IM or PM, and the frequency was similar to previously reported data from Asian populations. We also found evidence for a significant association between the efficacy of clopidogrel and CYP2C19 genotypes using PFA-200 platelet function tests.
Acknowledgments
This work was supported by the Dong-A University research fund.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1404–1411. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Park KJ, Chung HS, Kim SR, Kim HJ, Han JY, Lee SY. Clinical, pharmacokinetic, and pharmacogenetic determinants of clopidogrel resistance in Korean patients with acute coronary syndrome. Korean J Lab Med. 2011;31:91–94. doi: 10.3343/kjlm.2011.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237–242. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 4.Ferri N, Corsini A, Bellosta S. Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs. 2013;73:1681–1709. doi: 10.1007/s40265-013-0126-z. [DOI] [PubMed] [Google Scholar]
- 5.Jeong YH, Tantry US, Kim IS, Koh JS, Kwon TJ, Park Y, et al. Effect of CYP2C19*2 and *3 loss-of-function alleles on platelet reactivity and adverse clinical events in East Asian acute myocardial infarction survivors treated with clopidogrel and aspirin. Circ Cardiovasc Interv. 2011;4:585–594. doi: 10.1161/CIRCINTERVENTIONS.111.962555. [DOI] [PubMed] [Google Scholar]
- 6.Renda G, Zurro M, Malatesta G, Ruggieri B, De Caterina R. Inconsistency of different methods for assessing ex vivo platelet function: relevance for the detection of aspirin resistance. Haematologica. 2010;95:2095–2101. doi: 10.3324/haematol.2010.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lordkipanidzé M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28:1702–1708. doi: 10.1093/eurheartj/ehm226. [DOI] [PubMed] [Google Scholar]
- 8.Hu YF, Lu TM, Wu CH, Lin YJ, Chang SL, Lo LW, et al. Differences in high on-treatment platelet reactivity between intra-coronary and peripheral blood after dual anti-platelet agents in patients with coronary artery disease. Thromb Haemost. 2013;110:124–130. doi: 10.1160/TH13-01-0034. [DOI] [PubMed] [Google Scholar]
- 9.Koessler J, Kobsar AL, Rajkovic MS, Schafer A, Flierl U, Pfoertsch S, et al. The new INNOVANCE® PFA P2Y cartridge is sensitive to the detection of the P2Y12 receptor inhibition. Platelets. 2011;22:20–27. doi: 10.3109/09537104.2010.514967. [DOI] [PubMed] [Google Scholar]
- 10.Choi JL, Li S, Han JY. Platelet function tests: a review of progresses in clinical application. Biomed Res Int. 2014;2014:456569. doi: 10.1155/2014/456569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosiak M, Postula M, Kaplon-Cieslicka A, Kondracka A, Trzepla E, Czlonkowski A, et al. Effect of ASA dose doubling versus switching to clopidogrel on plasma inflammatory markers concentration in patients with type 2 diabetes and high platelet reactivity: the AVOCADO study. Cardiol J. 2013;20:545–551. doi: 10.5603/CJ.2013.0045. [DOI] [PubMed] [Google Scholar]
- 12.Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, et al. High on-treatment platelet reactivity by more than one agonist predicts 12-month follow-up cardiovascular death and non-fatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting. Thromb Haemost. 2010;104:279–286. doi: 10.1160/TH10-01-0007. [DOI] [PubMed] [Google Scholar]
- 13.Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 14.Stimpfle F, Karathanos A, Droppa M, Metzger J, Rath D, Müller K, et al. Impact of point-of-care testing for CYP2C19 on platelet inhibition in patients with acute coronary syndrome and early dual antiplatelet therapy in the emergency setting. Thromb Res. 2014;134:105–110. doi: 10.1016/j.thromres.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Dawson-Saunders B, Trapp RG, editors. Basic and clinical biostatistics. 4th ed. New York: Lange Medical Books/McGraw-Hill; 2004. [Google Scholar]
- 16.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakata T, Miyahara M, Nakatani K, Wada H, Tanigawa T, Komada F, et al. Relationship between CYP2C19 loss-of-function polymorphism and platelet reactivities with clopidogrel treatment in Japanese patients undergoing coronary stent implantation. Circ J. 2013;77:1436–1444. doi: 10.1253/circj.cj-12-1095. [DOI] [PubMed] [Google Scholar]
- 18.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HZ, Kim MH, Han JY, Jeong YH. Defining predictive values using three different platelet function tests for CYP2C19 phenotype status on maintenance dual antiplatelet therapy after PCI. Platelets. 2014;25:285–291. doi: 10.3109/09537104.2013.815340. [DOI] [PubMed] [Google Scholar]
- 20.Janssen PW. Platelet function testing and tailored antiplatelet therapy. J Cardiovasc Transl Res. 2013;6:316–328. doi: 10.1007/s12265-013-9458-z. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler GL, Braden GA, Steinhubl SR, Kereiakes DJ, Kottke-Marchant K, Michelson AD, et al. The Ultegra rapid platelet-function assay: comparison to standard platelet function assays in patients undergoing percutaneous coronary intervention with abciximab therapy. Am Heart J. 2002;143:602–611. doi: 10.1067/mhj.2002.121734. [DOI] [PubMed] [Google Scholar]
- 22.van Werkum JW, van der Stelt CA, Seesing TH, Hackeng CM, ten Berg JM. A head-to-head comparison between the VerifyNow P2Y12 assay and light transmittance aggregometry for monitoring the individual platelet response to clopidogrel in patients undergoing elective percutaneous coronary intervention. J Thromb Haemost. 2006;4:2516–2518. doi: 10.1111/j.1538-7836.2006.02187.x. [DOI] [PubMed] [Google Scholar]
- 23.Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]