Dear Editor,
Translocation t(8;9)(p22;p24) has been reported in diverse hematologic neoplasms, including acute leukemia, myeloproliferative neoplasm (MPN), and myelodysplastic syndromes/myeloproliferative neoplasm. These findings indicate that the mutation occurs in pluripotent, lymphoid-myeloid stem cells [1]. The pericentriolar material 1 (PCM1) gene, located on chromosome 8p22, encodes a protein with coiled-coil domains, and the Janus activated kinase 2 (JAK2) gene, located on chromosome 9p24, encodes non-receptor tyrosine kinases [2,3]. The t(8;9)(p22;p24) leads to a PCM1-JAK2 fusion gene, resulting in the continuous activation of JAK2 tyrosine kinase [2]. Here we report a case of myeloid sarcoma (MS) and concurrent myeloproliferative neoplasm, unclassifiable (MPN, U), associated this translocation.
A 42-yr-old man was referred to our hospital in April 2015 for left inguinal lymphadenopathy. He had visited another hospital 20 months earlier, in August 2013, owing to multiple cervical lymphadenopathies. At that time, complete blood count (CBC) revealed 10×109/L leukocytes, 9.9 g/dL hemoglobin, 166×109/L platelets, and peripheral blood (PB) smear demonstrated leukoerythroblastic reaction. Bone marrow (BM) biopsy showed both normal cellularity and a mixed pattern of fibrosis and normal cellularity. An abdominal computed tomography (CT) revealed splenomegaly. His neck lymph node (LN) was radically dissected and a pathologist interpreted the biopsy as showing metastatic malignancy of unknown origin. Based on this, the patient underwent neck radiotherapy and chemotherapy with 5-fluorouracil and cisplatin.
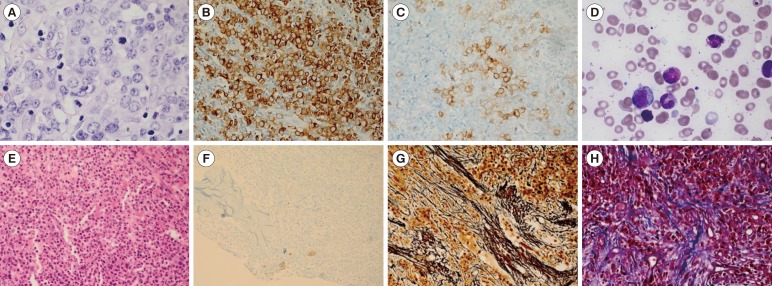
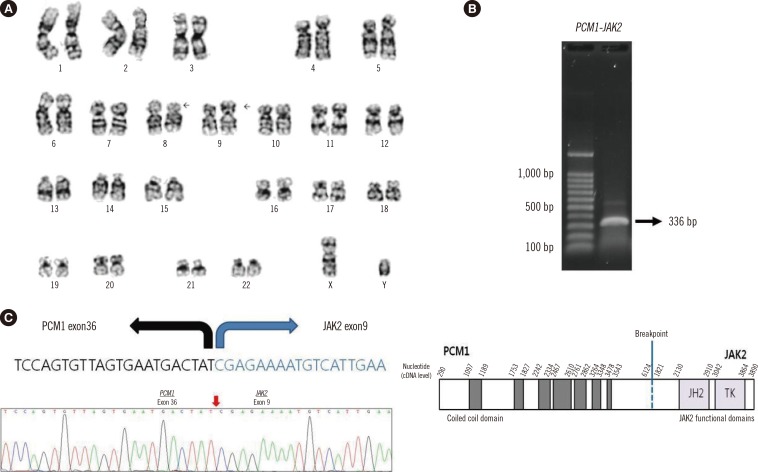
After 13 months, in March 2015, a chest CT revealed enlarged LNs in the right axillary and an LN gun biopsy revealed MS. One week later, the patient developed left inguinal lymphadenopathy and was admitted to our hospital. A inguinal LN biopsy at our hospital also confirmed MS, and immunohistochemistry demonstrated positive results for myeloperoxidase and CD117 (Fig. 1A-C). CBC revealed 5.8×109/L leukocytes, 7.7 g/dL hemoglobin, and 130×109/L platelets, and a PB smear showed leukoerythroblastic reaction (nucleated red blood cell [RBC]: 3/100 white blood cell [WBC]; metamyelocytes: 2%). BM aspiration indicated a normal myeloid:erythroid ratio and 3.2% eosinophils without myelodysplasia, and a biopsy confirmed hyperplasia and myelofibrosis without megakaryocytic proliferation and atypia (Fig. 1D-H). The patient tested negative for major/minor BCR-ABL1 rearrangement and JAK2, MPL, and CALR mutations were not detected. The lactate dehydrogenase level was 206 IU/L, and the C reactive protein level was 1.81 nmol/L. These results indicated features of MPN but did not meet specific criteria for this diagnosis; therefore, a diagnosis of "MPN, U" was made. The BM karyotype was 46,XY,t(8;9)(p22;p24)[20] (Fig. 2A), and reverse-transcription (RT)-PCR was performed with the following primers which we designed: PCM1 forward 5'-TAGTGCTGCCCATAAGGAGTC-3' and JAK2 reverse 5'-AGCGAACAGTTTCCATCTGGT-3'. The PCR product was directly sequenced by using Applied Biosystems 3130 Genetic Analyzers (Applied Biosystems, Foster City, CA, USA). Sanger sequencing revealed an in-frame fusion between exon 36 of PCM1 and exon 9 of JAK2 (Fig. 2B, C), which were shown in previous reports [4,5]. Inguinal LN culture yielded no mitotic cells for chromosome analysis, and RT-PCR of the PCM1-JAK2 fusion gene from a paraffin-embedded LN failed. Chromosomal analysis of PB showed a normal karyotype. The patient underwent chemotherapy with cytosine arabinoside and daunorubicin, and is now waiting for allogeneic hematopoietic stem-cell transplantation.
Fig. 1. Lymph node (LN) and bone marrow (BM) immunohistochemistry. (A) Myeloid sarcoma in an inguinal LN showing increased immature cells with less cytoplasms, round nuclei, and distinct prominent nucleoli (Hematoxylin & Eosin [H&E] stain, ×400); (B) Increased immature cells in an inguinal LN positive for myeloperoxidase (×400) and (C) CD117 (×400); (D) Diluted BM aspiration showing normal hematopoietic cells (Wright stain, ×1,000); (E) BM biopsy showing cellularity of nearly 100% (H&E stain, ×400); (F) Megakaryocytes positive for CD61 without proliferation and atypia (×200); (G) BM biopsy showing grade 2 myelofibrosis (on a 0-3 scale), with diffuse and dense reticulin fibers (Reticulin stain, ×400); (H) focal bundles of collagen fibers (Masson Trichrome stain, ×400).
Fig. 2. The t(8;9)(p22;p24) translocations and PCM1-JAK2 fusion gene. (A) Karyogram of bone marrow showing 46,XY,t(8;9)(p22;p24)[20]; (B) Reverse transcription-PCR product of the PCM1-JAK2 gene from bone marrow; (C) Genetic sequence and schematic representation of the chimeric PCM1-JAK2 gene.
JAK2 has several fusion partner genes, including PCM1, ETV6, and BCR [1,6]. Since the PCM1-JAK2 fusion gene was first detected in 2005 [5], there have been reports of at least 33 more patients with this fusion [1,2]. Many of the hematologic malignancy cases with the PCM1-JAK2 have shown common morphological features, such as myeloproliferation, eosinophilia, and myelofibrosis, and common clinical features such as splenomegaly and a male predominance [2,7]. The PCM1-JAK2 protein is believed to be a target of the JAK1/JAK2 inhibitor, and a recent report indicated that the use of ruxolitinib induced short-term (18 months) remission in a patient with myeloid neoplasms [6]. To the best of our knowledge, this is the first report of a patient with a t(8;9)(p22;p24) in Korea and the second report associated with MPN, U worldwide [3]. Although our patient showed similarities with previous cases, MS with t(8;9)(p22;p24) has not been previously reported. MS can develop synchronously or metachronously in a variety of hematologic malignancies, including MPN. According to one study, results of a FISH analysis of MS tissues and BM or PB karyotypes were concordant in 10 out of 14 cases [8]. Although we did not detect the fusion gene in the LN, it is possible that this fusion gene caused the MS.
In conclusion, t(8;9)(p22;p24) is rare and leads to a PCM1-JAK2 fusion gene. We report MS, concurrent with MPN, U and PCM1-JAK2 fusion gene. This is the first report of such a case in Korea.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (grant number 2013R1A1A3011696).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Bain BJ. Should myeloid and lymphoid neoplasms with PCM1-JAK2 and other rearrangements of JAK2 be recognized as specific entities? Br J Haematol. 2014;166:809–817. doi: 10.1111/bjh.12963. [DOI] [PubMed] [Google Scholar]
- 2.Patterer V, Schnittger S, Kern W, Haferlach T, Haferlach C. Hematologic malignancies with PCM1-JAK2 gene fusion share characteristics with myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, and FGFR1. Ann Hematol. 2013;92:759–769. doi: 10.1007/s00277-013-1695-3. [DOI] [PubMed] [Google Scholar]
- 3.Saba N. A myeloproliferative neoplasm with translocation t(8;9)(p22;p24) involving JAK2 gene. Blood. 2013;122:861. doi: 10.1182/blood-2013-03-487348. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet M, Quelen C, De Mas V, Duchayne E, Roquefeuil B, Delsol G, et al. The t(8;9)(p22;p24) translocation in atypical chronic myeloid leukaemia yields a new PCM1-JAK2 fusion gene. Oncogene. 2005;24:7248–7252. doi: 10.1038/sj.onc.1208850. [DOI] [PubMed] [Google Scholar]
- 5.Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 6.Schwaab J, Knut M, Haferlach C, Metzgeroth G, Horny HP, Chase A, et al. Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Ann Hematol. 2015;94:233–238. doi: 10.1007/s00277-014-2221-y. [DOI] [PubMed] [Google Scholar]
- 7.Hoeller S, Walz C, Reiter A, Dirnhofer S, Tzankov A. PCM1-JAK2-fusion: a potential treatment target in myelodysplastic-myeloproliferative and other hemato-lymphoid neoplasms. Expert Opin Ther Targets. 2011;15:53–62. doi: 10.1517/14728222.2011.538683. [DOI] [PubMed] [Google Scholar]
- 8.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]