This article reviews the safety, efficacy, and pharmacokinetics of several approved formulations of intravitreal corticosteroids for the treatment of diabetic macular edema (including triamcinolone acetonide, dexamethasone, and fluocinolone acetonide) and explores the relationship between kinetics, efficacy, and the development of adverse events.
Key words: diabetic macular edema, intravitreal corticosteroids, pharmacokinetics, bioerodible implants, triamcinolone acetonide, dexamethasone, fluocinolone acetonide, best-corrected visual acuity, intraocular pressure, cataract
Purpose:
To review the relationship between kinetics, efficacy, and safety of several corticosteroid formulations for the treatment of diabetic macular edema.
Methods:
Reports of corticosteroid use for the treatment of diabetic macular edema were identified by a literature search, which focused on the pharmacokinetics, efficacy, and safety of these agents in preclinical animal models and clinical trials.
Results:
Available corticosteroids for diabetic macular edema treatment include intravitreal triamcinolone acetonide, dexamethasone, and fluocinolone acetonide. Because of differences in solubility and bioavailability, various delivery mechanisms are used. Bioerodible delivery systems achieve higher maximum concentrations than nonbioerodible formulations. There is a relationship between visual gains and drug persistence in the intravitreal compartment. Safety effects were more complex; level of intravitreal triamcinolone acetonide exposure is related to development of elevated intraocular pressure and cataract; this does not seem to be the case for dexamethasone, where two different doses showed similar mean intraocular pressure and incidence of cataract surgery. With fluocinolone acetonide, rates of intraocular pressure elevations requiring surgery seem to be dose related; rates of cataract extraction were similar regardless of dose.
Conclusion:
Available corticosteroids for diabetic macular edema exhibit different pharmacokinetic profiles that impact efficacy and adverse events and should be taken into account when developing individualized treatment plans.
Diabetic macular edema (DME) is a common microvascular complication of diabetes, characterized by a breakdown in the blood–retina barrier that causes intracellular and extracellular accumulation of excess fluid and lipid exudates, leading to retinal thickening. Center-involved DME is the most common cause of moderate vision loss in patients with diabetes and is the leading cause of visual impairment in the working-age population. The goal of therapy in DME is to preserve or improve retinal function and vision by reducing or resolving retinal thickening and edema. Therapeutic measures that have been proven to be effective in reducing risk of visual loss in DME include intensive glycemic and blood pressure control,1–3 fenofibrate therapy,3 laser photocoagulation,4,5 and intravitreally administered anti–vascular endothelial growth factor (anti-VEGF) agents6–8 or corticosteroids.9,10
The efficacy of laser photocoagulation in reducing risk of visual loss in patients with DME has been well established since it was found to reduce the risk of moderate visual loss from 24% to 12% over 3 years in patients with DME without macular ischemia in the Early Treatment Diabetic Retinopathy Study in 1985.4 More recently, in a 2-year study comparing laser with intravitreal triamcinolone acetonide (IVTA), moderate visual loss occurred in 14% of patients treated with laser.5 In 2010, the Diabetic Retinopathy Clinical Research Network (DRCR.net) study (Protocol I) reported a moderate visual loss rate of 12% in eyes treated with laser at 2 years; however, the proportion of patients with ≥15-letter vision gain in the laser arm was only 17%.11 In the same study, 29% of patients treated with ranibizumab and deferred laser gained ≥15 letters at 2 years. Recently, other anti-VEGF agents, namely bevacizumab and aflibercept, have also been shown to have similarly improved outcomes compared with laser, but all three agents require monthly or bimonthly injections to maintain efficacy.7,12
Another approach to treatment of DME has been to use intravitreal corticosteroids. Whereas VEGF inhibitors act to reduce excess vascular permeability by acting only on VEGF, corticosteroids act on several pathways involved in the pathogenesis of DME to reduce the action of inflammatory cytokines and VEGF, reduce leukostasis, and improve other physiologic and structural changes, such as tight junction integrity. Corticosteroids that have been extensively evaluated in DME include IVTA, dexamethasone (DEX), and fluocinolone acetonide (FAc).5,9,10,13–15 These three agents are potent and selective glucocorticoid receptor agonists with very limited activity on the mineralocorticoid receptor. It is likely that intravitreal corticosteroids will become an increasingly important element in our armamentarium for therapy in DME. The purpose of this article was to review the published data on these three corticosteroid agents to highlight the differences in potency, solubility, and pharmacokinetic profiles and to consider the relevance of these differences to the observed differences in therapeutic effects seen in clinical studies on DME.
Formulations of Triamcinolone Acetonide, Dexamethasone, and Fluocinolone Acetonide
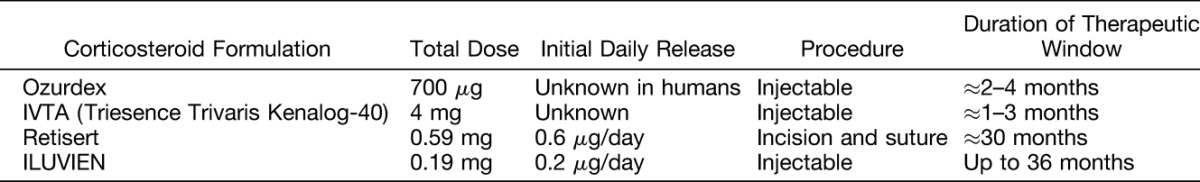
Triamcinolone acetonide (TA; Figure 1A) is a synthetic corticosteroid formulated as an injectable suspension and has a 7.5-fold higher anti-inflammatory potency than cortisone.16 Because TA is water insoluble, it can remain in the vitreous for much longer than other corticosteroids, which are usually eliminated within a few days. Available formulations include Kenalog, Trivaris, and Triesence, which are indicated in the United States for ocular inflammatory conditions unresponsive to topical corticosteroids.17–19 In the European Union, Kenalog is contraindicated for intraocular injection, and Trivaris and Triesence are not approved for any indication.
Fig. 1.
Chemical structures of (A) TA,17–19 (B) DEX,21,22 and (C) FAc.24,25
Dexamethasone (DEX; Figure 1B), which is a less-potent glucocorticoid receptor agonist than TA, has higher solubility in water than TA and requires a slow release delivery system to maintain therapeutic doses in the eye.20 The DEX 700-μg posterior segment drug delivery system (Ozurdex) is a bioerodible implant delivered intravitreally using a 22-gauge injector. It is indicated in the United States, the European Union, and other countries worldwide for the treatment of DME, as well as for macular edema following branch retinal vein occlusion or central retinal vein occlusion and for inflammation of the posterior segment of the eye presenting as noninfectious uveitis.21,22
Fluocinolone acetonide (FAc; Figure 1C), a more potent glucocorticoid receptor agonist than TA, has slightly higher solubility in water than TA and, similar to DEX, requires a delivery system for sustained ocular dosing.20 Two sustained-release devices containing FAc, Retisert and ILUVIEN, are indicated for ocular use. Retisert contains 0.59 mg of FAc and has an initial release of approximately 0.6 μg/day in the vitreous. It is surgically implanted into the posterior segment of the eye through a pars plana incision.23 In the United States, it is indicated for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye. ILUVIEN is a nonbioerodible insert implanted in the eye via injection through the pars plana using a 25-gauge needle. ILUVIEN contains 0.19 mg of FAc. It is indicated in Europe for the treatment of vision impairment associated with chronic DME considered insufficiently responsive to available therapies and in the United States for the treatment of DME in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure (IOP).24,25 In the development of ILUVIEN, 2 doses were studied, one having an initial release of approximately 0.2 μg/day and the other having an initial release of 0.5 μg/day (similar to Retisert). The lower dose elutes FAc from one end of a nonbioerodible polyimide tube, whereas the higher dose elutes drug from both ends of the polyimide tube. Only the lower dose implant has been approved for the treatment of DME.24,25
Intravitreal Release Kinetics
Steroids are known to have complex dose–response curves. Efficacy of steroids depends not only on the potency and dose of drug administered but also on the ability to maintain drug availability in the posterior segment of the eye. This is dependent on the pharmacokinetic profiles produced by the different release kinetics of the short- and sustained-release formulations in terms of burst height and duration of release. An increase in dose of steroid released does not necessarily result in a corresponding increase in efficacy.10,26 Release kinetics differ between the various formulations of intravitreal corticosteroid agents. Evidence from animal and human studies demonstrates the pharmacokinetic profiles of TA, DEX, and FAc.
Similar release kinetics of IVTA in rabbits' eyes have been reported in several studies. Kaamppeter et al27 studied 18 white New Zealand rabbits, each receiving an injection of 6 mg and followed up for 8 months. The authors found that TA concentrations were significantly higher in the vitreous samples than in the anterior chamber at all time points (Figure 2). In the vitreous, the concentrations of triamcinolone were highest the first day (14,434.0 ± 10,768 μg/L), whereas in the anterior chamber, the highest concentrations were observed 3 days after injection (28.9 ± 24.5 μg/L). In both sections of the eye, triamcinolone levels followed a 2-compartment model of elimination: an exponential decrease in concentration within the first 4 weeks (burst period), followed by a steady decline over the following months (duration period). At 8 months, concentrations were 3.3 ± 1.6 μg/L in the anterior chamber and 70.7 ± 37.0 μg/L in the vitreous, approximately 200 times lower than the concentration in the burst period. In another similar study, Ye et al28 examined the pharmacokinetics of IVTA in rabbits that received 4 mg or 8 mg of IVTA and then had vitreous drug concentrations measured using high-performance liquid chromatography. They found that both doses demonstrated peak at the time of initial concentration measurement (burst period) and decreased gradually over time (duration period). Following injection of 4 mg of IVTA, a concentration of 0.0524 mg/mL was detected in the vitreous after 130 days; with 8 mg of IVTA, 0.2606 mg/mL was present at 150 days after injection. Of note, no significant differences in IOP were seen between the two dosing groups, and neither group showed any signs of retinal damage.
Fig. 2.
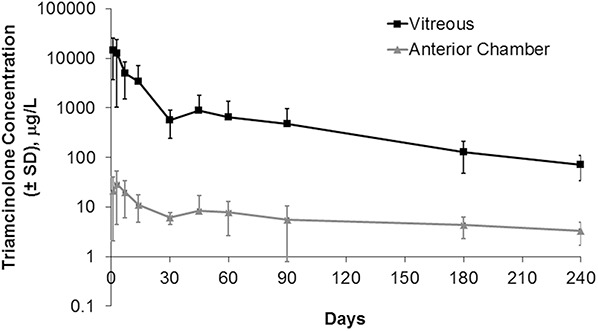
Triamcinolone in the vitreous and anterior chamber of rabbits after injection of 6 mg of TA.27
The effects of vitrectomy on the pharmacokinetics of IVTA and DEX have also been studied extensively. In vitrectomized rabbit eyes, triamcinolone concentration decreased 1.5 times more rapidly versus nonvitrectomized rabbit eyes; half-life was also shorter in vitrectomized versus nonvitrectomized eyes (1.57 days vs. 2.89 days).29,30 The release kinetics of the DEX implant were examined in 25 vitrectomized and 25 nonvitrectomized Dutch-belted rabbits. In nonvitrectomized eyes, time maximal concentration was 22 days for both retina and vitreous humor; by Day 31, 5.0 ± 3.3% of DEX remained in the implant. Results were similar for nonvitrectomized eyes, with time to maximal concentration being shorter in the retina (15 days); 4.2 ± 5.4% of DEX remained after 31 days in vitrectomized eyes. Bhagat et al31 also demonstrated that the release profile of DEX in rabbit eyes was similar with an intact (one-piece) DEX implant and a three-piece implant, suggesting no clinical significance would be associated with fragmentation of the DEX implant.
The release kinetics of DEX also were studied in 34 monkeys after bilateral implantation of 0.7 mg of sustained-release DEX implants.32 Chang-Lin et al32 showed that this bioerodible dose formulation releases with a significant burst—for approximately 2 months—before an exponential decline in release (Figure 3). After 6 months, DEX was below the limits of detection for this assay (0.001 ng/mL in the vitreous humor, 0.001 ng/mL for the retina, and 0.200 ng/mL for plasma). The difference between intravitreal injection of a bolus dose of TA and a biodegradable release form of DEX is most visible in the duration of the burst phase, during which the highest concentration of drug is released.
Fig. 3.
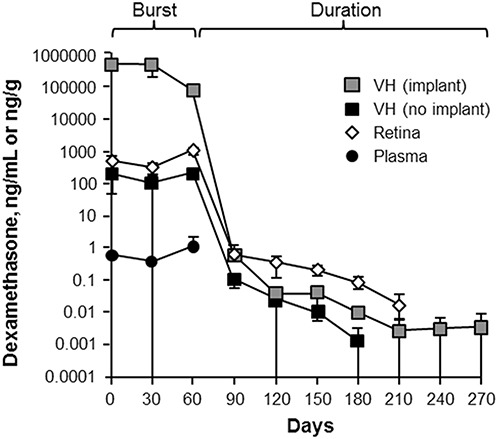
Concentration of DEX in monkey eyes.32 Republished with permission of the Association for Research in Vision and Ophthalmology, from Chang-Lin JE, et al. Invest Ophthalmol Vis Sci 2011;52:80–86; permission conveyed through Copyright Clearance Center, Inc. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Because the implant was difficult to separate from the vitreous humor, the globes were bisected transversely into two hemispheres, with and without the DEX implant; VH indicates vitreous humor.
The release kinetics of FAc sustained-release implants have been studied in rabbit vitreous with both the Retisert and the ILUVIEN delivery systems. The FAc-releasing Retisert implant was surgically placed into the eyes of anesthetized rabbits via sclerotomy and suture.33 Near zero–order kinetics were noted with vitreous humor levels relatively constant from the first time point (2 hours) through 1 year after implantation.33 Plasma levels were below the lower limit of quantitation (200 pg/mL for Retisert) at all times. Release kinetics of ILUVIEN FAc implants were studied in pigmented rabbits.34 After intravitreal injection of different doses of FAc implants, exposure in the vitreous humor was assessed (Figure 4). Quantifiable FAc concentrations were not observed at any dose level in the plasma (lower limit of quantitation, 100 pg/mL for ILUVIEN). The ILUVIEN delivery system and the Retisert system exhibited near zero–order release kinetic profiles but differed in intraocular exposure to FAc based on observed vitreous concentrations. The overall burst heights of corticosteroid concentration in the vitreous found with FAc implants were orders of magnitude lower than either TA or the bioerodible system releasing DEX (1–20 ng/g for FAc vs. >1100 ng/g for DEX and >10,000 ng/g for TA).
Fig. 4.
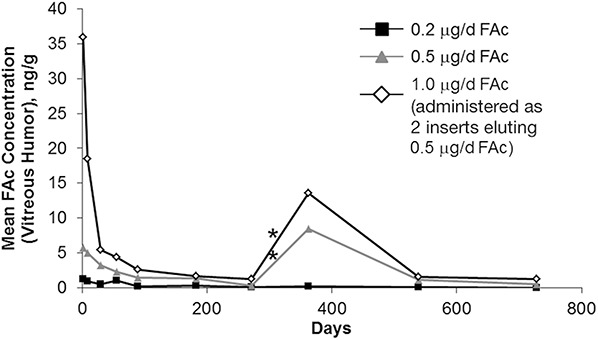
Mean (±standard error of the mean) FAc concentration in the vitreous humor of rabbits.34 Asterisk indicates that a second randomized treatment was administered during Week 52.
In Vivo Pharmacokinetics in Human Eyes
Intravitreal Triamcinolone Acetonide
A small study examined the release kinetics of a single injection of 4 mg of IVTA in human eyes (N = 5).35 They determined from aqueous humor samples that the pharmacokinetics followed a 2-compartment model and the half-life ranged from 76 hours to 635 hours. The mean elimination half-life was 18.6 days in nonvitrectomized eyes, suggesting that measurable concentrations of triamcinolone would be expected to last for approximately 3 months (93 ± 28 days). Audren et al36 examined the pharmacokinetics of IVTA in a larger group of patients with diffuse DME (51 injections in 37 eyes). In this study, they used population pharmacokinetic–pharmacodynamic modeling without triamcinolone concentration measurements. They found that the pharmacokinetic profile of the effect of triamcinolone on central macular thickness (CMT) was characterized by a curve in 3 phases: a fast decrease, a steady state, and a relapse (Figure 5). Similar to results of the smaller study, the mean estimated half-life of triamcinolone ± standard deviation (SD) was 15.4 ± 1.9 days. The mean (±SD) maximum duration of the effect of a single injection was 140 ± 17 days, slightly longer than previously observed. They suggested that the difference could be because of the interval between the elimination of TA and cessation of its pharmacologic activity, which is typical of corticosteroid agents. The authors further suggest that functional measurements such as mean CMT may be even more useful in the clinical field than measuring the TA concentration because this measurement reflects both the concentration and activity of TA.
Fig. 5.
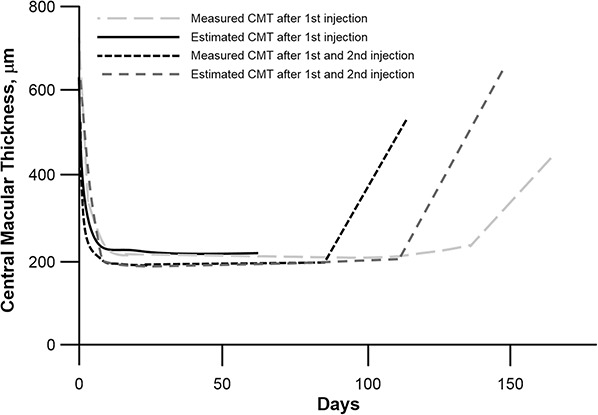
Central macular thickness curves calculated from four individual CMT values after a single injection of IVTA.36
Measured and estimated CMT after first injection are after the first injection of IVTA in the right and left eye of the same patient. Measured and estimated CMT after first and second injection are after the first and second injection in the same eye of another patient.
Dexamethasone
There are no published studies on the pharmacokinetics of DEX 700-μg implants in the human eye. Plasma levels in the majority of patients were below the lower limit of quantitation (50 pg/mL) on Days 7, 30, 60, and 90 after administration but were detectable in 10 of 73 samples at a range of 52 pg/mL to 94 pg/mL.22
Fluocinolone Acetonide
The pharmacokinetics of FAc in human aqueous was examined in the Fluocinolone Acetonide in Human Aqueous study, which compared the levels of FAc released in patients with DME receiving the ILUVIEN delivery system.14 In this study, 37 patients with DMO and 7 with uveitis had aqueous levels of FAc measured after receiving either a 0.2 µg/day insert or a 0.5 µg/day insert. Follow-up assessments were done at 1 week after treatment and then again at 1, 3, 6, 9, 12, 15, 18, 21, 24, 30, and 36 months. A companion study assessed the aqueous levels of FAc associated with Retisert in patients with noninfectious posterior uveitis.37 Overall, results were similar to what was seen in the rabbit studies, with the highest burst obtained with the Retisert implant (Figure 6).37 The 0.5-μg/day FAc implant had a higher mean burst than the 0.2-μg/day ILUVIEN implant. The initial release was followed by a relatively flat profile of release, with a similar submicrogram dose released during the follow-up period for the two doses studied with the ILUVIEN system. Through Month 24, the 0.5-μg/day implant released at a slightly higher level than the 0.2-μg/day implant. The 0.2-μg/day implant continued to release FAc through 36 months (end of follow-up). Both Retisert and ILUVIEN were below the lower limit of quantitation in plasma (200 pg/mL and 100 pg/mL, respectively).23,37 The observed pharmacokinetic properties of various formulations of these corticosteroids are presented in Table 1.
Fig. 6.
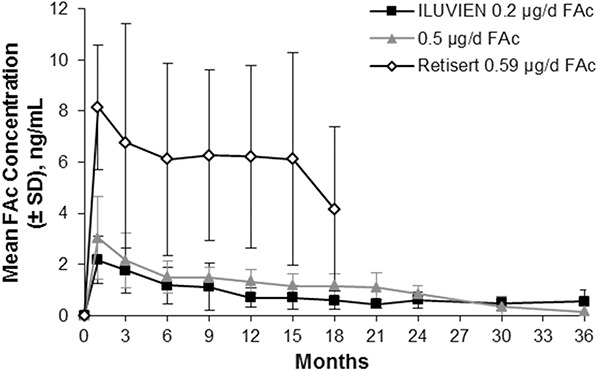
Mean human aqueous concentrations of FAc implants.37
Table 1.
Observed Pharmacokinetic Properties of Approved Ocular Steroid Formulations
Impact of Pharmacokinetics on Efficacy in DME
Intravitreal Triamcinolone Acetonide
Intravitreal triamcinolone acetonide has been evaluated for the treatment of DME in numerous small and a few large clinical trials. The majority of data regarding the efficacy of IVTA in patients with DME suggests that efficacy is greatest 1 month after injection and that these effects are no longer significant at 6 months. A single injection of ITVA (4 mg/0.1 mL) or bevacizumab (1.5 mg/0.06 mL) was examined in patients with refractory DME, defined as having at least 1 previous macular laser photocoagulation treatment.38 The single intravitreal injection resulted in peak functional and anatomical improvement (best-corrected visual acuity [BCVA] and CMT) between Weeks 4 and 8, after which these parameters returned to baseline by Week 24 (Figure 7). Similarly, in a prospective controlled trial of IVTA for refractory diffuse DME, CMT decreased after injection and was significantly lower at Weeks 4 and 12; however, this was no longer significant at Week 24 because of recurrence of macular edema in 5 of 12 eyes.39
Fig. 7.
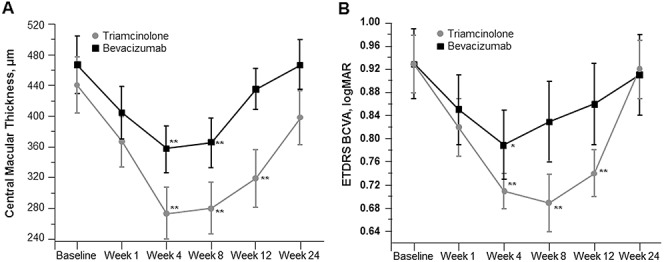
Central macular thickness (A) and BCVA (B) after a single injection of triamcinolone or bevacizumab.38 *P < 0.05 and **P < 0.01 for within-group changes from baseline. Reproduced from Paccola L, et al. Br J Ophthalmol 2008;92:76–80, with permission from BMJ Publishing Group Ltd. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Bonini-Filho et al40 examined intravitreal injection versus sub-Tenon's infusion of TA for refractory DME in a randomized clinical trial of 28 eyes. Visual acuity significantly improved from baseline among patients receiving IVTA at 4, 8, and 12 weeks after injection but was no longer significant at Week 24. These improvements, and the improvements in CMT, were greater with IVTA versus sub-Tenon's infusion, suggesting that intravitreal injections may be more effective in terms of both functional and anatomical outcomes.40 A meta-analysis of visual acuity after IVTA for DME refractory to laser treatment indicated that the maximum improvement in BCVA was found 1 month after injection.41 Finally, in the large, randomized DRCR.net Protocol B study, patients with center-involved DME received either focal/grid laser photocoagulation (control group), 1 mg of IVTA, or 4 mg of IVTA, with eligibility for retreatment at 4-month intervals if persistent or recurrent DME was present. The mean number of injections received by patients in the 4-mg IVTA group was 3.1 over 2 years. At the first follow-up visit (4 months), the patients randomized to the 4-mg IVTA group had the greatest improvement in visual acuity (mean difference compared with laser, adjusted for prior photocoagulation and baseline visual acuity = +3.8 letters); however, at the Month 24 visit, patients randomized to laser had the greatest improvement in visual acuity (+1 with laser, −2 with 1 mg of IVTA, and −3 with 4 mg of IVTA).5 Although loss of visual gains in these patients could occur through a variety of mechanisms, the timing of both the visual gains and the loss are concordant with the timing of the measured intravitreal concentrations of IVTA in rabbit eyes.27 This may reflect a correlation between intravitreal concentration of IVTA and therapeutic effect.
Dexamethasone
The clinical effect of the pharmacokinetics of DEX intravitreal implants can be seen in the PLACID study, which compared DEX intravitreal implants plus laser to laser alone in patients with DME, most of whom had not received previous medical treatment for DME.42 The observed pattern in mean change in BCVA after the first implant reflects the kinetics of drug release in vivo (Figure 8). Mean BCVA improved by slightly more than 6 letters at 1 month and subsequently declined through Month 6. After that point, two thirds of patients were retreated, resulting in a second spike in the BCVA curve.27 Similarly, in vitrectomized eyes, the largest increase in BCVA (6 letters) was noted 8 weeks after implant; by 26 weeks, mean increase in BCVA had dropped to 3 letters.43
Fig. 8.
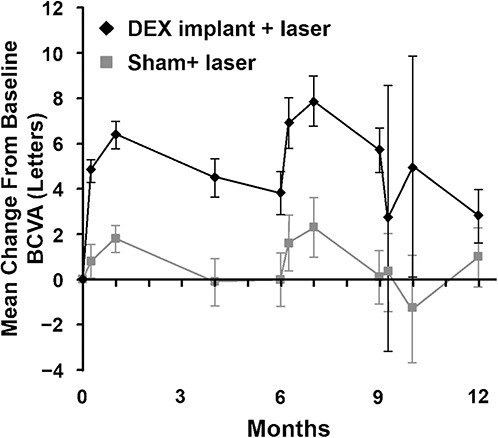
Mean change from baseline BCVA with DEX implant versus sham in the PLACID study.42 Reprinted from Callanan DG, et al. Ophthalmology 2013;120:1843–1851, with permission from Elsevier. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Similar to the mean BCVA profile in the PLACID study, results of the Phase 3 Macular Edema: Assessment of Implantable Dexamethasone in Diabetes (MEAD) study of Ozurdex for DME showed improvement after injection at Months 1.5 and 3, followed by a decline through Month 6 when patients were eligible for reinjection.9 The decline after the 3-month time point was evident even among patients who were pseudophakic at baseline. Overall, these studies suggested that a retreatment interval shorter than 6 months may be needed to provide sustained benefit with DEX implants, consistent with the pharmacokinetic profile.
Fluocinolone Acetonide
Results of the 3-year Retisert in DME trial demonstrated that differences in retinal thickness and visual acuity were significant for Retisert versus standard of care at 2 years, but these differences were no longer significant at 3 years.15 The timing of these results is consistent with the 30-month duration of release from the implant. Similarly, consistent with the sustained release of drug through the end of the 36-month Fluocinolone Acetonide in Human Aqueous study, 0.2-μg/day FAc ILUVIEN implants resulted in sustained efficacy through 36 months.10,13,14,37
In the FAME trials, which examined the safety and efficacy of ILUVIEN in patients with DME, the greatest treatment effect was seen in patients with chronic DME.17 To further examine efficacy in the context of the pharmacokinetic results, the mean change in BCVA was determined for patients with chronic DME who received only a single ILUVIEN implant during FAME. Figure 9A demonstrates that efficacy was maintained through Month 36 in these patients (data on file, Alimera Sciences). A majority of patients who were phakic at baseline (80%) required cataract surgery; however, those patients who underwent cataract surgery during the study still experienced a visual benefit. In patients who were pseudophakic at baseline (Figure 9B; data on file, Alimera Sciences), there was stability of visual acuity on follow-up, particularly in a subgroup of patients with chronic DME and pseudophakia at baseline. Overall, these data suggest that a sustained, low dose of steroid delivered to the eye can result in long-term efficacy.
Fig. 9.
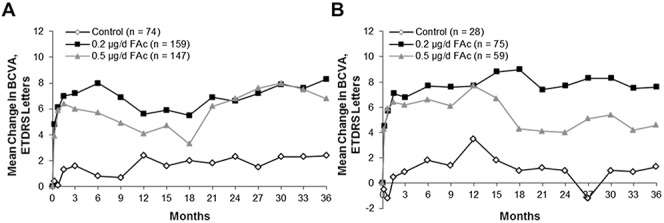
Mean change in BCVA with a single treatment (sham control or FAc implant) in the FAME study among all patients with chronic DME (A) and patients with chronic DME who were pseudophakic at baseline (B) (data on file, Alimera Sciences).
Impact of Pharmacokinetics on Safety in DME
The most common complications associated with ocular steroids are elevated IOP and cataract development. Elevated IOP associated with corticosteroid injection results from the interaction of the steroid with the trabecular meshwork of the anterior chamber of the eye.44 It is possible that minimizing steroid exposure in the front of the eye may decrease the interaction with the trabecular meshwork and thereby decrease the incidence of IOP-related events. Risk factors for developing ocular hypertension in response to steroid exposure include documented or suspected glaucoma, family history of glaucoma, and younger age.45,46 The potential impact on the release profile of IVTA, DEX, and FAc were examined in published trials.
Intravitreal Triamcinolone Acetonide
Elevated IOP usually occurs 1 to 2 months after injection of IVTA but has been found to occur anywhere between 1 and 13 weeks postinjection.44 Following a single injection of IVTA in patients with refractory DME in the Intravitreal Triamcinolone Acetonide Versus Intravitreal Bevacizumab for Refractory Diabetic Macular Edema (IBEME) study, the highest IOP was observed by Week 4, followed by a decline to baseline over time, consistent with the timing of maximum efficacy.38 In the DRCR.net Protocol B trial, IOP elevations of ≥10 mmHg were observed in 33% of patients receiving 4 mg of IVTA, and 30% of these patients required IOP-lowering medications.5 These results are based on 2 years of follow-up and a mean of 3.1 injections of 4 mg of IVTA. A single dose of IVTA in the IBEME study did not result in cataract progression during the 24-month follow-up38; however, a dose–response relationship was observed between IVTA exposure and cataract formation in the DRCR.net Protocol B study, where 51% of patients receiving 4 mg of IVTA injections required cataract surgery compared with 23% in the 1-mg IVTA arm (P < 0.001).5
Dexamethasone
Elevated IOP was seen following treatment with Ozurdex treatment in the PLACID study, with 16.8% and 4% of eyes experiencing an IOP of ≥25 mmHg and ≥35 mmHg, respectively, at some point during the study.42 By Month 12, no eyes had IOP ≥25 mmHg. Haller et al47 observed that most cases of elevated IOP occurred during the first 60 days after treatment, during the initial burst phase of release. In the MEAD study, the highest mean change in IOP in the overall population occurred within 3 months after Ozurdex implant and subsequently declined by Month 6. These results occurred for each retreatment throughout the study.9 Mean IOP was similar in the 700-μg and 350-μg dose groups. In studies with shorter follow-up, such as the PLACID study, rates of cataract surgery were not greater in patients treated with Ozurdex implants than in patients treated with laser alone; however, cataract-related adverse events were more frequent in the DEX group versus the laser group (22.2% vs. 9.5%; P = 0.017).42 In studies with longer follow-up, such as the 3-year MEAD study, cataract surgery was performed on 59.2% of patients receiving 700 μg of DEX and 52.3% receiving 350 μg of DEX (35.9% and 33.7% of patients discontinued the study in the 700-μg and 350-μg DEX arms, respectively).9
Fluocinolone Acetonide
With FAc treatment, dose seemed to have an effect on the incidence of IOP-related adverse events. With Retisert treatment of DME, IOP ≥30 mmHg was recorded in 61.4% of implanted eyes, and 33.8% required surgery for ocular hypertension by 4 years.15 Fewer IOP-related events were associated with ILUVIEN, particularly IOP-lowering surgery (over 3 years, 4.8% of patients in the 0.2-μg/day FAc group and 8.1% of those in the 0.5-μg/day FAc group).10 As with IVTA, timing of IOP elevation seemed to mirror the release of steroid from the FAc implant (Figure 10). The dose of FAc did not seem to impact the rate of cataract, with cataract extraction occurring in 91% of phakic eyes treated with Retisert, 87.2% treated with 0.5 μg/day FAc, and 80% treated with ILUVIEN 0.2 μg/day FAc.10,15
Fig. 10.
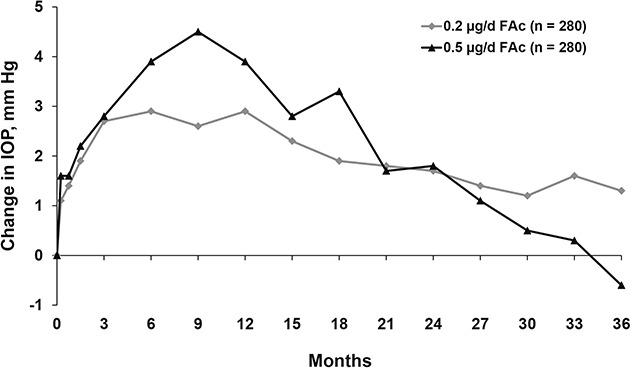
Mean change from baseline IOP in the FAME study (data on file, Alimera Sciences).
Overall, the timing of IOP elevation with the steroids studied suggests that elevated IOP mirrors drug release in the eye and returns to baseline as drug is depleted. Cataract incidence is not related to the pharmacokinetics observed with a single short-term release corticosteroid but rather to sustained dosage over an extended time. This was seen both with sustained-release steroid implants and with repeated injections of short-release steroids formulations.
Discussion
The importance of corticosteroids in the treatment of DME has been established, including emerging evidence from Phase 3 trials supporting the need for multifactorial treatment in patients with longer duration disease who do not respond to highly specific treatments (i.e., VEGF inhibitors).10,42 The corticosteroids that have been examined for treatment of DME include TA, DEX, and FAc, which differ in potency, solubility, and pharmacokinetic profile. Because of the insolubility of TA, a bolus injection is used, which forms a crude depot inside the eye that slowly dissolves. Dexamethasone, the most soluble of the corticosteroids, must be delivered via a bioerodible insert. Currently, only FAc is delivered via a nonbioerodible system. These different delivery mechanisms contribute to the different therapeutic durations, which range from months to years. For drugs with a high burst (IVTA, DEX implant), the greatest effect is seen during this initial phase, followed by waning during the duration phase. For those with a low burst and near zero–order kinetics (FAc implant), efficacy is maintained throughout the duration phase. There could be many explanations for this effect, one of which is that a very high initial dose could “shock the system,” making cells dependent on a high concentration of drug for continued effect.
Because of the known adverse events related to corticosteroid use in the eye, one of the goals of corticosteroid administration is maximizing efficacy while minimizing cataract development and IOP elevation. Minimization of exposure of the trabecular meshwork through posterior positioning and a low dose of corticosteroid may ameliorate the IOP events, as evidenced by the difference seen with the two FAc delivery systems, Retisert and ILUVIEN. The rate of IOP-lowering surgery was 7-fold lower with ILUVIEN than Retisert, possibly because of the lower dose and more posterior positioning of ILUVIEN.
The studies described herein also have limitations in the interpretation of results. The patients in each trial had different baseline characteristics, including some who were treatment-naive and some who were refractory to laser. Differences in the resiliency of their photoreceptor systems could impact cellular response to steroid exposure. Some trials included only the use of a steroid injection, whereas in others, patients received laser or other treatments, which could further impact the efficacy and confound results. Data in all cases are not directly comparable because of differing use of standard of care treatments; however, the totality of evidence across trials can give insights to the duration and clinical profile of various steroid treatments.
In conclusion, the published data suggest that there are distinct differences in pharmacokinetic profiles between IVTA formulations, the bioerodible DEX implant, and nonbioerodible FAc implants. These differences may well account for the differences in duration of treatment response and adverse events, such as cataract formation and elevated IOP. Given the presence of multiple pathogenic mechanisms that drive the development of macular edema in a variety of retinal diseases, it is likely that corticosteroids will form an important part of our armamentarium in the therapeutic management of patients with such retinal conditions. Given these corticosteroids' different profiles of efficacy, duration, and safety, it may be of some advantage in clinical practice to select a particular corticosteroid for a particular clinical scenario based on our understanding of how the differences in pharmacokinetic profiles can be considered for the individual needs of patients.
Footnotes
The authors received editorial assistance from MediTech Media, Hamilton, NJ, funded by Alimera Sciences.
Yit Yang reports personal fees from Alimera Sciences, Allergan, Alcon, Bayer, Novartis, and Thrombogenics, during the conduct of the study. Clare Bailey reports the following: Bristol Eye Hospital undertakes clinical trial work funded by Novartis pharmaceuticals, honoraria and occasional lectures for attending occasional advisory board meetings; Bristol Eye Hospital took part in the FAME studies, therefore received commercial funding for recruitment into this trial from Alimera Sciences; Bristol Eye Hospital received research funding for clinical trials work from Bayer. Anat Loewenstein reports personal fees from Allergan, Alcon, Alimera Sciences, Bayer, La Roche, Novartis, Teva, during the conduct of the study; reports personal fees from ForsightLabs, Orabio, Lumenis, Notal Vision, outside of the submitted work. Pascale Massin reports consultancy for Novartis, Allergan, Alimera Sciences, Bayer, and Sanofi, during the conduct of the study.
References
- 1.Holman RR, Paul SK, Bethel MA, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–1576. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.ACCORD Study Group; ACCORD Eye Study Group; Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985;103:1796–1806. [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008;115:1447–1449; 1449.e1–1449.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham ET, Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112:1747–1757. [DOI] [PubMed] [Google Scholar]
- 7.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 2012;130:972–979. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120:2013–2022. [DOI] [PubMed] [Google Scholar]
- 9.Boyer DS, Yoon YH, Belfort R, Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904–1914. [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012;119:2125–2132. [DOI] [PubMed] [Google Scholar]
- 11.Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064–1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen QD. Two-year outcomes of the VISTA/VIVID trials of intravitreal aflibercept injection in diabetic macular edema. 2014. AAO Meeting Abstracts 2014; [abstract PA083]. Available at: https://secure.aao.org/apps/MeetingArchive/tabid/433/Default.aspx. Accessed July 28, 2015. [Google Scholar]
- 13.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011;118:626–635.e2. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Hafiz G, Shah SM, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology 2010;117:1393–1399.e3. [DOI] [PubMed] [Google Scholar]
- 15.Pearson PA, Comstock TL, Ip M, et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology 2011;118:1580–1587. [DOI] [PubMed] [Google Scholar]
- 16.Mansoor S, Kuppermann BD, Kenney MC. Intraocular sustained-release delivery systems for triamcinolone acetonide. Pharm Res 2009;26:770–784. [DOI] [PubMed] [Google Scholar]
- 17.TRIESENCE (triamcinolone acetonide injectable suspension) 40 mg/mL [package insert]. Fort Worth, TX: Alcon Laboratories; 2013. Available at: http://ecatalog.alcon.com/pi/Triesence_us_en.pdf. Accessed July 28, 2015.
- 18.TRIVARIS (triamcinolone acetonide injectable suspension) 80 mg/mL [package insert]. II, Irvine, CA: Allergan, Inc. 2008. Available at: http://www.drugs.com/pro/trivaris.html. Accessed July 28, 2015.
- 19.KENALOG-40 INJECTION (triamcinolone acetonide injectable suspension, USP) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2011. Available at: http://packageinserts.bms.com/pi/pi_kenalog-40.pdf. Accessed July 28, 2015.
- 20.Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica 2010;224:S25–S30. [DOI] [PubMed] [Google Scholar]
- 21.electronic Medicines Compendium (eMC). Ozurdex. Available at: http://www.medicines.org.uk/emc/medicine/23422. Accessed October 23, 2014.
- 22.Ozurdex (dexamethasone intravitreal implant) [package insert]. Irvine, CA: Allergan, Inc; 2014. Available at: http://www.allergan.com/assets/pdf/ozurdex_pi.pdf. Accessed October 6, 2014.
- 23.Bausch & Lomb. RETISERT (fluocinolone acetonide intravitreal implant) 0.59 mg [package insert]. Rochester, NY: Bausch & Lomb; 2012. Available at: http://www.bausch.com/Portals/107/-/m/BL/United%20States/USFiles/Package%20Inserts/Pharma/retisert-prescribing-information.pdf. Accessed July 28, 2015. [Google Scholar]
- 24.ILUVIEN summary of product characteristics. Aldershot, United Kingdom: Alimera Sciences F Limited; 2013. Available at: https://www.medicines.org.uk/emc/medicine/27636. Accessed July 28, 2015. [Google Scholar]
- 25.ILUVIEN (fluocinolone acetonide intravitreal implant) [package insert]. Alpharetta, GA: Alimera Sciences, Inc; 2014. Available at: http://iluvien.com/downloads/iluvien-prescribing-information.pdf. Accessed October 6, 2014.
- 26.Hauser D, Bukelman A, Pokroy R, et al. Intravitreal triamcinolone for diabetic macular edema: comparison of 1, 2, and 4 mg. Retina 2008;28:825–830. [DOI] [PubMed] [Google Scholar]
- 27.Kamppeter BA, Cej A, Jonas JB. Intraocular concentration of triamcinolone acetonide after intravitreal injection in the rabbit eye. Ophthalmology 2008;115:1372–1375. [DOI] [PubMed] [Google Scholar]
- 28.Ye YF, Gao YF, Xie HT, Wang HJ. Pharmacokinetics and retinal toxicity of various doses of intravitreal triamcinolone acetonide in rabbits. Mol Vis 2014;20:629–636. [PMC free article] [PubMed] [Google Scholar]
- 29.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005;25:556–560. [DOI] [PubMed] [Google Scholar]
- 30.Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci 2011;52:4605–4609. [DOI] [PubMed] [Google Scholar]
- 31.Bhagat R, Zhang J, Farooq S, Li XY. Comparison of the release profile and pharmacokinetics of intact and fragmented dexamethasone intravitreal implants in rabbit eyes. J Ocul Pharmacol Ther 2014;30:854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci 2011;52:80–86. [DOI] [PubMed] [Google Scholar]
- 33.Driot JY, Novack GD, Rittenhouse KD, et al. Ocular pharmacokinetics of fluocinolone acetonide after retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther 2004;20:269–275. [DOI] [PubMed] [Google Scholar]
- 34.Kane FE, Green KE. Ocular pharmacokinetics of fluocinolone acetonide following iluvien implantation in the vitreous humor of rabbits. J Ocul Pharmacol Ther 2015;31:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003;110:681–686. [DOI] [PubMed] [Google Scholar]
- 36.Audren F, Tod M, Massin P, et al. Pharmacokinetic-pharmacodynamic modeling of the effect of triamcinolone acetonide on central macular thickness in patients with diabetic macular edema. Invest Ophthalmol Vis Sci 2004;45:3435–3441. [DOI] [PubMed] [Google Scholar]
- 37.Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology 2013;120:583–587. [DOI] [PubMed] [Google Scholar]
- 38.Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). Br J Ophthalmol 2008;92:76–80. [DOI] [PubMed] [Google Scholar]
- 39.Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology 2004;111:218–224; discussion 224–225. [DOI] [PubMed] [Google Scholar]
- 40.Bonini-Filho MA, Jorge R, Barbosa JC, et al. Intravitreal injection versus sub-tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci 2005;46:3845–3849. [DOI] [PubMed] [Google Scholar]
- 41.Rudnisky CJ, Lavergne V, Katz D. Visual acuity after intravitreal triamcinolone for diabetic macular edema refractory to laser treatment: a meta-analysis. Can J Ophthalmol 2009;44:587–593. [DOI] [PubMed] [Google Scholar]
- 42.Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013;120:1843–1851. [DOI] [PubMed] [Google Scholar]
- 43.Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina 2011;31:915–923. [DOI] [PubMed] [Google Scholar]
- 44.Razeghinejad MR, Katz LJ. Steroid-induced iatrogenic glaucoma. Ophthalmic Res 2012;47:66–80. [DOI] [PubMed] [Google Scholar]
- 45.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol 1963;70:492–499. [DOI] [PubMed] [Google Scholar]
- 46.Davies TG. Tonographic survey of the close relatives of patients with chronic simple glaucoma. Br J Ophthalmol 1968;52:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol 2010;128:289–296. [DOI] [PubMed] [Google Scholar]


