Significance
Decadal-scale metabolic responses of plants to environmental changes, including the magnitude of the “CO2 fertilization” effect, are a major knowledge gap in Earth system models, in agricultural models, and for societal adaptation. We introduce intramolecular isotope distributions (isotopomers) as a methodology for detecting shifts in plant carbon metabolism over long times. Trends in a deuterium isotopomer ratio allow quantification of a biogeochemically relevant shift in the metabolism of C3 plants toward photosynthesis, driven by increasing atmospheric CO2 since industrialization. Isotopomers strongly increase the information content of isotope archives, and may therefore reveal long-term acclimation or adaptations to environmental changes in general. The metabolic information encoded in isotopomers of plant archives bridges a fundamental gap between experimental plant science and paleoenvironmental studies.
Keywords: isotopomer, acclimation, deuterium, CO2 fertilization, atmospheric change
Abstract
Terrestrial vegetation currently absorbs approximately a third of anthropogenic CO2 emissions, mitigating the rise of atmospheric CO2. However, terrestrial net primary production is highly sensitive to atmospheric CO2 levels and associated climatic changes. In C3 plants, which dominate terrestrial vegetation, net photosynthesis depends on the ratio between photorespiration and gross photosynthesis. This metabolic flux ratio depends strongly on CO2 levels, but changes in this ratio over the past CO2 rise have not been analyzed experimentally. Combining CO2 manipulation experiments and deuterium NMR, we first establish that the intramolecular deuterium distribution (deuterium isotopomers) of photosynthetic C3 glucose contains a signal of the photorespiration/photosynthesis ratio. By tracing this isotopomer signal in herbarium samples of natural C3 vascular plant species, crops, and a Sphagnum moss species, we detect a consistent reduction in the photorespiration/photosynthesis ratio in response to the ∼100-ppm CO2 increase between ∼1900 and 2013. No difference was detected in the isotopomer trends between beet sugar samples covering the 20th century and CO2 manipulation experiments, suggesting that photosynthetic metabolism in sugar beet has not acclimated to increasing CO2 over >100 y. This provides observational evidence that the reduction of the photorespiration/photosynthesis ratio was ca. 25%. The Sphagnum results are consistent with the observed positive correlations between peat accumulation rates and photosynthetic rates over the Northern Hemisphere. Our results establish that isotopomers of plant archives contain metabolic information covering centuries. Our data provide direct quantitative information on the “CO2 fertilization” effect over decades, thus addressing a major uncertainty in Earth system models.
Atmospheric CO2 levels have increased from ∼200 ppm during the last ice age to currently 400 ppm, and they may, according to pessimistic scenarios, exceed 1,000 ppm in the year 2100 (1). Understanding plant responses to increasing CO2 is currently hampered by two fundamental limitations: First, it is unknown how well manipulation experiments represent responses to the gradual CO2 increase over decades and centuries. In Free-Air CO2 Enrichment (FACE) experiments, which most closely mimic natural conditions, increases in [CO2] generally increase plant growth, but this “CO2 fertilization” effect often declines after a few years of enrichment (2). Such transient responses may be related to the step increases in [CO2] used in the experiments, their limited duration (2), or factors other than CO2 becoming limiting (3). Second, in response to the [CO2] increase since industrialization, genetic (4) and phenotypic plant responses (5–7) have been observed. Although century-scale changes have been detected in carbon isotopes (δ13C) and attributed to [CO2], these responses are tied to differences in intercellular substrate concentrations that reflect several metabolic fluxes and diffusion processes (8). However, the responses do not measure the metabolic fluxes directly. The consequently poor constraint of the magnitude of the CO2 fertilization effect over decadal to century time scales (1, 9) is a major source of uncertainty in parameterizations of Earth system models (1, 10) and crop productivity models (11, 12). Therefore, observational data are essential to enable global carbon models to provide reliable long-term predictions.
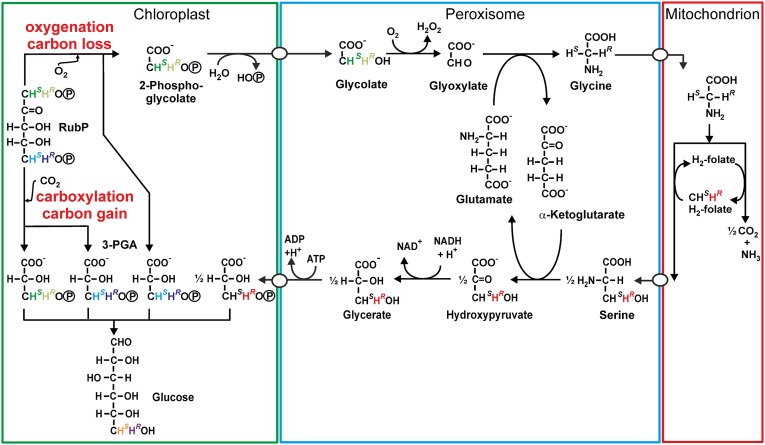
Plants that fix carbon by the C3 photosynthetic pathway (C3 plants) account for most (ca. 75%) global primary production and human food production (13). In C3 plants, CO2 initially reacts with D-ribulose-1,5-bisphosphate (RubP) catalyzed by the enzyme D-ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), but this enzyme also has a competing O2 fixation activity (14). The resulting carboxylation and oxygenation processes create photosynthetic and photorespiratory metabolic fluxes (15), which, respectively, cause C gains and losses for plants (Fig. 1), and which, globally, are among the largest biogeochemical C fluxes (16). Thus, the balance between these metabolic fluxes is a major determinant of the net primary productivity of C3 plants in response to increasing [CO2], and therefore is a major influence on the CO2 sink strength of terrestrial vegetation, including the performance of C3 crop plants.
Fig. 1.
Overview of metabolic pathways emanating from RubP by Rubisco activity. Upon carboxylation, 3-phosphoglycerate (3-PGA) molecules are formed from C1−C2 and C3−C5 of RuBP. Upon oxygenation, one 3-PGA molecule is formed from C3−C5 of RubP, and up to one-half 3-PGA is formed via the photorespiration pathway through the peroxisome and mitochondrion. The color coding of hydrogen atoms tracks the biochemical origins of hydrogens at C3 of 3-PGA, which give rise to the isotopomer signal encoded in the colored C6H2 group of glucose.
Stable isotopes such as deuterium (D) are key tools for probing plants’ ecological and biogeochemical interactions with their environment. Conventional compound-specific stable isotope methods provide the D/H ratio, usually expressed as δD (17), of an entire molecule, that is, averaged over all intramolecular positions in the molecule. This means that conventional stable isotope methods are based on isotopologues, which, by definition, differ in the number of isotopic substitutions. The same situation applies to the carbon isotope ratio 13C/12C expressed as δ13C. The δD or δ13C signals of plant metabolites largely depend on three mechanisms: the isotope signatures of the plant’s H2O and CO2 sources, isotope fractionation during substrate uptake, and isotope fractionation during biosynthesis, primarily enzyme isotope effects.
It is well established that enzyme isotope effects influence stable isotope abundance in specific intramolecular positions (18, 19). A molecule carrying an isotope substitution in a specific intramolecular position is termed an isotopomer. Deuterium isotopomer abundances can be measured by NMR. Glucose, measured as a derivative that gives highly resolved deuterium NMR spectra (see Methods), gives rise to seven signals with variable integrals (Fig. 2A), which reflect the abundance of each of the seven D isotopomers of glucose. Isotopomer variation encodes metabolic information (18, 19), but isotopomers have not yet been used for unraveling long-term metabolic changes induced by environmental drivers.
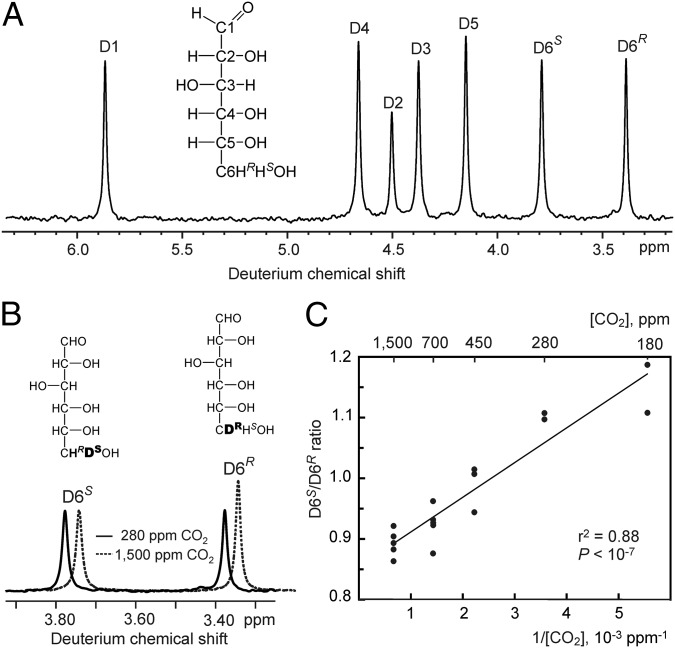
Fig. 2.
Effect of [CO2] on the D6S/D6R isotopomer ratio of photosynthetically generated glucose moieties in sunflower (H. annuus). (A) Deuterium NMR spectrum of a glucose derivative displaying one signal for each of the seven isotopomers of glucose. The signals’ integrals are proportional to the isotopomer abundances. (B) Excerpts of deuterium NMR spectra of glucose prepared from sunflower leaf starch, showing signals arising from the D6S and D6R isotopomers of the C6H2 group of glucose. The solid and dashed spectra were acquired from glucose formed at 280 ppm and 1,500 ppm CO2, respectively. The dashed spectrum has been shifted sideways to ease comparison. (C) Dependence of the D6S/D6R ratio of glucose from sunflower leaf starch on 1/[CO2] (in units of 10−3 ppm−1) during growth [r2 = 0.88, slope 0.057 ±0.006 (SEM), P < 10−7, n = 17, individual plants except for 180 and 280 ppm, where material from two to four plants had to be pooled for each sample].
Here, we establish isotopomers as a ground-breaking technique for evaluating long-term effects of gradual [CO2] increase on plant metabolism at the biochemical level. We first use CO2 manipulation experiments to identify an isotopomer signal that reflects the Rubisco oxygenation/carboxylation ratio of C3 plants. We then trace the signal in herbarium samples of wild plants, crops, and Sphagnum mosses, to estimate changes in metabolic flux ratios in photosynthetic carbon metabolism of C3 plants over the past century.
Results
An Isotopomer Signal of the Photorespiration/Photosynthesis Ratio.
We first exposed sunflower (Helianthus annuus), a C3 plant, to CO2 concentrations ranging from 180 ppm to 1,500 ppm. Expansions of deuterium NMR spectra of a glucose derivative (Fig. 2B) show signals that reflect the abundances of the D isotopomers of the C6H2 group of glucose; these hydrogens are stereochemically and biochemically distinct, which is indicated by the labeling as D6S and D6R. The integral ratio of the signals, the D6S/D6R ratio, differs between the two spectra, which were obtained from glucose samples formed at 280 ppm and 1,500 ppm [CO2], respectively. When CO2 is varied, the D6S/D6R ratio shows a linear dependence on 1/CO2 (Fig. 2C and Table S1). Because sunflower is a C3 plant, the oxygenation/carboxylation metabolic flux ratio at Rubisco is proportional to the concentration ratio of the competing substrates O2/CO2 (20, 21), which, at constant [O2], yields a linear dependence on 1/[CO2]. That the D6S/D6R ratio reflects this metabolic flux ratio is supported by leaf gas exchange measurements (Fig. S1).
Table S1.
Gas exchange and isotopomer data obtained from experiments with sunflower leaves (group A)
| Variable | CO2 treatment, ppm | ||||
| 180 | 280 | 450 | 700 | 1,500 | |
| Ci, µM | 5.2 ± 0.1 | 7.8 ± 0.2 | 11.65 ± 0.3 | 19.7 ± 0.6 | 49.1 ± 0.7 |
| ϕ | 0.51 | 0.34 | 0.23 | 0.14 | 0.05 |
| D6S/D6R | 1.187 | 1.107 | 0.944 | 0.962 | 0.883 |
| 1.108 | 1.097 | 1.014 | 0.877 | 0.893 | |
| 1.007 | 0.925 | 0.904 | |||
| 0.931 | 0.863 | ||||
| 0.923 | 0.921 | ||||
The leaf temperature was 22 °C, giving an intercellular (dissolved) O2 concentration of 277 μM and Ksp of 104 (59). Ci was derived from gas exchange measurements on sunflower leaves, and D6S/D6R ratios were measured on leaf starch. Values of Ci are averages ± SE. Samples represent individual plants, except for 180 ppm and 280 ppm, where material from two to four plants had to be pooled to obtain sufficient sample amount.
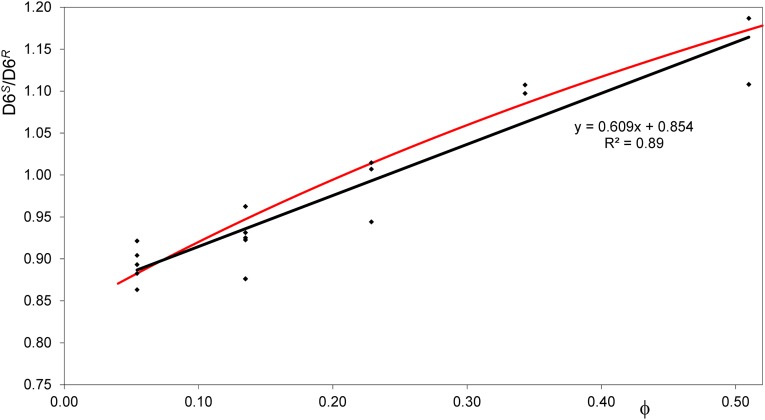
Fig. S1.
The D6S/D6R isotopomer ratio as a function of ϕ (the oxygenation to carboxylation ratio). Glucose derivative was prepared from structural carbohydrates of sunflower leaves; input data are given in Table S1. The red curve shows a root-least-squares fit of the data to Eq. S1, and the black line shows a linear fit of the data.
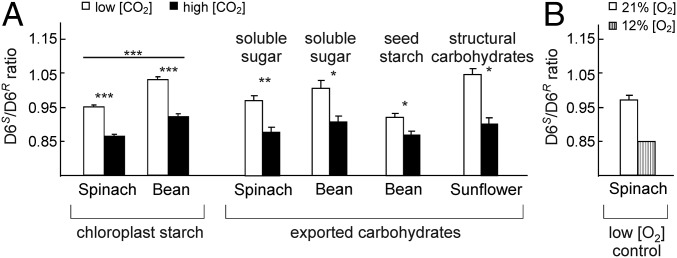
Photosynthetic oxygen production first appeared ca. 3 billion years ago, before which atmospheric [O2] was low (22). Since then, it has risen greatly, with a concomitant increase in the wasteful Rubisco oxygenation reaction. To cope with oxygenation, C3 plants have evolved the photorespiration pathway to recycle oxygenation products (23). It appears that evolutionary changes could not suppress Rubisco’s oxygenation activity, because of a strong trade-off between Rubisco’s reaction rate and its CO2/O2 selectivity (8, 23–25). In contrast, C4 plants largely suppress oxygenation by locally increasing [CO2] (23). Thus, [CO2] dependence of the D6S/D6R ratio should be observable in all C3 plants but should be absent in C4 species. Accordingly, analysis of the D6S/D6R ratio of chloroplast starch of two additional C3 species grown at experimentally increased [CO2], spinach (Spinacia oleracea) and bean (Phaseolus vulgaris) (Fig. 3A and Table S2), confirmed the findings from sunflowers. In both species, the D6S/D6R ratio decreased by ∼0.1 upon doubling [CO2], in accordance with the results from the experiment with sunflower (Fig. 2C). In marked contrast, but in agreement with the suppression of photorespiration in C4 plants, the D6S/D6R ratios of glucose formed under 450 ppm and 1,200 ppm in the C4 species maize (Zea mays) were not significantly different (P = 0.9). Furthermore, reducing the O2 concentration influenced the D6S/D6R ratio in the same way as increasing [CO2] in the tested C3 plants (Fig. 3B), as expected for suppression of photorespiration. These mechanistic tests lead us to conclude that the D6S/D6R ratio is indeed a robust measure of the oxygenation/carboxylation metabolic flux ratio in C3 plants.
Fig. 3.
Response of the D6S/D6R ratio of different species and metabolites to manipulation of the [CO2]/[O2] ratio. (A) [CO2] manipulation; white bars represent low and black bars represent high [CO2] of 360 ppm and 700 ppm, respectively, except for sunflower (200 ppm and 1,000 ppm). (B) O2 manipulation; white bar represents ambient atmosphere, and gray bar represents ambient [CO2] with reduced [O2] (12%); glucose from soluble sugars was analyzed. Values are averages ± SEM (n = 2–5), except for one single sample pooled from several plants. *P < 0.05; **P < 0.01; ***P < 0.001 (ANOVA).
Table S2.
Summary of plant material, growth conditions, CO2 treatments, and measured D6S/D6R values for greenhouse and growth chamber experiments
| Group | Species | Growth conditions, duration of CO2 treatment | Sugar analyzed | Samples | CO2 treatment, ppm | D6S/D6R | |||
| Low CO2 | High CO2 | Difference | ANOVA P | ||||||
| A | sunflower | greenhouse, 2 d | leaf starch | a | 180/280/450/700/1,500 | see Table S1 | see Table S1 | see Table S1 | — |
| B | sunflower | growth chamber, from germination | leaf structural carbohydrates | b | 200 and 1,000 | 1.043 ± 0.018 | 0.862 | 0.0181 | — |
| C | maize* | greenhouse, 2 d | leaf starch | c | 450 and 1,200 | 1.269 ± 0.013 | 1.270 ± 0.001 | −0.001 | 0.966 |
| D | spinach | growth chamber, from germination | leaf starch | d | 360 and 700 | 0.950 ± 0.007 | 0.865 ± 0.005 | 0.085 | <0.001† |
| leaf soluble sugars | e | 360 and 700 | 0.968 ± 0.014 | 0.877 ± 0.015 | 0.091 | 0.007 | |||
| leaf soluble sugars | f | 360‡ | 0.849 | 0.101‡ | — | ||||
| E | bean | growth chamber, from germination | leaf starch | g | 360 and 700 | 1.032 ± 0.006 | 0.923 ± 0.007 | 0.109 | <0.001† |
| leaf soluble sugars | h | 360 and 700 | 1.004 ± 0.022 | 0.906 ± 0.017 | 0.098 | 0.024 | |||
| seed starch | i | 360 and 700 | 0.920 ± 0.013 | 0.868 ± 0.011 | 0.052 | 0.037 | |||
Plants with C3, C4, and crassulacean acid metabolism (CAM) show distinct overall D isotopomer patterns, and high D6S/D6R values are characteristic of C4 metabolism (28). Importantly, the D6S/D6R ratio of the maize metabolite is independent of [CO2].
Two-way ANOVA with species (spinach or bean) and CO2 treatment as variables shows that D6S/D6R ratios differ between species (P = 10−9) and depend on [CO2] (P = 10−7), with no interaction between the variables (P = 0.14), indicating that although D6S/D6R values are species-dependent, their shifts as function of [CO2] are conserved among species. Such variation may reflect species-dependent D fractionation connected to the regulation of carbon metabolism.
Twelve percent O2 and difference is taken relative to 360-ppm treatment, sample e.
Next, we tested if the 1/[CO2] dependence of the D6S/D6R ratio is preserved in glucose units of metabolites formed after export of carbohydrates from the chloroplast (leaf soluble sugars, leaf structural carbohydrates, and bean seed starch). The D6S/D6R ratio in all metabolites depended on [CO2] as well (Fig. 3A), and statistical comparison of metabolites in bean and spinach revealed the same CO2 response in leaf starch and soluble sugars, whereas, in bean seed starch, the response was smaller (two-way ANOVA, Table S2). This suggests that the [CO2] dependence of the D6S/D6R ratio in initial photosynthates of C3 plants may be weakened by subsequent metabolic transformations. A likely explanation is hydrogen isotope exchange with cellular water, which is known to occur during many enzyme reactions (26). If so, observed variations in the D6S/D6R ratio would put lower bounds on the underlying change in the oxygenation/carboxylation ratio. Despite the exchange reactions, the data on exported metabolites show that physiological information encoded in the isotopomers is transmitted to long-lived metabolites.
CO2 Increase Since Industrialization Has Shifted the Photorespiration/Photosynthesis Ratio.
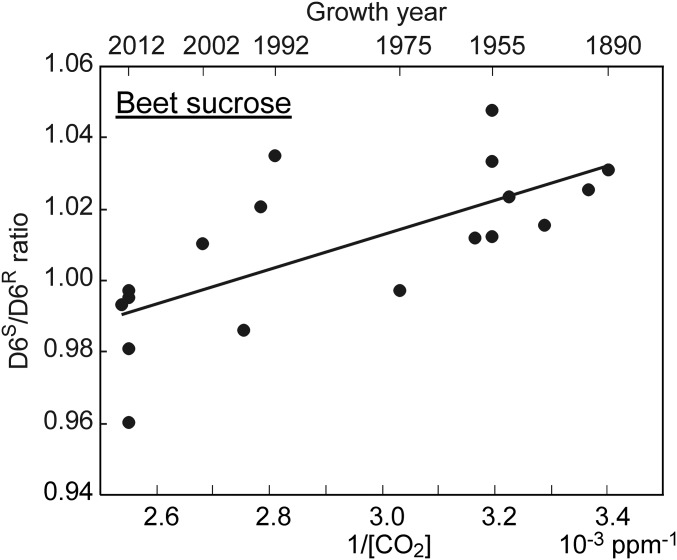
The D6S/D6R ratio is sensitive to [CO2] across a concentration range including preindustrial to current levels (Fig. 2C). Therefore, analysis of the isotopomer ratio may retrospectively reveal metabolic shifts induced by increasing atmospheric [CO2]. For a series of 18 archived sucrose samples from sugar beet, spanning the period 1890–2012 and a [CO2] increase from ∼295 ppm to ∼395 ppm, the D6S/D6R ratio shows a highly significant linear dependence on 1/[CO2] (Fig. 4 and Table S3). The regression slope (0.048 ± 0.012, SEM) is not significantly different from the slope determined for glucose moieties in greenhouse-grown sunflowers (Fig. 2C), showing that increasing atmospheric [CO2] has reduced the oxygenation/carboxylation ratio in sugar beet to the same degree as in our greenhouse manipulation experiments. The [CO2] dependence in archived beet sugar samples demonstrates that the photorespiration signal is highly robust, despite probable differences among the samples in agricultural practices, cultivars, and locations. This is remarkable, given that the modern samples and their progenitors experienced increasing [CO2] over more than 100 growing seasons. Furthermore, all modern plant breeding has occurred during this time period—after rediscovery of Mendel’s laws in 1900—but has not detectably influenced the CO2-driven change of the photosynthesis/photorespiration ratio. Finally, the agreement implies that the [CO2]-driven metabolic shift during the past 100 y has not been counteracted by homeostatic adjustments of physiological properties, such as stomatal function or regulation of photosynthesis, in sugar beet.
Fig. 4.
Isotopomer response to changes in [CO2] in archived samples of beet sugar. D6S/D6R ratios of the glucose moiety of beet sucrose samples as a function of 1/[CO2] (in units of 10−3 ppm−1) during the year of growth, shown on the upper x axis (r2 = 0.49, P = 0.001, n = 18).
Table S3.
Origin, growth year, and D6S/D6R values of samples from spinach, beet sugar, E. angustifolium, and S. fuscum
| Species/sample ID | Origin or growth location | Growth year* | D6S/D6R ratio± SEM† |
| Sugar beet | Danmarks Sukkermuseum, Nakskov, Denmark (sugar from “Odense Sukkerfabrik”) | 1890 | 1.031 ± 0.008 |
| Sugar beet/ NM.0209.401a | Nordiska Museet, Stockholm, Sweden (sugar from Tanto sugar factory) | 1900–1906 | 1.025 ± 0.004 |
| Sugar beet/ NM.0261.873 | Nordiska Museet, Stockholm, Sweden | 1920–1925 | 1.016 ± 0.003 |
| Sugar beet | Zuckerforschung Tulln, Austria | 1940 | 1.023 ± 0.008 |
| Sugar beet | Karin Rädel, Zuckersammler-Klub, Germany | 1950–1960 | 1.033 ± 0.008 |
| Sugar beet | Karin Rädel, Zuckersammler-Klub, Germany | 1950–1960 | 1.048 ± 0.004 |
| Sugar beet | Karin Rädel, Zuckersammler-Klub, Germany | 1950–1960 | 1.012 ± 0.011 |
| Sugar beet | Haus der Geschichte der Bundesrepublik Deutschland, Bonn, Germany | 1955–1962 | 1.012 ± 0.004 |
| Sugar beet | “Kölner Zucker” brand, Pfeifer & Langen Germany | 1970–1980 | 0.997 ± 0.005 |
| Sugar beet | Zuckerforschung Tulln, Austria | 1992 | 1.035 ± 0.003 |
| Sugar beet | Zuckerforschung Tulln, Austria | 1994 | 1.025 ± 0.006 |
| Sugar beet | Zuckerforschung Tulln, Austria | 1996 | 0.986 ± 0.006 |
| Sugar beet | Zuckerforschung Tulln, Austria | 2002 | 1.010 ± 0.004 |
| Sugar beet | “Dansukker” brand, sugar cube | 2011 | 0.981 ± 0.004 |
| Sugar beet | “Dansukker,” granulated sugar, Sweden | 2011 | 0.960 ± 0.008 |
| Sugar beet | Diamant Zucker, Pfeifer & Langen, Germany | 2011 | 0.995 ± 0.007 |
| Sugar beet | Südzucker, Germany | 2011 | 0.997 ± 0.008 |
| Sugar beet | Zuckerforschung Tulln, Austria | 2012 | 0.993 ± 0.004 |
| Spinach | Stadsliden, Umeå, Sweden; herbarium UME, Umeå | 1905 | 0.961 ± 0.008 |
| Spinach | Orsa, Dalarna, Sweden; herbarium UME, Umeå | 1908 | 0.912 ± 0.004 |
| Spinach | Sparreholm, Södermanland Sweden; herbarium UME, Umeå | 1903 | 0.931 ± 0.010 |
| Spinach | Lidingö, Stockholm, Sweden; Naturhistoriska riksmuseet, Stockholm | 1892 | 0.956 ± 0.010 |
| Spinach | commercial: Findus, grown in Sweden | 2011 | 0.888 ± 0.005 |
| Spinach | commercial: Findus, grown in Sweden | 2014 | 0.875 ± 0.003 |
| Spinach | commercial: ICA, grown in EU | 2014 | 0.918 ± 0.004 |
| Spinach | commercial: Findus, grown in Sweden | 2014 | 0.885 ± 0.003 |
| Spinach | commercial: COOP, grown in EU | 2014 | 0.914 ± 0.004 |
| Spinach | commercial: COOP, grown in EU | 2014 | 0.915 ± 0.003 |
| E. angustifolium | Jukkasjärvi, Sweden; herbarium UME, Umeå | 1949 | 0.968 ± 0.006 |
| E. angustifolium | Åvike, Medelpad, Sweden; herbarium UME, Umeå | 1944 | 0.942 ± 0.009 |
| E. angustifolium | Grangärde, Sweden; herbarium UME, Umeå | 1943 | 0.986 ± 0.004 |
| E. angustifolium | Åby parish, Kalmar län, Sweden; herbarium UME, Umeå | 1954 | 0.991 ± 0.004 |
| E. angustifolium | Umeå, Sweden | 2014 | 0.942 ± 0.003 |
| E. angustifolium | Umeå, Sweden | 2014 | 0.936 ± 0.002 |
| E. angustifolium | Umeå, Sweden | 2014 | 0.916 ± 0.002 |
| E. angustifolium | Umeå, Sweden | 2014 | 0.932 ± 0.002 |
| S. fuscum UME 176929 | Dorotea, Västerbotten, Sweden; herbarium UME, Umeå | 1921 | 0.877 ± 0.004 |
| S. fuscum | Östergotland, Sweden, Naturhistoriska riksmuseet, Stockholm | 1888 | 0.875 ± 0.003 |
| S. fuscum | Västergotland, Sweden, Naturhistoriska riksmuseet, Stockholm | 1923 | 0.874 ± 0.002 |
| S. fuscum | nutrient-poor minerogenic mire, Småland, Sweden (56°56’00N; 15°25’25E) | 2013 | 0.836 ± 0.001 |
| S. fuscum | nutrient-poor minerogenic mire, Småland, Sweden (56°56’00N; 15°25’25E) | 2013 | 0.865 ± 0.002 |
| S. fuscum | nutrient-poor minerogenic mire Västerbotten, Sweden (63°52’01N; 20°30’02E) | 2014 | 0.824 ± 0.004 |
| S. fuscum | nutrient-poor minerogenic mire Västerbotten, Sweden (63°52’01N; 20°30’02E) | 2014 | 0.831 ± 0.002 |
| S. fuscum | nutrient-poor minerogenic mire Västerbotten, Sweden (63°52’01N; 20°30’02E) | 2014 | 0.852 ± 0.002 |
When only an interval could be determined, the middle of the interval was assumed to be the growth year. Atmospheric [CO2] for growth years is from Carbon Dioxide Information Analysis Center (cdiac.ornl.gov/ftp/trends/co2/lawdome.smoothed.yr20).
SEM represents measurement precision from five replicate spectra.
The magnitude of CO2 fertilization is a major uncertainty in models of the global carbon cycle and of crop productivity (1, 11). Assuming constant leaf and environmental properties, oxygenation/carboxylation ratios and net photosynthesis of C3 plants can be modeled as a function of [CO2] using Rubisco kinetic parameters (21, 27). For the [CO2] increase from 295 ppm (∼A.D. 1900) to 395 ppm (∼A.D. 2012), such modeling predicts that the oxygenation/carboxylation ratio declined by ∼25%, which contributed to an increase in net photosynthesis of about 35%. The agreement of the regression slopes obtained for our greenhouse-grown plants and the beet sugar samples indicates that this putative shift in metabolism has actually occurred in field-grown sugar beet between 1890 and 2012.
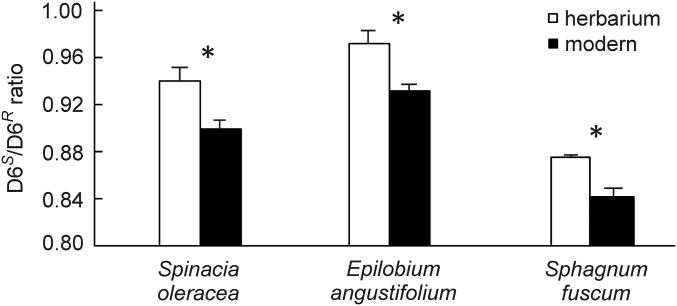
To further explore the generality of our observations, we compared herbarium and modern samples of an additional crop and two undomesticated C3 species: the herbaceous crop spinach (S. oleracea L.), the vascular plant rosebay willowherb (or fireweed, Epilobium angustifolium), and a common peat moss species, Sphagnum fuscum. The modern samples of each species show significantly lower D6S/D6R ratios, by 0.034–0.041, compared with the older herbarium samples (Fig. 5 and Table S3) that were produced at lower [CO2]. This reduction is again consistent with a reduction in oxygenation due to the [CO2] increase during their respective growth periods. These results provide experimental evidence that the CO2 increase since industrialization has shifted the photorespiration/photosynthesis ratio toward photosynthesis not only in crops grown under fertilized conditions but also in natural vegetation. E. angustifolium and S. fuscum both occur widely across the Northern Hemisphere. Because the underlying mechanism based on Rubisco properties is general for C3 plants, these observations suggest that a substantial shift in the oxygenation/carboxylation ratio has occurred in terrestrial C3 plants and it can be quantified using isotopomers.
Fig. 5.
Isotopomer response comparing herbarium and modern samples. D6S/D6R ratios of structural carbohydrates of herbarium samples (open bars) and modern samples (black bars). Modern samples grew between 2011 and 2014, herbarium spinach samples grew between 1892 and 1908 ([CO2] ∼295 ppm), herbarium Epilobium samples grew between 1943 and 1954 ([CO2] ∼310 ppm), and herbarium Sphagnum samples grew between 1888 and 1923 ([CO2] ∼295 ppm). Values are averages ± SEM (n = 3–6); *P < 0.02.
Discussion
Origin of the Isotopomer Signal.
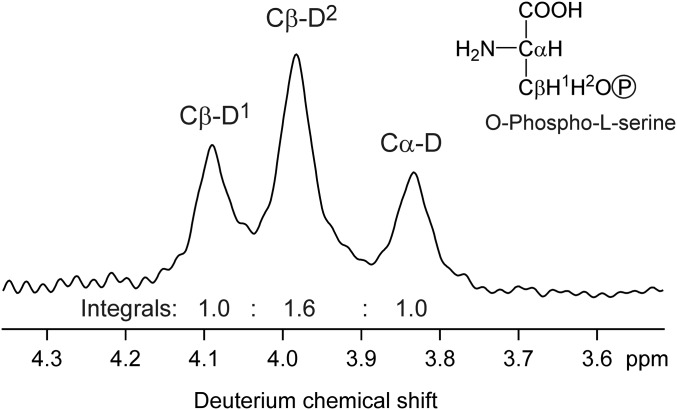
We observed a dependence of the D6S/D6R ratio on 1/[CO2] in controlled experiments and in archival plant material. In the following, we explain this dependence by differences in isotope fractionation in the metabolic pathways originating from carboxylation and oxygenation, respectively, at Rubisco. The hydrogen atoms of the C6H2 group of glucose can be traced back to their origins after carboxylation or oxygenation of ribulose bisphosphate (RuBP) in C3 metabolism, as shown in Fig. 1. Although photorespiration leads to C loss, 3-phosphoglycerate (3-PGA) is formed after both carboxylation and oxygenation, but with differing stoichiometries and along differing paths. Numerous observations have shown that synthesis of a compound via different pathways induces different isotopomer distributions (18, 19, 28–32). No exception to this rule has been observed, and it agrees with the theory of isotope effects. Therefore, it is rational to assert that the PGA molecules formed from C3−C5 of RuBP, from C1−C2 of RuBP, and via the photorespiration pathway have differing D isotopomer ratios in their C3H2 groups, as indicated by color coding in Fig. 1. In the photorespiration cycle, the C3H2 group of 3-PGA originates from serine, which is formed by serine hydroxymethyl transferase. Commercial serine is produced by the same enzyme (33) and shows a strongly uneven isotopomer distribution in its C3H2 group (Fig. S2). This supports the proposition that 3-PGA originating from photorespiration has a distinct isotopomer composition, and that serine hydroxymethyl transferase contributes to creation of the oxygenation/carboxylation signal. The ratio of metabolic fluxes originating from carboxylation and from oxygenation determines the contributions of the alternative PGA sources to the PGA pool, and the D isotopomer distribution of the PGA pool is a flux-weighted average of the PGA sources. During the conversion of PGA into glucose, no reaction occurs at the C3H2 group; thus, there is no scrambling of hydrogen isotopes (34), and the D isotopomer ratio of the C6H2 group of the resulting glucose will be identical to that of the PGA pool. Hence the weighted isotopomer distribution is transmitted from the 3-PGA pool to glucose, so that the D6S/D6R ratio of glucose becomes dependent on the oxygenation/carboxylation ratio.
Fig. S2.
Deuterium NMR spectrum of O-Phospho-l-serine (Santa Cruz Biotechnolgy), indicating D isotopomer abundances of serine formed by serine hydroxymethyl transferase during fermentation (33). The integrals of the signals, obtained by deconvolution, are proportional to the abundances of the Cα-D isotopomer and the two D isotopomers at Cβ, labeled Cβ-D1 and Cβ-D2. Although the stereochemical assignments of Cβ-D1 and Cβ-D2 are unknown, their strongly differing integrals show that serine hydroxymethyl transferase creates a strongly uneven D isotopomer ratio at C3 of serine, which, in the photorespiration pathway, is converted into an uneven D isotopomer ratio at C3 of 3-phosphoglycerate.
This dependence is the first example, to our knowledge, of an isotopomer shift induced by an environmental driver that can be mechanistically interpreted in terms of a metabolic response to this driver. We conclude that isotopomers carry metabolic signals that can be identified using laboratory experiments, and may be retrieved from archives of plant material.
Methodological Advantages of Isotopomer Signals.
An important advantage of isotopomer ratios is that they can be interpreted without any reference to the δD of source water. This is possible because the δD of a plant’s source and leaf water affect the abundance of all isotopomers to the same degree, so isotopomer ratios are independent of δD (35). Therefore, isotopomer ratios are exclusively determined by biochemical isotope effects. In contrast, interpretations of δD of plant archives are often complicated (36) because the required data on δD of source water and on evaporative enrichment of leaf water are not available. Because hydrogen isotope signatures are stable for >104 y (37), isotopomers may yield information on physiological changes on long time scales, including interglacial cycles. The complete D NMR spectrum of glucose (Fig. 2A) is fully described by seven isotopomer abundances, or by six isotopomer ratios and the overall amplitude of the spectrum, which is given by δD of the entire molecule. Thus, six out of seven degrees of freedom that describe the spectrum are isotopomer ratios, and hence the complete isotopomer pattern contains much more information than δD of the entire molecule.
Isotopomer information complements manipulation studies such as FACE experiments, in that information on metabolism can be retrieved on long time scales. Here we show that the primary metabolic response to elevated CO2—suppression of photorespiration—has, in the species studied here, persisted during the 20th century. In contrast to the persistent suppression of photorespiration observed here, CO2-driven increases in plant growth observed in FACE experiments often decline after a few years of CO2 enrichment (2). Thus, combining FACE and isotopomer data may allow assessment of the role of factors besides photosynthesis that limit growth on the ecosystem level (38). Further studies will have to address whether long-term suppression of photorespiration has occurred for C3 plants in general, how photorespiration will develop under scenarios for future CO2 levels and climate change, and how the global photorespiration flux will influence efforts to stabilize CO2 levels.
Properties of Rubisco and the photorespiration cycle are current targets for crop improvement (20, 39). The D6S/D6R ratio may be used to detect differences in CO2 responses between crop species, or cultivars, and isotopomers in general to monitor metabolic effects of crop engineering. Isotopomer variation exists for many other metabolites and isotopes, notably 13C (40, 41). Therefore, time series of δ13C of entire molecules may also be affected by isotopomer trends, and 13C isotopomer analysis of plant archives can yield much more information than is available from δ13C.
Implications for Biogeochemistry and Plant Ecophysiology.
Among the little that is known about responses of plants to increasing CO2 over recent centuries, δ13C time series of annual plant material or tree rings are arguably the strongest evidence. From δ13C, increases in assimilation have been deduced, and the reduction in the oxygenation/carboxylation ratio is a large part of this. Our isotopomer results provide the first empirical data on the magnitude of the shift in this central biogeochemical flux ratio.
Forests make a big contribution to the global carbon cycle, and tree rings are one of the most prominent paleoarchives. Therefore, it is important to note that isotopomer information may be retrieved from tree rings, because the glucose derivative can be produced from whole wood or cellulose (35). Furthermore, we have previously shown that metabolic signatures produced on the leaf level are preserved in the glucose moieties obtained from tree rings (42). This indicates that it will be possible to derive long-term physiological information from tree rings. Because glucose can sometimes be produced from fossil leaves, paleoatmospheric reconstruction of atmospheric CO2 may also be possible.
The peat mosses belonging to the genus Sphagnum are the main constituents of peat of high-latitude mires (43, 44). This peat has accumulated during the Holocene and currently represents ∼25% of the global soil carbon pool. The amount of carbon stored in peat has caused a lowering of the contemporary atmospheric CO2 concentration by 35 ppm (45). Due to the extraordinarily large carbon store in these peatlands, their feedback to changing climate and rising CO2 constitutes a major scientific and societal concern with respect to predicting future biosphere−atmosphere CO2 exchange and storage (44, 46). Recent studies have revealed net primary production as the main driver for peatland carbon accumulation (46, 47), which is also supported by the observation that incoming photosynthetic active radiation during the growing season is the main driver for geographic variation in Sphagnum moss productivity (48). The studied species S. fuscum grows in hummocks and is thus directly exposed to atmospheric CO2. Such hummock-forming sphagnum species dominate peat formation at high-latitude mires (48). The trend in the isotopomer ratio observed in S. fuscum constitutes the first evidence of increased photosynthesis among peat-forming Sphagnum mosses in response to the century-scale CO2 increase under natural conditions. Thus, by applying isotopomers to a geographic and species range of peat-forming species, it may be possible to assess general trends in the physiology of peat-forming species. Trends in net photosynthesis may then be scaled up to the global scale, to obtain a mechanistic understanding of the carbon balance of peatlands.
Reducing the photorespiration/photosynthesis ratio increases the light use efficiency of photosynthesis. Some measure of light use efficiency is frequently used to parameterize photosynthetic algorithms in models of ecosystem response to climate change (49–51). These parameterizations have been based on models of Rubisco substrate specificity (27) scaled up to the ecosystem level or, more often, empirical fitting from remote sensing (52), eddy covariance (53), or biomass (54) data that was scaled downward and thus lacked a mechanistic description of this key metabolic flux ratio. The isotopomer approach thus provides a mechanistically based means of parameterizing light use efficiency models, allowing us to improve our predictions. These improvements will appear in both paleoarchive data, looking backward, and the use of experimental CO2 enrichment experiments, to look forward.
Information on effects of long-term increases in [CO2] on the metabolism of crops and natural vegetation is essential for understanding underlying mechanisms and robustly modeling C exchange fluxes between terrestrial vegetation and the atmosphere. The presented methodology may be used to detect variation in the CO2 responses between species or crop lines, and changes in isotopomer patterns may be used to detect long-term acclimation or adaptation. Retrieving such information will enable the time frames of plant physiological studies to be extended to centuries, thereby bridging the gap between manipulation experiments and paleoenvironmental studies.
Methods
CO2 Manipulation Experiments.
In greenhouse and growth chamber experiments, Helianthus annuus, Z. mays, S. oleracea, and P. vulgaris plants were exposed to different atmospheric [CO2] levels, and S. oleracea was exposed to reduced [O2]. Gas exchange parameters of H. annuus leaves were determined using a two-channel fast response measurement system (Fast-Est), described in detail in ref. 55. Details of growth and measurement conditions are provided in Supporting Information.
Historic Plant Material.
Eighteen archived sugar beet sucrose samples that grew between 1890 and 2012 were obtained from sources listed in Table S3 (specified growth years of these samples are as stated by the contributors). Current samples (2011−2012) are dated to the previous growing season. Herbarium samples of S. oleracea, E. angustifolium, and S. fuscum were obtained from the herbarium at Umeå University and from Naturhistoriska Riksmuseet. Modern samples for comparison were either commercial frozen products (spinach) or were collected in Sweden. Exact origins, growth locations, and growth years are provided in Table S3. Structural carbohydrates were analyzed.
Sample Preparation for NMR Isotopomer Measurements.
For deuterium NMR measurements, pure samples of a glucose derivative were prepared from all samples, as follows. Soluble sugars were extracted from leaf material according to previously used methods (35, 56), starting with 10 g of fresh material. Starch from chloroplasts was extracted from the pellet resulting from extraction of soluble sugars, according to ref. 57, with the following modifications: 200 mL of 50 mM KOH was added, and the solution was kept at 90 °C for 2 h. After cooling on ice, the pH was adjusted to 4.7 with 1 M acetic acid, and 42 units of amyloglucosidase (Roche) were added. After incubation at 37 °C overnight, samples were centrifuged for 20 min at 4,000 × g, and the supernatant was collected. Starch from bean fruits was hydrolyzed to glucose by amyloglucosidase treatment. Leaf structural carbohydrates were obtained by removing soluble sugars and starch from leaves by extraction and amyloglucosidase hydrolysis, respectively. Structural carbohydrates were hydrolyzed to glucose according to Betson et al. (35). Sugar samples prepared from plants were dried in vacuum and were converted into a pure glucose derivative suitable for deuterium NMR analysis (35).
Isotopomer Quantification.
Deuterium NMR measurements and subsequent data analysis followed Schleucher et al. (19), modified as previously described (35). For NMR measurements, we used a DRX600 spectrometer (Bruker) equipped with a 5-mm broadband observe probe and a 19F lock device, or an AVANCE III 850 spectrometer (Bruker) equipped with a cryogenic probe optimized for deuterium detection and equipped with a 19F lock. Deuterium NMR spectra were integrated by deconvolution with a Lorentzian line shape fit, using TopSpin software (version 3.1; Bruker). The D6S/D6R isotopomer ratio was determined as the ratio of the integrals of the D6S and D6R signals.
Statistical Analysis.
For each sample, five or six replicate spectra were recorded, the average isotopomer ratio among the spectra was used, and its SE was calculated to estimate measurement precision. For biological replicate samples, SEs were calculated. The significance of between-treatment differences in isotopomer ratios was tested using ANOVA, and linear regression analysis was applied to test relationships between the ratios and [CO2] (in both cases using Excel).
Description of CO2 Manipulation Experiments
In the following text, “groups A−E” refer to independent laboratory experiments and “samples a−i” refer to samples of materials subjected to various treatments (as described in the following text) applied in the experiments. Isotopomer results are summarized in Tables S2 and S3.
Group A.
Sunflower (H. annuus L. cv Zebulon) plants were grown in 1.4-L pots in a greenhouse in Umeå, Sweden, during fall 2000. The photoperiod was set at 16 h with a light intensity of 300–400 µmol photons m−2⋅s−1. The [CO2], as measured by an LCA2 infrared gas analyzer (ADC), was slightly higher than atmospheric (around 450 ppm). The plants were watered daily and fertilized, twice a week, with a commercial nutrient solution (RIKA-S, NPK 7–1-B; Weibulls). After 7–8 wk in the greenhouse, plants were transferred to a growth chamber in groups of eight, where they were exposed to a range of CO2 concentrations (180 ppm, 280 ppm, 450 ppm, 700 ppm, or 1,500 ppm). CO2 concentrations of 450 ppm and higher were created by introducing pure CO2 via a valve system into the growth chamber, while concentrations of 180 ppm and 280 ppm were attained by mixing flows of air and CO2-free air. Temperature and relative humidity in the growth chamber were 22/18 °C and 60/70% during day/night, respectively. During the first day in the growth chamber, the plants were kept in the dark at the same [CO2] as in the greenhouse (450 ppm). During the following 2 d, the plants were exposed to the aforementioned photoperiod and the selected [CO2]. During the second day of CO2 treatment, gas exchange measurements were performed and leaf samples were collected. Leaf material was stored at −20 °C until leaf starch was extracted (Table S2, samples a).
Group B.
Sunflower plants were grown, from seeds, during summer 2003 in Freising, Germany, in two growth chambers with [CO2] set at 200 ppm and 1,000 ppm. Further details about the experiment and growth conditions can be found in ref. 58. Glucose moieties in structural carbohydrates of freeze-dried leaves were isolated and prepared as described in Methods (Table S2, samples b).
Group C.
Maize (Z. mays) plants were grown in 5-L pots in a greenhouse in Umeå, Sweden, during summer 2000. The growth conditions and [CO2] treatments were identical to those for sunflowers (group A) except that the maize plants were exposed only to 450 ppm and 1,200 ppm [CO2], and leaf samples were collected during the second day of CO2 treatment. Leaf material was stored at −20 °C until its soluble sugars and starch contents were extracted (Table S2, samples c).
Group D.
Spinach (S. oleracea cv. Giant noble) plants were grown from seeds in a growth chamber providing the following conditions: 11-h photoperiods (600 µmol photons m−2⋅s−1), 22/18 °C light/dark temperatures and 60% relative humidity. Two [CO2] concentration treatments (ambient ≈ 360 ppm and 700 ppm) were applied from germination. In a third treatment, five spinach plants (∼6 wk old, grown at 360 ppm [CO2]) were exposed for 2 d to air with the oxygen content reduced to 12%. Plants subjected to each of the three treatments were harvested 6 wk after germination and stored at −20 °C until leaf soluble sugars and leaf starch were extracted (Table S2, samples d−f).
Group E.
Bean (P. vulgaris L. cv. Linden) plants were grown from seeds under the same conditions as for spinach (group D). Leaves and fruits of mature plants were harvested and stored at −20 °C until leaf soluble sugars and leaf starch were extracted (Table S2, samples g−i).
Leaf Gas Exchange Measurements
Gas exchange parameters of sunflowers’ leaves (group A) were determined using a two-channel fast-response measurement system (Fast-Est), described in detail in ref. 55. For each measurement, part of the leaf (8.04 cm2) was enclosed in a leaf chamber, its upper part was attached with starch gel to a thermostated glass window maintained at 22 °C [to calculate the dissolved O2 concentration and CO2/O2 specificity constant of Rubisco (59)] and it was exposed to an airflow of 0.5 mmol⋅s−1. Light was provided by a Schott KL 1500 source (Heinz Walz) and measured by a Li-Cor Quantum Sensor. Gas exchange was measured only through the lower side of the leaf, although sunflower leaves have stomata on both sides. Gas exchange measurements were performed at 300 µmol photons m−2⋅s−1 and the [CO2] to which plants had been exposed for 2 d in the growth chamber. From the gas exchange data, the intercellular CO2 concentration (Ci) was obtained. In the afternoon of the day of gas exchange measurements, leaves were harvested and stored at −20 °C, until extraction of their starch contents (Table S2, sample a).
Functional Dependence of the D6S/D6R Ratio on the Oxygenation to Carboxylation Ratio
The 3-PGA molecules derived from oxygenation and carboxylation differ in their isotopomer ratios (Fig. 1). This leads to a functional dependence of the D6S/D6R isotopomer ratio on the oxygenation/carboxylation flux ratio. The ratio of oxygenation flux (Vo) to carboxylation flux (Vc) is ϕ = Vo/Vc = 1/Ksp [O2]/Ci, where Ksp is the specificity constant of Rubisco (21, 60), and [O2] is the dissolved oxygen concentration. Two 3-PGA molecules are formed upon RuBP carboxylation. Upon RuBP oxygenation, one 3-PGA is formed directly, and up to 0.5 additional 3-PGA are formed via the photorespiration cycle (Fig. 1). We assign the value m, 1 ≤ m ≤ 1.5, as the total 3-PGA production due to oxygenation. If the oxygenation/carboxylation ratio is ϕ, then, in total, (2 + mϕ) 3-PGA are formed. Of these, the fractional contribution due to oxygenation is mϕ/(2 + mϕ), and the fractional contribution due to carboxylation is 2/(2 + mϕ). For 3-PGA molecules formed via oxygenation and carboxylation, we define Ro and Rc, respectively, as variables describing their D isotopomer ratios at the C3H2 group. The isotopomer ratio of the PGA pool used to synthesize glucose will be an average of Ro and Rc, weighted by the respective fractional contributions,
| [S1] |
Experimental values for Ci and ϕ from gas exchange measurements and associated D6S/D6R ratios are given in Table S1.
From the intercellular O2 and CO2 concentrations and the specificity constant of Rubisco, the ratio of oxygenation to carboxylation, ϕ, was calculated as ϕ = Vo/Vc = 1/Ksp × [O2]/Ci. In Fig. S1, the D6S/D6R ratios are plotted as a function of ϕ. Photorespiratory metabolites such as Gly and Ser may be removed from the PCO pathway, but only in small amounts (61); therefore, the number of PGA molecules formed following oxygenation (m) will be close to 1.5. Assuming m = 1.5, the data can be fitted to Eq. S1 (best-fit parameters Rc = 0.83, Ro = 2.06). The curvature of the fitted line is small; therefore, the fit to Eq. S1 is well approximated by a linear function. Thus, the D6S/D6R ratio can be described by a linear function of ϕ. Because ϕ is proportional to 1/Ci, and typical variation of the Ci/Ca (Ca, atmospheric CO2 concentration) ratio is small, the D6S/D6R ratio is also a linear function of 1/Ca.
Acknowledgments
We thank the late Stefan Ericsson, Herbarium UME, Umeå University, for assistance with acquiring herbarium plant samples. We thank several institutions and colleagues for providing samples and for valuable advice on the manuscript, and Vaughan Hurry and Agu Laisk for help with the gas exchange measurements. This study was supported by the National Magnetic Resonance Facility at Madison, the Centre for Environmental Research in Umeå, the Swedish Research Council VR, the Kempe foundations, and the “NMR for Life” facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504493112/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, et al., editors. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 2.Leuzinger S, et al. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol Evol. 2011;26(5):236–241. doi: 10.1016/j.tree.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Drake JE, et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett. 2011;14(4):349–357. doi: 10.1111/j.1461-0248.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 4.Jump AS, Hunt JM, Martínez-Izquierdo JA, Peñuelas J. Natural selection and climate change: Temperature-linked spatial and temporal trends in gene frequency in Fagus sylvatica. Mol Ecol. 2006;15(11):3469–3480. doi: 10.1111/j.1365-294X.2006.03027.x. [DOI] [PubMed] [Google Scholar]
- 5.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424(6951):901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 6.Penuelas J, Canadell JG, Ogaya R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob Ecol Biogeogr. 2011;20(4):597–608. [Google Scholar]
- 7.Woodward FI. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature. 1987;327(6123):617–618. [Google Scholar]
- 8.Franks PJ, et al. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013;197(4):1077–1094. doi: 10.1111/nph.12104. [DOI] [PubMed] [Google Scholar]
- 9.Leakey ADB, Lau JA. Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2] Philos Trans R Soc Lond B Biol Sci. 2012;367(1588):613–629. doi: 10.1098/rstb.2011.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Quéré C, et al. Trends in the sources and sinks of carbon dioxide. Nat Geosci. 2009;2(12):831–836. [Google Scholar]
- 11.Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312(5782):1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- 12.Lobell DB, Schlenker W, Costa-Roberts J. Climate trends and global crop production since 1980. Science. 2011;333(6042):616–620. doi: 10.1126/science.1204531. [DOI] [PubMed] [Google Scholar]
- 13.Beer C, et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science. 2010;329(5993):834–838. doi: 10.1126/science.1184984. [DOI] [PubMed] [Google Scholar]
- 14.Ogren WL, Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- 15.Laing WA, Ogren WL, Hageman RH. Regulation of soybean net photosynthetic CO2 fixation by interaction of CO2, O2, and ribulose 1,5-diphosphate carboxylase. Plant Physiol. 1974;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beerling DJ, Woodward FI. Vegetation and the Terrestrial Carbon Cycle: Modelling the First 400 Million Years. Cambridge Univ Press; Cambridge, UK: 2001. [Google Scholar]
- 17.Wolfsberg M, Van Hook WA, Paneth P, Rebelo LP. Isotope Effects in the Chemical, Geological, and Bio Sciences. Springer; Heidelberg: 2010. pp. 290–292. [Google Scholar]
- 18.Schmidt H-L. Fundamentals and systematics of the non-statistical distributions of isotopes in natural compounds. Naturwissenschaften. 2003;90(12):537–552. doi: 10.1007/s00114-003-0485-5. [DOI] [PubMed] [Google Scholar]
- 19.Schleucher J, Vanderveer P, Markley JL, Sharkey TD. Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant Cell Environ. 1999;22(5):525–533. [Google Scholar]
- 20.Parry MAJ, et al. Rubisco activity and regulation as targets for crop improvement. J Exp Bot. 2013;64(3):717–730. doi: 10.1093/jxb/ers336. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey TD. Estimating the rate of photorespiration in leaves. Physiol Plant. 1988;73(1):147–152. [Google Scholar]
- 22.Tomitani A, Knoll AH, Cavanaugh CM, Ohno T. The evolutionary diversification of cyanobacteria: Molecular−phylogenetic and paleontological perspectives. Proc Natl Acad Sci USA. 2006;103(14):5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foyer CH, Bloom AJ, Queval G, Noctor G. Photorespiratory metabolism: Genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol. 2009;60:455–484. doi: 10.1146/annurev.arplant.043008.091948. [DOI] [PubMed] [Google Scholar]
- 24.Fernie AR, et al. Perspectives on plant photorespiratory metabolism. Plant Biol (Stuttg) 2013;15(4):748–753. doi: 10.1111/j.1438-8677.2012.00693.x. [DOI] [PubMed] [Google Scholar]
- 25.Tcherkez GGB, Farquhar GD, Andrews TJ. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA. 2006;103(19):7246–7251. doi: 10.1073/pnas.0600605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose IA. Mechanism of the aldose-ketose isomerase reactions. Adv Enzymol Relat Areas Mol Biol. 1975;43:491–517. doi: 10.1002/9780470122884.ch6. [DOI] [PubMed] [Google Scholar]
- 27.Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B-L, et al. Hydrogen isotopic profile in the characterization of sugars. Influence of the metabolic pathway. J Agric Food Chem. 2002;50(6):1574–1580. doi: 10.1021/jf010776z. [DOI] [PubMed] [Google Scholar]
- 29.Martin GJ, Martin ML, Zhang B-L. Site-specific natural isotope fractionation of hydrogen in plant products studied by nuclear magnetic resonance. Plant Cell Environ. 1992;15(9):1037–1050. [Google Scholar]
- 30.Zhang B-L, Quemerais B, Martin ML, Martin GJ, Williams JM. Determination of the natural deuterium distribution in glucose from plants having different photosynthetic pathways. Phytochem Anal. 1994;5(3):105–110. [Google Scholar]
- 31.Tenailleau EJ, Lancelin P, Robins RJ, Akoka S. Authentication of the origin of vanillin using quantitative natural abundance 13C NMR. J Agric Food Chem. 2004;52(26):7782–7787. doi: 10.1021/jf048847s. [DOI] [PubMed] [Google Scholar]
- 32.Fronza G, et al. The positional δ(18O) values of extracted and synthetic vanillin. Helv Chim Acta. 2001;84(2):351–359. [Google Scholar]
- 33.Elvers B, editor. Ullmann’s Fine Chemicals. Vol 3 Wiley-VCH; Weinheim, Germany: 2014. [Google Scholar]
- 34.Hanson KR. Stereochemical determination of carbon partitioning between photosynthesis and photorespiration in C3 plants: Use of (3R)-D-[3-3H1, 3-14C]glyceric acid. Arch Biochem Biophys. 1984;232(1):58–75. doi: 10.1016/0003-9861(84)90521-6. [DOI] [PubMed] [Google Scholar]
- 35.Betson TR, Augusti A, Schleucher J. Quantification of deuterium isotopomers of tree-ring cellulose using nuclear magnetic resonance. Anal Chem. 2006;78(24):8406–8411. doi: 10.1021/ac061050a. [DOI] [PubMed] [Google Scholar]
- 36.McCarroll D, Loader NJ. Stable isotopes in tree rings. Quat Sci Rev. 2004;23(7-8):771–801. [Google Scholar]
- 37.Sessions AL, Sylva SP, Summons RE, Hayes JM. Isotopic exchange of carbon-bound hydrogen over geologic timescales. Geochim Cosmochim Acta. 2004;68(7):1545–1559. [Google Scholar]
- 38.Norby RJ, Zak DR. Ecological lessons from Free-Air CO2 Enrichment (FACE) experiments. Annu Rev Ecol Evol Syst. 2011;42:181–203. [Google Scholar]
- 39.Nölke G, Houdelet M, Kreuzaler F, Peterhänsel C, Schillberg S. The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnol J. 2014;12(6):734–742. doi: 10.1111/pbi.12178. [DOI] [PubMed] [Google Scholar]
- 40.Rossmann A, Butzenlechner M, Schmidt HL. Evidence for a nonstatistical carbon isotope distribution in natural glucose. Plant Physiol. 1991;96(2):609–614. doi: 10.1104/pp.96.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaintreau A, et al. Site-specific 13C content by quantitative isotopic 13C nuclear magnetic resonance spectrometry: A pilot inter-laboratory study. Anal Chim Acta. 2013;788:108–113. doi: 10.1016/j.aca.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Augusti A, Betson TR, Schleucher J. Deriving correlated climate and physiological signals from deuterium isotopomers in tree rings. Chem Geol. 2008;252(1-2):1–8. [Google Scholar]
- 43.Gorham E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol Appl. 1991;1(2):182–195. doi: 10.2307/1941811. [DOI] [PubMed] [Google Scholar]
- 44.Loisel J, et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene. 2014;24(9):1028–1042. [Google Scholar]
- 45.Frolking S, Roulet NT. Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions. Glob Change Biol. 2007;13(5):1079–1088. [Google Scholar]
- 46.Charman DJ, et al. Climate-related changes in peatland carbon accumulation during the last millennium. Biogeosciences. 2013;10(2):929–944. [Google Scholar]
- 47.Lund M, et al. Variability in exchange of CO2 across 12 northern peatland and tundra sites. Glob Change Biol. 2010;16(9):2436–2448. [Google Scholar]
- 48.Loisel J, Gallego-Sala AV, Yu Z. Global-scale pattern of peatland Sphagnum growth driven by photosynthetically active radiation and growing season length. Biogeosciences. 2012;9(7):2737–2746. [Google Scholar]
- 49.Medlyn BE. Physiological basis of the light use efficiency model. Tree Physiol. 1998;18(3):167–176. doi: 10.1093/treephys/18.3.167. [DOI] [PubMed] [Google Scholar]
- 50.Sitch S, et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob Change Biol. 2003;9(2):161–185. [Google Scholar]
- 51.Turner DP, et al. Scaling gross primary production (GPP) over boreal and deciduous forest landscapes in support of MODIS GPP product validation. Remote Sens Environ. 2003;88(3):256–270. [Google Scholar]
- 52.Hilker T, Coops NC, Wulder MA, Black TA, Guy RD. The use of remote sensing in light use efficiency based models of gross primary production: A review of current status and future requirements. Sci Total Environ. 2008;404(2-3):411–423. doi: 10.1016/j.scitotenv.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Yuan W, et al. Deriving a light use efficiency model from eddy covariance flux data for predicting daily gross primary production across biomes. Agric For Meteorol. 2007;143(3-4):189–207. [Google Scholar]
- 54.Landsberg JJ, Waring RH. A generalised model of forest productivity using simplified concepts of radiation-use efficiency, carbon balance and partitioning. For Ecol Manage. 1997;95(3):209–228. [Google Scholar]
- 55.Laisk A, Edwards GE. CO2 and temperature-dependent induction in C4 photosynthesis: An approach to the hierarchy of rate-limiting processes. Aust J Plant Physiol. 1997;24(4):505–516. [Google Scholar]
- 56.Augusti A, Betson TR, Schleucher J. Hydrogen exchange during cellulose synthesis distinguishes climatic and biochemical isotope fractionations in tree rings. New Phytol. 2006;172(3):490–499. doi: 10.1111/j.1469-8137.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- 57.Hurry V, Strand A, Furbank R, Stitt M. The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. Plant J. 2000;24(3):383–396. doi: 10.1046/j.1365-313x.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 58.Lehmeier CA, Schäufele R, Schnyder H. Allocation of reserve-derived and currently assimilated carbon and nitrogen in seedlings of Helianthus annuus under sub-ambient and elevated CO growth conditions. New Phytol. 2005;168(3):613–621. doi: 10.1111/j.1469-8137.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 59.Sumberg A. Laisk A. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. Vol 5. Kluwer Acad; Dordrecht, The Netherlands: 1995. pp. 615–618. [Google Scholar]
- 60.Laisk A, Sumberg A. Partitioning of the leaf CO2 exchange into components using CO2 exchange and fluorescence measurements. Plant Physiol. 1994;106(2):689–695. doi: 10.1104/pp.106.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tcherkez G. Is the recovery of (photo) respiratory CO2 and intermediates minimal? New Phytol. 2013;198(2):334–338. doi: 10.1111/nph.12101. [DOI] [PubMed] [Google Scholar]