Significance
The second messenger cAMP exerts potent immunosuppressive effects on the innate immune system, in part by activating class IIa histone deacetylases, and thereby inhibiting NF-κB–dependent transcription. We found that cAMP also promotes M2 macrophage polarization by stimulating the cyclic AMP-responsive element binding (CREB)/cAMP-regulated transcriptional coactivators TORC1 pathway. In the setting of acute overnutrition, macrophage CREB inhibits the production of inflammatory mediators and contributes to the maintenance of insulin sensitivity.
Keywords: cAMP, CREB/CRTC, M2 macrophage, insulin resistance
Abstract
Obesity is thought to promote insulin resistance in part via activation of the innate immune system. Increases in proinflammatory cytokine production by M1 macrophages inhibit insulin signaling in white adipose tissue. In contrast, M2 macrophages have been found to enhance insulin sensitivity in part by reducing adipose tissue inflammation. The paracrine hormone prostaglandin E2 (PGE2) enhances M2 polarization in part through activation of the cAMP pathway, although the underlying mechanism is unclear. Here we show that PGE2 stimulates M2 polarization via the cyclic AMP-responsive element binding (CREB)-mediated induction of Krupple-like factor 4 (KLF4). Targeted disruption of CREB or the cAMP-regulated transcriptional coactivators 2 and 3 (CRTC2/3) in macrophages down-regulated M2 marker gene expression and promoted insulin resistance in the context of high-fat diet feeding. As re-expression of KLF4 rescued M2 marker gene expression in CREB-depleted cells, our results demonstrate the importance of the CREB/CRTC pathway in maintaining insulin sensitivity in white adipose tissue via its effects on the innate immune system.
Under obese conditions, macrophage infiltration and activation in adipose tissue leads to a chronic inflammatory state with increased secretion of proinflammatory cytokines (1). The activation of IkB and Jun N-terminal kinases impairs insulin signaling in metabolic tissues and thereby contributes to insulin resistance (2–4). Classically activated M1 macrophages secrete proinflammatory cytokines, such as TNF-α and IL-12, which promote insulin resistance. Alternatively activated M2 macrophages are thought to protect adipocytes from the development of insulin resistance in response to IL-4 signaling (5, 6). Increases in STAT6 activity stimulate the expression of Krupple-like factor 4 (KLF4), which in turn promotes expression of the M2 program. Obesity causes an M2-to-M1 shift in adipose tissue that leads to insulin resistance (7).
The eicosanoid prostaglandin E2 (PGE2) has been found to promote M2 macrophage polarization in part via induction of the cAMP pathway. Indeed, circulating catecholamines also exert potent anti-inflammatory effects on macrophage function via cAMP signaling (8). In this regard, a number of bacteria appear to evade the innate immune system by producing toxins that enhance cAMP production. cAMP stimulates the expression of cellular genes in part via the phosphorylation of CREB at Ser133 and via the dephosphorylation of the cAMP regulated transcriptional coactivators (CRTC) family of coactivators (9). Following its activation, the cyclic AMP-responsive element binding (CREB) pathway appears to block M1 macrophage function in part via the induction of the anti-inflammatory cytokine IL-10 (10, 11). Superimposed on these effects, cAMP also inhibits the expression of proinflammatory cytokines via the induction of class IIa histone deacetylases (HDACs) and subsequent de-acetylation of NF-κB.
Here we explore the potential roles of the class IIa HDAC and CREB/CRTC pathways in M2 macrophages. We found that, although PGE2 stimulates both pathways, only one of these is required for M2 macrophage polarization. As disruption of this pathway increases insulin resistance in the setting of dietary obesity, our results suggest that small molecules that enhance its activity in macrophages may provide therapeutic benefit to diabetic individuals.
Results
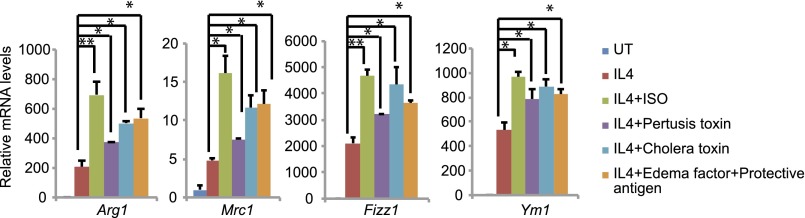
We evaluated the effect of cAMP signaling on the expression of M2 macrophage marker genes, including arginase-1 (Arg1), the mannose receptor (Mrc1), resistin-like α (Fizz1, Retnla), and chitinase 3-like 3 (Ym1, Chi3l3) in cultured bone marrow macrophages (BMMs) (12, 13). Exposure to PGE2, potentiated IL-4–induced increases in M2 marker gene expression (Fig. 1 A and B). We observed similar effects using other cAMP agonists, including the β2 adrenergic receptor agonist, isoproterenol, as well as a number of bacterial toxins (Fig. S1A).
Fig. 1.
cAMP promotes M2 macrophage polarization. (A and B) Effect of PGE2 exposure on M2 macrophage marker mRNA amounts (A) (Arg1, Mrc1, Fizz1, Ym1) and Arg1 protein level (B) in BMMs treated with IL-4. (C and D) Effects of leptin on M2 macrophage marker mRNA (C) and Arg1 protein amounts (D) in fat pads of ob/ob mice. Effect of β-adrenergic antagonist (Propanolol; pro) shown. Body weights indicated (n = 4 per group). (E and F) Effect of normal chow (NC) or HFD feeding on M2 macrophage marker mRNA amounts (E) and on Arg1 protein levels (F) in WAT. Coinjection of phospho-diesterase inhibitor Rolipram (5 mg/kg) in HFD mice indicated. (n = 4 per group). (G) Effect of PGE2 exposure on amounts of phosphorylated CREB, CRTC2/3, or HDAC4 in wild-type BMMs. *P < 0.05 and **P < 0.01.
Fig. S1.
Effect of cotreatment with IL-4 and agonist for β adrenergic receptor (isoproterenol) or bacterial toxins (pertussis, cholera, edema factor) on M2 macrophage marker mRNA amounts in BMMs. *P < 0.05 and **P < 0.01.
Overnutrition triggers leptin-mediated increases in catecholamine signaling to white and brown adipose tissues (WAT and BAT) that promote fat burning. Indeed, leptin injection into ob/ob mice up-regulated circulating concentrations of norepinephrine, leading to elevations in intracellular cAMP (14, 15). In keeping with these effects, leptin administration augmented M2 marker expression in WAT from leptin-sensitive ob/ob mice; these effects were blocked by pretreatment with β-adrenergic receptor antagonist propranolol (Fig. 1 C and D).
In contrast with acute effects of overnutrition, chronic high-fat diet (HFD) feeding causes leptin resistance and attenuates sympathetic catecholamine signaling to WAT (14). As a result, M2 marker gene expression was down-regulated in WAT from HFD mice; these effects were reversed by administering the phospho-diesterase 4 (PDE4) inhibitor Rolipram (5 mg/kg) (Fig. 1 E and F) and increasing intracellular concentrations of cAMP. Taken together, these results show that increases in cAMP signaling promote M2 macrophage polarization.
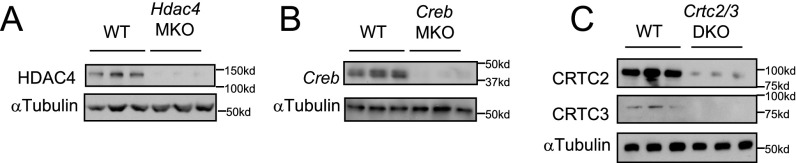
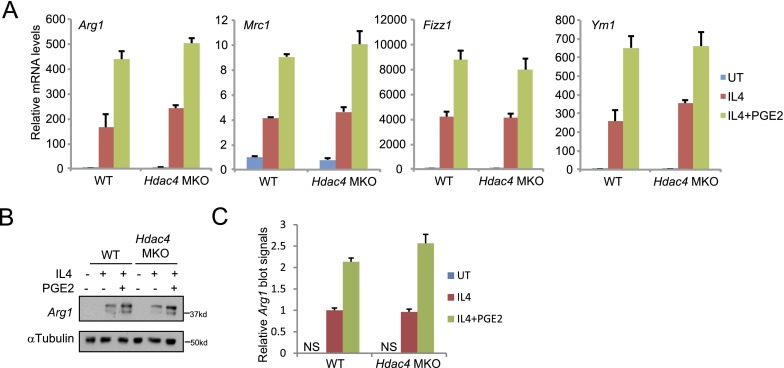
cAMP triggers the activation of the CREB/CRTC and class IIa HDAC pathways (9, 16, 17) (Fig. S1B). Of the three members of the class IIa HDAC family (HDAC4, -5, and -7), HDAC4 is expressed at highest levels in macrophages, prompting us to use cells from mice with a macrophage-specific knockout of HDAC4 (HDAC4 MKO) to evaluate the importance of this pathway for M2 polarization. Although exposure of BMMs to PGE2 plus IL-4 promoted HDAC4 dephosphorylation and activation (Fig. 1G), M2 marker gene expression was up-regulated comparably between wild-type and HDAC4 MKO BMMs (Figs. S2A and S3 A and B), arguing against a significant role for the HDAC4 pathway in this setting.
Fig. S2.
(A) Immunoblot analysis of HDAC4 protein amounts in HDAC4 MKO BMMs. (B) Immunoblot analysis of CREB protein amounts in CREB MKO BMMs. (C) Immunoblot analysis of CRTC2/3 protein amounts in CRTC2/3 DKO BMMs.
Fig. S3.
(A and B) Effect of IL-4 and PGE2 on M2 macrophage marker mRNA amounts (A) and Arg1 protein level (B) in BMMs from HDAC4 MKO or control littermates. (C) Quantitated results for Arg1 protein level shown in B.
Next we tested the importance of the CREB pathway for M2 polarization using homozygous CREB floxed (CREB fl/fl) mice expressing a macrophage-specific LysM-cre transgene. Exposure of wild-type BMMs to PGE2 increased CREB Ser133 phosphorylation and augmented CRTC2 and CRTC3 dephosphorylation (Fig. 1G) (14). Exposure to IL-4 and PGE2 increased M2 marker gene expression cooperatively in wild-type cells, and these effects were potently disrupted in CREB mutant BMMs (Fig. 2 A and B, and Fig. S2B).
Fig. 2.
PGE2 stimulates M2 polarization via the CREB pathway. (A and B) Effect of IL-4 and PGE2 on M2 macrophage marker mRNA (A) and Arg1 protein amounts (B) in in BMMs from mice with a deletion of the CREB gene in macrophages (CREB MKO) or control littermates. (C and D) Effect of IL-4 and PGE2 on M2 macrophage marker mRNA (C) and Arg1 protein amounts (D) in wild-type and CRTC2/3 DKO BMMs. *P < 0.05 and **P < 0.01.
We further evaluated the role of CREB in M2 polarization by expressing the dominant-negative CREB inhibitor ACREB, a synthetic polypeptide that selectively heterodimerizes with and disrupts the DNA binding activity of all CREB family members (CREB1, CREM, and ATF1). Similar to CREB1 knockout cells, ACREB expression in BMMs disrupted M2 marker gene expression following exposure to IL-4 plus PGE2 (Fig. S4 A and B).
Fig. S4.
(A and B) Effect of IL-4 and PGE2 on M2 macrophage marker mRNA amounts (A) and Arg1 protein level (B) in BMMs infected with either GFP or ACREB lentivirus. (C) Quantitated results for Arg1 protein level shown in B. *P < 0.05 and **P < 0.01.
CRTC proteins are potent coactivators of CREB. Sequestered in the cytoplasm under basal conditions through phosphorylation-dependent interactions with 14-3-3 proteins, CRTCs shuttle to the nucleus following their dephosphorylation in response to cAMP, where they bind to CREB over relevant promoters (9). Of the three family members, CRTC2 and CRTC3 proteins, but not CRTC1, were detected in cultured BMMs by immunoblot assay (Fig. S2C).
Based on their overlapping effects on CREB target gene expression, we evaluated the importance of both CRTC2 and CRTC3 (CRTC2/3 DKO) for macrophage polarization. M2 marker expression was reduced in CRTC2/3 DKO BMMs exposed to IL-4 plus PGE2 (Fig. 2 C and D, and Fig. S2C). Taken together, these results demonstrate that the CREB/CRTC pathway is required for cAMP induction of M2 marker genes (Fig. 2D).
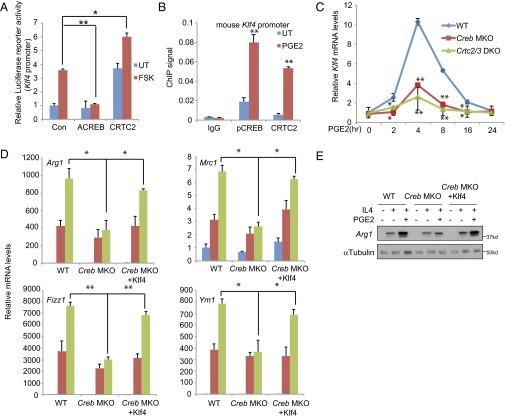
Although CREB and CRTC2/3 appeared important for induction of M2 marker genes by PGE2, the absence of conserved CREB binding sites on these promoters pointed to the involvement of additional transcriptional activators in mediating these effects. In this regard, KLF4 has been shown to cooperate with STAT6 in promoting M2 macrophage polarization; deletion of the KLF4 gene in macrophages disrupts M2 function and increases proinflammatory gene expression (13). Moreover, the mouse KLF4 promoter contains CREB binding sites at −269 and −232 relative to the transcription start site. Correspondingly, exposure to FSK up-regulated KLF4-luciferase reporter activity in HEK293T cells. CRTC2 overexpression further enhanced KLF4 promoter activity, whereas A-CREB blocked it (Fig. 3A). Consistent with a direct effect on KLF4 expression, exposure to PGE2 increased phospho-CREB and CRTC2 occupancy over the KLF4 promoter in BMMs (Fig. 3B). Exposure of wild-type BMMs to PGE2 increased endogenous KLF4 mRNA amounts maximally after 4 h; KLF4 expression was substantially down-regulated in CREB MKO as well as CRTC2/3 DKO BMMs (Fig. 3C).
Fig. 3.
The CREB/CRTC pathway promotes M2 function via induction of KLF4. (A) Transient assay of HEK293T cells showing effects of FSK on KLF4 reporter activity. Cotransfection with ACREB or CRTC2 indicated. (B) ChIP (26) assay of pCREB and CRTC2 recruitment to the KLF4 promoter in BMMs exposed to PGE2. (C) Effect of PGE2 exposure for various times on KLF4 mRNA amounts in BMMs from wild-type, CREB MKO, and CRTC2/3 DKO mice. (D and E) Effect of lentiviral KLF4 expression on M2 macrophage marker mRNA amounts (D) and Arg1 protein level (E) in wild-type and CREB MKO BMMs exposed to IL-4 and PGE2. *P < 0.05 and **P < 0.01.
We tested whether the induction of KLF4 is required for the up-regulation of M2 marker genes in response to PGE2. Supporting this idea, IL-4/PGE2-dependent induction of M2 marker genes were down-regulated in CREB and CRTC2/3 mutant cells relative to control; these effects were rescued following lentiviral expression of KLF4 (Fig. 3D). We detected similar changes in Arg1 protein expression following KLF4 expression (Fig. 3E). Taken together, these results indicate that the CREB/CRTC pathway promotes M2 polarization via the up-regulation of KLF4 in response to IL-4/PGE2 signaling.
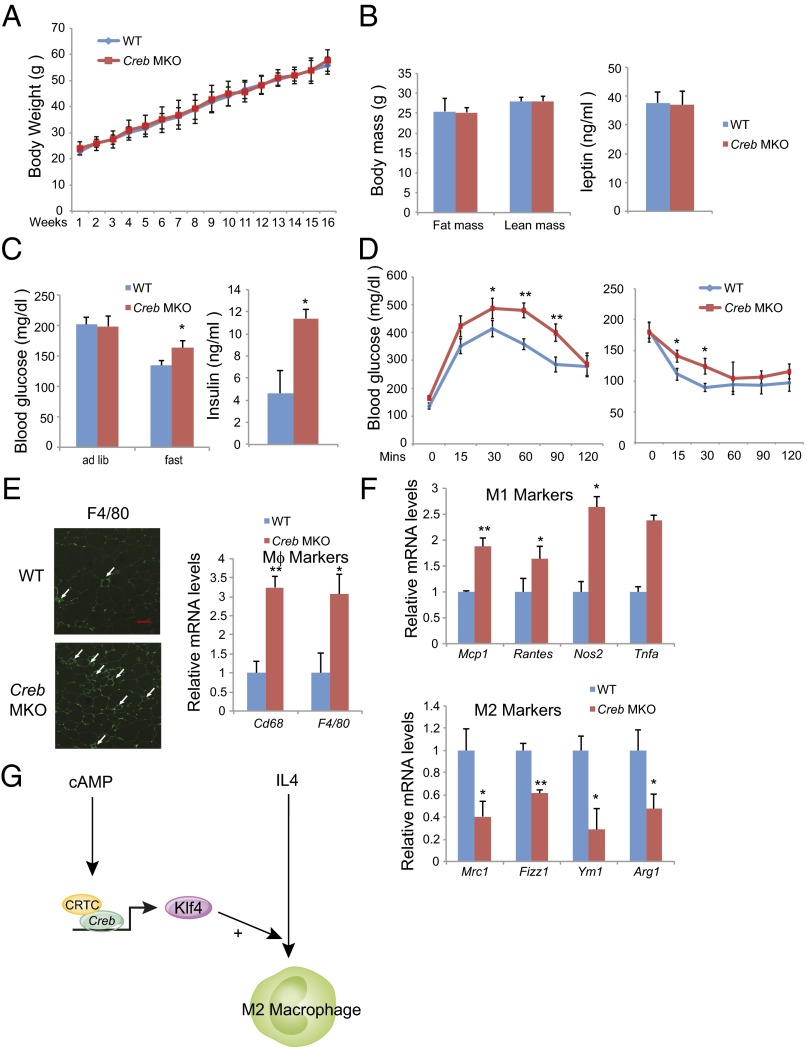
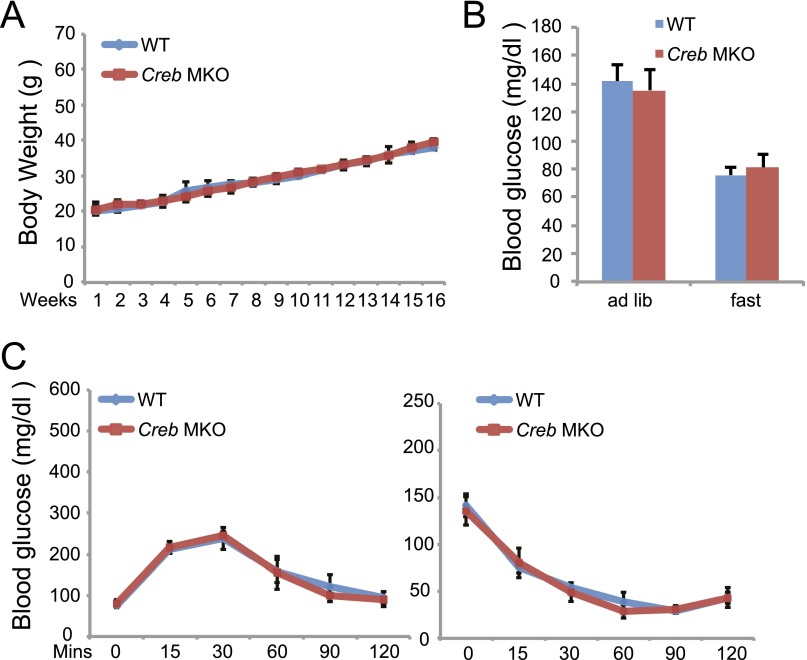
M2 macrophages have been shown to maintain insulin sensitivity in adipose tissue by reducing proinflammatory cytokine expression (18). The loss of M2 macrophages leads to reciprocal increases in M1 polarized macrophages in adipose tissue that promote insulin resistance. Based on its effects on M2 marker genes, we speculated that the CREB/CRTC pathway may protect against the development of insulin resistance in this setting. To test this notion, we evaluated effects of HFD feeding on insulin sensitivity in CREB MKO versus wild-type littermates. Although body weights, adiposity, and leptin levels were comparable between the two groups (Fig. 4 A and B and Fig. S5A), CREB MKO mice exhibited higher fasting glucose and insulin concentrations in the context of HFD feeding compared with wild-type (Fig. 4C and Fig. S5B); they were also more glucose intolerant and had reduced glucose clearance relative to controls by glucose and Insulin tolerance testing (Fig. 4D and Fig. S5C). Indeed, macrophage infiltrates in WAT were more pronounced in CREB MKO mice compared with wild-type (Fig. 4E). M1 macrophage markers were significantly elevated in CREB mutants, whereas M2 markers were reduced (Fig. 4F). Taken together, these results demonstrate that the macrophage CREB/CRTC pathway promotes M2 polarization that protects adipose tissue from insulin resistance in the setting of obesity.
Fig. 4.
Loss of M2 macrophages and insulin resistance in CREB MKO mice. (A and B) Relative weight gain (A) (n = 8 per group) as well as fat mass (n = 4 per group) and circulating leptin levels (B) (n = 8 per group) in CREB MKO and control littermates under HFD. (C) Circulating glucose and insulin concentrations in CREB MKO compared with control littermates maintained on a HFD for 12 wk (n = 8 per group). (D) Glucose tolerance (Left) and insulin tolerance (Right) testing of CREB MKO and control littermates maintained on HFD (n = 12 per group). (E) Relative macrophage infiltration in WAT by immunohistochemical (Left) and quantitative PCR (Right) analyses. (Scale bar, 50 μm.) (F) Expression of M1 and M2 marker genes in WAT by quantitative PCR analyses. (G) Schematic of proposed mechanism. *P < 0.05 and **P < 0.01.
Fig. S5.
(A) Relative weight gain (n = 8 per group) in CREB MKO and control littermates under normal chow (NC). (B) Circulating glucose concentrations in CREB MKO compared with control littermates maintained on a NC (n = 8 per group). (C) Glucose and Insulin tolerance testing of CREB MKO and control littermates maintained on NC (n = 12 per group).
Discussion
In addition to its role as a starvation state signal, the second messenger cAMP exerts potent anti-inflammatory effects through the inhibition of macrophage function. cAMP attenuates proinflammatory M1 macrophage gene expression, for example, via induction of the anti-inflammatory cytokine IL-10 (10, 11) and via the induction of HDAC4. Superimposed on these effects, we found that cAMP promotes anti-inflammatory M2 macrophage gene expression through up-regulation of the CREB/CRTC pathway (Fig. 4G). Both CREB and class IIa HDAC pathways inhibit the production of inflammatory mediators and contribute to the maintenance of insulin sensitivity in the setting of overnutrition.
In addition to KLF4, a number of other CREB target genes, most notably CEBPβ and SOCS3, have been shown to promote macrophage polarization (19, 20). Future studies should provide further insight into these and other mechanisms by which cAMP signaling modulates innate immunity.
Experimental Procedures
Cells.
HEK293T cells were maintained in DMEM and exposed to FSK (10 μM) for transient transfection assays. BMMs were prepared as described previously (21). Cells were treated with IL-4 (10 ng/mL) and PGE2 (10 nm), isoproterenol (10 μm), pertusis toxin (1 μg/mL), cholera toxin (1 μg/mL), edema factor (0.1 μg/mL) + protective antigen (0.3 μg/mL) for 16 h, and mRNA or protein was extracted for examination.
Mice.
C57BL/6J, ob/ob, and LysMcre mice were purchased from The Jackson Laboratory. CREB1 fl/fl mice were a generous gift of E. Nestler (Mount Sinai Hospital, New York, NY). Macrophage-specific knockout of CREB was obtained by a two-step cross of CREB1 fl/fl mice with LysMcre mice according to The Jackson Laboratory protocol. CRTC2 fl/fl mice were as described previously (22). CRTC3 fl/fl mice were generated with loxP sites flanking exon 4 (ES cell clone obtained from KOMP). Floxed CRTC2/3 mice were obtain by cross of CRTC2 fl/fl with CRTC3 fl/fl. In studies with CRTC2/3 DKO macrophages, BMMs from floxed CRTC2/3 mice were infected with cre-expressing or control lacz-expressing lentivirus. For studies with KO mice, age-matched wild-type littermates were used as controls. For HFD studies, 6-wk-old mice were transferred to a 60% HFD (Research Diets, D12492) for 12 wk. MRI scans for fat and lean mass were performed using an Echo MRI-100 instrument according to the manufacturer’s instructions. All mice were housed in colony cages with a 12-h light/dark cycle in a temperature-controlled environment. Animal studies were approved by the Institutional Animal Care and Use Committee at the Salk Institute.
Glucose Tolerance Testing and Insulin Tolerance Testing.
For glucose tolerance testing, mice were fasted for 16 h and then injected intraperitoneally with glucose (1.5 mg/kg). For insulin tolerance testing, mice were fasted 2 h and injected intraperitoneally with insulin (Humulin; 1 U/kg). Blood was collected from the tail vein at indicated times and glucose levels were measured with a One Touch Ultra Glucometer (Johnson & Johnson).
ChIP and Quantitative PCR.
BMMs were plated in 150-mm plates and exposed to PGE2 (100 nM) for 1 h. ChIP assays were performed as described previously (23). BMMs were stimulated for indicated times. RNA was isolated by RNeasy kit (Qiagen). Quantitative PCR was carried out with SYBR Green, as described previously (23).
Blotting and Immunostaining.
Immunoblot and immunostaining assays were performed as described previously (24). Quantitated results for Arg1 protein level are shown in Fig. S6. Mouse adipose tissues were fixed and paraffin-embedded. Sections (5 μm) were used for immunostaining with F4/80 antibody.
Fig. S6.
Quantitated results for Arg1 protein levels: A for Fig. 1B; B for Fig. 1D; C for Fig. 1F; D for Fig. 2B; E for Fig. 2D; and F for Fig. 3E. **P < 0.01.
Luciferase Reporter Assay.
HEK293T cells were transfected with KLF4-Luc reporter, RSV-βgal, and indicated plasmids for 48 h, and luciferase assays were performed as described previously (25).
Statistical Analyses.
All studies were performed on at least three independent occasions. Results are reported as mean ± SEM. The comparison of different groups was carried out using two-tailed unpaired Student’s t test or two-way ANOVA test. Differences were considered statistically significant at *P < 0.05 and **P < 0.001.
Acknowledgments
This work was supported by National Institutes of Health Grants R37DK083834 (to M.M.) and P01-DK049210 (to K.H.K.); the Clayton Foundation for Medical Research; the Kieckhefer Foundation; and the Leona M. and Harry B. Helmsley Charitable Trust Grant #2012-PG-MED002 (to M.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519644112/-/DCSupplemental.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 3.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 4.Han MS, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 6.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32(7):307–314. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: Macrophage inhibition by cyclic AMP (cAMP): Differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174(2):595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 9.Altarejos JY, Montminy M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark K, et al. Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc Natl Acad Sci USA. 2012;109(42):16986–16991. doi: 10.1073/pnas.1215450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avni D, Ernst O, Philosoph A, Zor T. Role of CREB in modulation of TNFalpha and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Mol Immunol. 2010;47(7-8):1396–1403. doi: 10.1016/j.molimm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao X, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121(7):2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luan B, et al. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 2014;19(6):1058–1065. doi: 10.1016/j.cmet.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibuya I, et al. Regulation of catecholamine synthesis by leptin. Ann N Y Acad Sci. 2002;971:522–527. doi: 10.1111/j.1749-6632.2002.tb04517.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang B, et al. A hormone-dependent module regulating energy balance. Cell. 2011;145(4):596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihaylova MM, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145(4):607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 19.Ruffell D, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin H, et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189(7):3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weischenfeldt J, Porse B. 2008. Bone marrow-derived macrophages (BMM): Isolation and applications. CSH Protoc 2008:pdb prot5080. [DOI] [PubMed]
- 22.Blanchet E, et al. Feedback inhibition of CREB signaling promotes beta cell dysfunction in insulin resistance. Cell Reports. 2015;10(7):1149–1157. doi: 10.1016/j.celrep.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119(1):61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Altarejos JY, et al. The Creb1 coactivator Crtc1 is required for energy balance and fertility. Nat Med. 2008;14(10):1112–1117. doi: 10.1038/nm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. Effect of normal lung definition on lung dosimetry and lung toxicity prediction in radiation therapy treatment planning. Int J Radiat Oncol Biol Phys. 2013;86(5):956–963. doi: 10.1016/j.ijrobp.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]