Significance
Stress-related disorders are prevalent worldwide, but the pathophysiology of these disorders and specific therapeutic targets remain elusive. Two hypothetical frameworks show great promise: decreased neurotrophic support and decreased responsiveness to glucocorticoids. Our study shows that the glucocorticoid receptor is a prominent target of the brain-derived neurotrophic factor (BDNF) signaling, which gives rise to unique functional attributes essential for the neuroplasticity to an antidepressant. We propose a unifying mechanism of BDNF-priming of glucocorticoid signaling, which provides new prospects for discovering innovative treatments for disorders featuring unpaired BDNF and glucocorticoid activities. Eliciting glucocorticoid receptor phosphorylation is an attractive means to confront resistance to antidepressants.
Keywords: BDNF, glucocorticoid, coincidence detection, stress, antidepressant
Abstract
Neurotrophins and glucocorticoids are robust synaptic modifiers, and deregulation of their activities is a risk factor for developing stress-related disorders. Low levels of brain-derived neurotrophic factor (BDNF) increase the desensitization of glucocorticoid receptors (GR) and vulnerability to stress, whereas higher levels of BDNF facilitate GR-mediated signaling and the response to antidepressants. However, the molecular mechanism underlying neurotrophic-priming of GR function is poorly understood. Here we provide evidence that activation of a TrkB-MAPK pathway, when paired with the deactivation of a GR-protein phosphatase 5 pathway, resulted in sustained GR phosphorylation at BDNF-sensitive sites that is essential for the transcription of neuronal plasticity genes. Genetic strategies that disrupted GR phosphorylation or TrkB signaling in vivo impaired the neuroplasticity to chronic stress and the effects of the antidepressant fluoxetine. Our findings reveal that the coordinated actions of BDNF and glucocorticoids promote neuronal plasticity and that disruption in either pathway could set the stage for the development of stress-induced psychiatric diseases.
Glucocorticoids can either facilitate or deteriorate the structure and function of brain circuits involved in perception, cognition, and mood by modulating neurotransmission and remodeling dendritic spines as a function of time at exposure, dose, and duration (1, 2). Neuronal growth defects and cognitive impairment manifest upon disruption of circadian oscillations and of stress-mediated peaks of glucocorticoids, common features of major depression (3). Paradoxically, despite the often high glucocorticoid concentrations in patients with depression, signaling through glucocorticoid receptors (GR) appears defective, as evidenced by the inability of the endocrine stress response to be suppressed by dexamethasone, and reduction in expression of GR-sensitive genes and an inability of GR to suppress inflammation (4, 5). This so called “glucocorticoid-resistant” state is not solely the result of GR down-regulation (6, 7). What accounts for this lack of GR responsiveness during chronic stress is not understood.
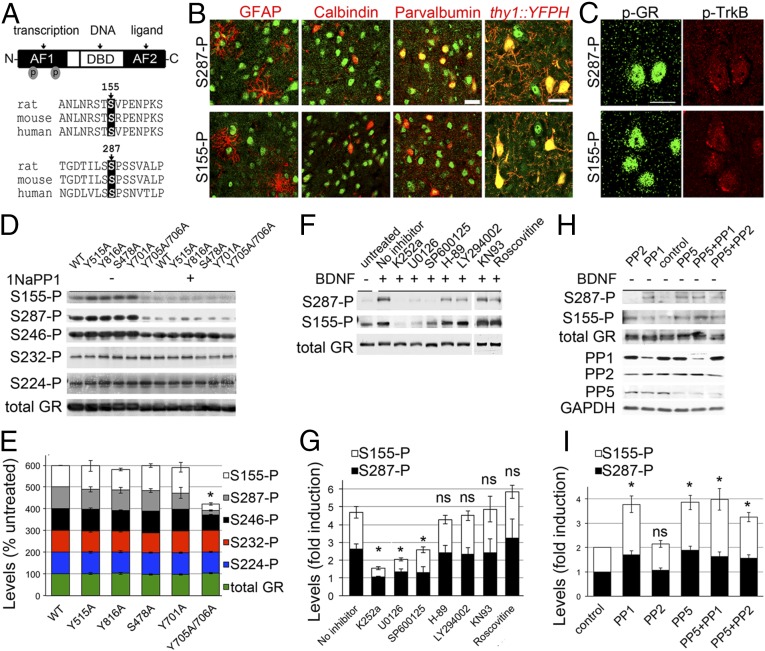
One hypothesis to account for glucocorticoid resistance is that additional stress-sensitive pathways influence GR function (8). For example, brain-derived neurotrophic factor (BDNF), is essential for the enhancement of contextual fear memories by glucocorticoids (9, 10). In contrast, BDNF-deficiency diminished the complexity of hippocampal dendritic arborization without further atrophy by stress (11). This raises the interesting question of whether BDNF initiates a cellular pathway that could modulate GR activity during stress. Using mass spectrometry, we previously found that BDNF promotes the phosphorylation of GR at two conserved phosphorylation sites (Fig. 1A) involved in the expression of select glucocorticoid-regulated genes when BDNF and glucocorticoid stimulation were paired in vitro (12).
Fig. 1.
Phosphorylation of GR by BDNF signaling. (A) Conserved GR phospho-sites sensitive to BDNF. (B) Cells expressing S287-P and S155-P in the mouse cortex S1. YFP labels LV excitatory cortical neurons. (C) Cells coexpressing phospho-GR and phospho-TrkB in the cortex S1. (D) GR phosphorylation and TrkB(F669A) inactivation in transfected 293 cells stimulated with 20 ng/mL BDNF and 10 ng/mL 1NaPP1 for 30 min. (E) Percentage of GR phosphorylation relative to the TrkB-WT −1NaPP1 (mean ± SEM of four experiments, t test *P < 0.001). (F) GR phosphorylation and kinase inhibitors: 100 nM K252a, 10 μM U0126, 5 μM SP600125, 10 μM H-89, 50 μM LY294002, 5 μM KN93, and 50 μM roscovitine for 30 min prior to 50 ng/mL BDNF for 30 min. (G) GR phosphorylation normalized to total GR levels and expressed as fold-change relative to untreated cells (mean ± SEM of three experiments, t test *P < 0.05). (H) GR phosphorylation induced in absence of BDNF stimulation of primary cortical neurons by the knockdown of the indicated serine phosphatases. (I) Levels of GR phosphorylation normalized to total GR and expressed as fold change relative to cells expressing the shRNA control (t test *P < 0.05, mean ± SEM of seven experiments). ns, not significant. (Scale bars, 20 μm.)
Here, we test physiological roles of BDNF-induced GR phosphorylation in vivo by substituting endogenous GR with BDNF-insensitive phospho-deficient mutants. We demonstrate that fluoxetine prevented the neuroplasticity of chronic stress by priming GR phosphorylation at BDNF-sensitive sites. Mechanistically, activation of a tropomyosin related kinase B-mitogen activated protein kinase (TrkB-MAPK) pathway coincident with the deactivation of a GR-protein phosphatase 5 (PP5) complex triggered GR phosphorylation and the expression of neuroplasticity genes when BDNF and glucocorticoid signaling were paired. Disruption of GR priming by BDNF signaling could explain antidepressant resistance.
Results
Phosphorylation of GR at BDNF-Sensitive Sites in Vivo.
To reveal GR phosphorylation at S155 and S287 in the brain, we performed immunohistochemistry using site-specific antibodies (Fig. S1). S287-P was detected in astrocytes (GFAP), interneurons (parvalbumin), and excitatory neurons (thy1::YFP) of the cerebral cortex in juveniles and adults. In contrast, S155-P was predominant in excitatory neurons, although some parvalbumin interneurons also stained (Fig. 1B). To address if GR phosphorylation coincides with BDNF signaling in vivo, we detected TrkB-P, the active form of the BDNF receptor, with S155-P or S287-P. We found a high degree of coexpression in all layers of the sensory cortex notably in LII/III (Fig. 1C), in the hippocampus, and hypothalamus (Fig. S2).
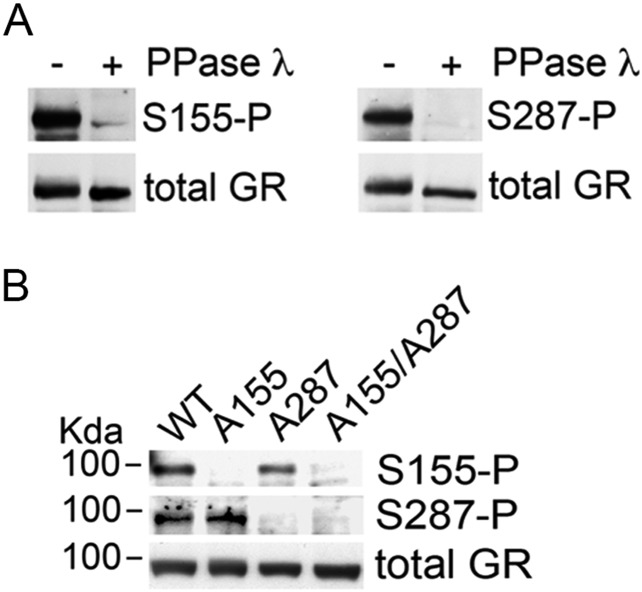
Fig. S1.
Specificity of the phospho-site–specific antisera against GR. Western blot used to probe antibodies on lysates from cortical neurons stimulated with 50 ng/mL BDNF for 30 min. (A) Samples were split in two, one treated with the phosphatase λ at 37 °C for 30 min, the other at 37 °C for 30 min without the enzyme. (B) Cultured neurons were infected with lentivirus bearing the molecular replacement constructs to substitute the endogenous GR by the indicated recombinant mutant.
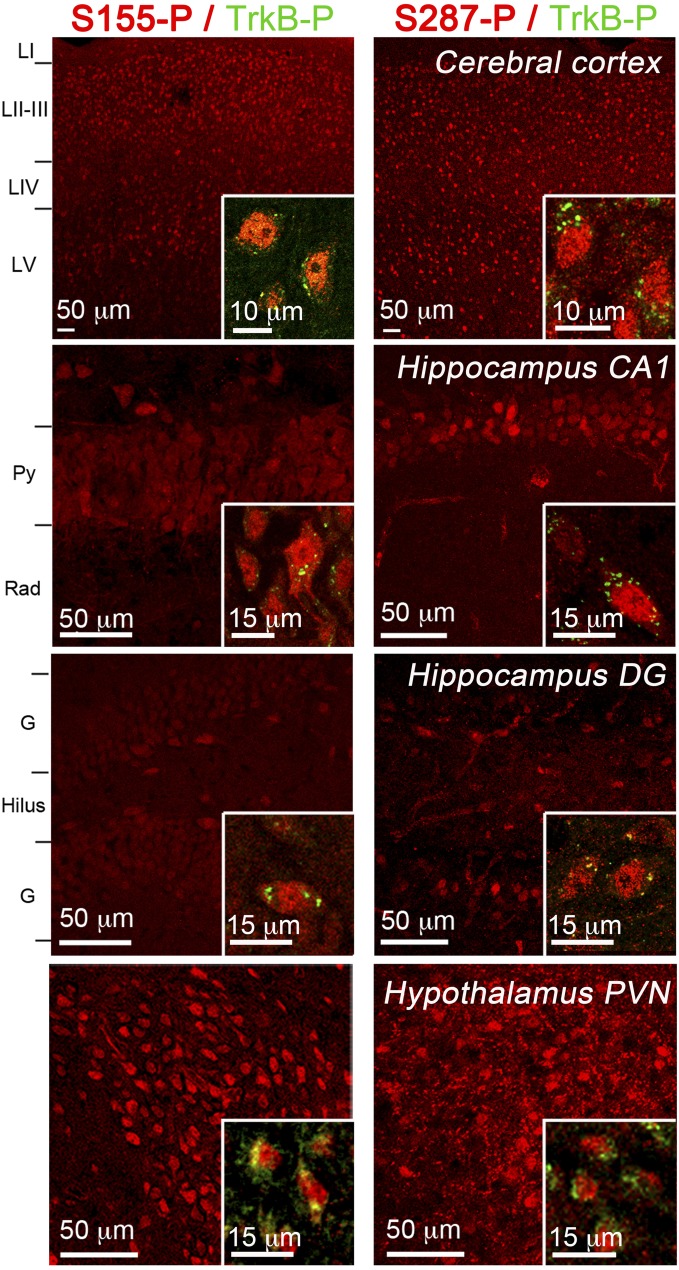
Fig. S2.
GR phosphorylation at S155 and S287 sites in the cortex, hippocampus, and hypothalamus. TrkB phosphorylation at Y816 (green) is shown (Insets) together with GR[S155-P] or GR[S287-P] in (red). Annotations are cortical layers (LI–V), pyramidal cell layer (Py), stratum radiatum (rad), granular cell layer (G).
GR Phosphorylation at BDNF-Sensitive Sites Responded to TrkB-MAPK and GR-PP5 Pathways.
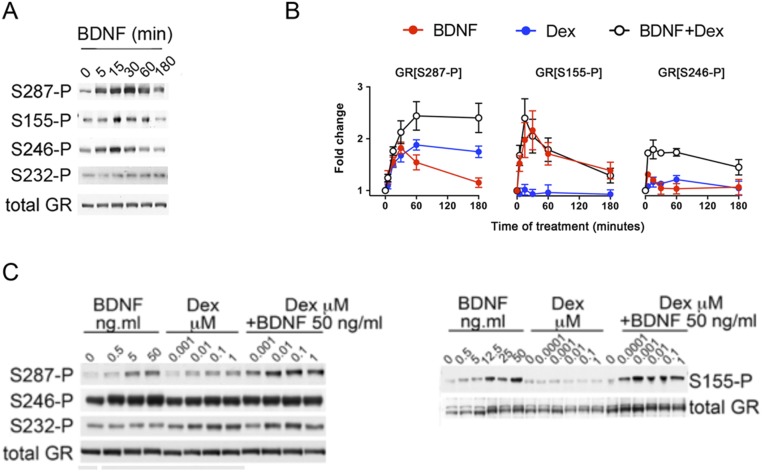
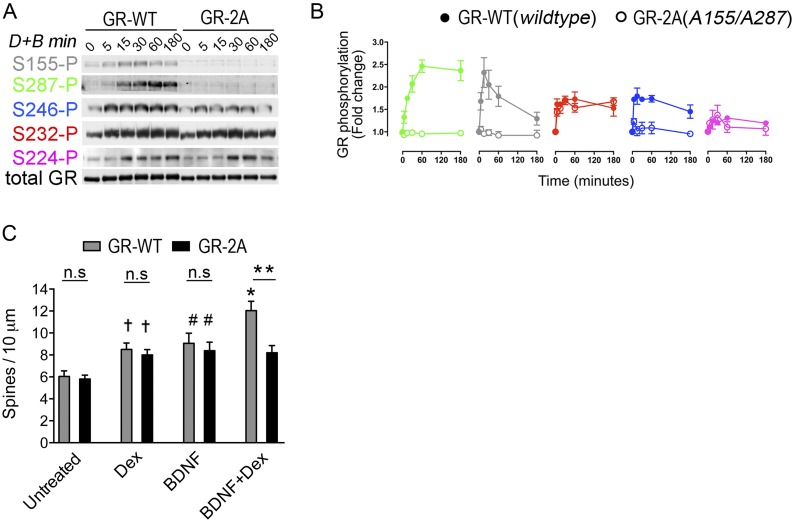
To gain insight into the BDNF signaling pathways responsible for GR phosphorylation, we tested TrkB mutants in a reconstituted system. We cotransfected 293 cells with GR and the TrkB(F669A) mutant, which is inactivated by 1NaPP1 (13). Stimulation with BDNF induced specific phosphorylation of TrkB(F669A) and GR at S155-P, S287-P, and to a lesser extent at S246-P because TrkB signaling was blocked by 1NaPP1 (Fig. 1D and Fig. S3). Of the established intracellular residues of TrkB required for BDNF signaling [Y515 for SHC, Y816 for PLCγ, S478 for Tiam1, Y701/Y705/Y706 for the activation loop (14, 15)], we found that Y705 and Y706 are critical for triggering GR phosphorylation at BDNF-sensitive sites but not S232-P and S224-P (Fig. 1 D and E). Dose–response and time-course experiments in primary neurons confirmed that S155-P, S287-P, and S246-P are BDNF-sensitive sites (Fig. S4), unlike S224-P and S232-P (Fig. S5).
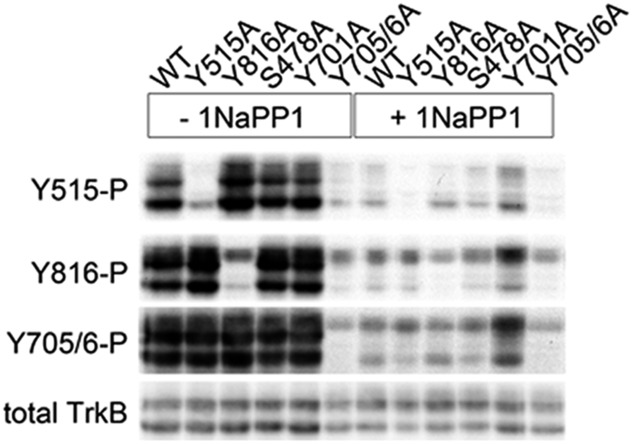
Fig. S3.
Deactivation of the TrkB(F669A) mutant by 1NaPP1. Western blot used to probe the phospho-site–specific Trk antibodies on lysates from 293 cells stimulated with 20 ng/mL BDNF for 30 min in the presence or absence of a pretreatment with 10 ng/mL 1NaPP1. Cells were transfected with the indicated TrkB mutants.
Fig. S4.
Characterization of GR phosphorylation by BDNF. (A) GR phosphorylation by BDNF (50 ng/mL) in primary cortical neurons occurs within minutes of stimulation and returned to baseline faster for S246 than S155 and S287. (B) Time-course analysis of GR phospho-sites (mean ± SEM, n = 5) in primary cortical neurons stimulated with 1 μM Dex and 50 ng/mL BDNF alone or in combination. (C) GR phosphorylation in cortical neurons as a function of the dose of BDNF, Dex alone, or in combination for 30 min. BDNF and Dex have additive effects on GR[S246-P] and GR[S287-P]. In contrast, GR[S155-P] responded only to BDNF, and GR[S232-P] responded only to Dex.
Fig. S5.
Inhibition of BDNF-induced GR phosphorylation with K252a (Trk inhibitor). (A) Primary cortical neurons stimulated with 50 ng/mL BDNF and increasing concentrations of Dex for 30 min in the presence or absence of 100 nM K252a. (B) Quantification of GR phosphorylation as a function of BDNF/TrkB signaling. Two-way ANOVA with post hoc Bonferroni’s test for the effect of BDNF+Dex at S287-P (P = 0.004), S155-P (P = 0.4846), S246-P (P < 0.0001), S232-P (P < 0.0001), S224-P (P = 0.0034). Two-way ANOVA post hoc Bonferroni’s test for the effect of K252a at S287-P (P < 0.0001), S155-P (P = 0.0048), S246-P (P < 0.019), S232-P (P = 0.4257), S224-P (P = 0.6638).
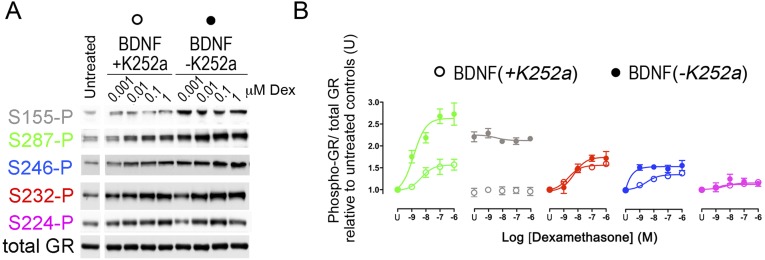
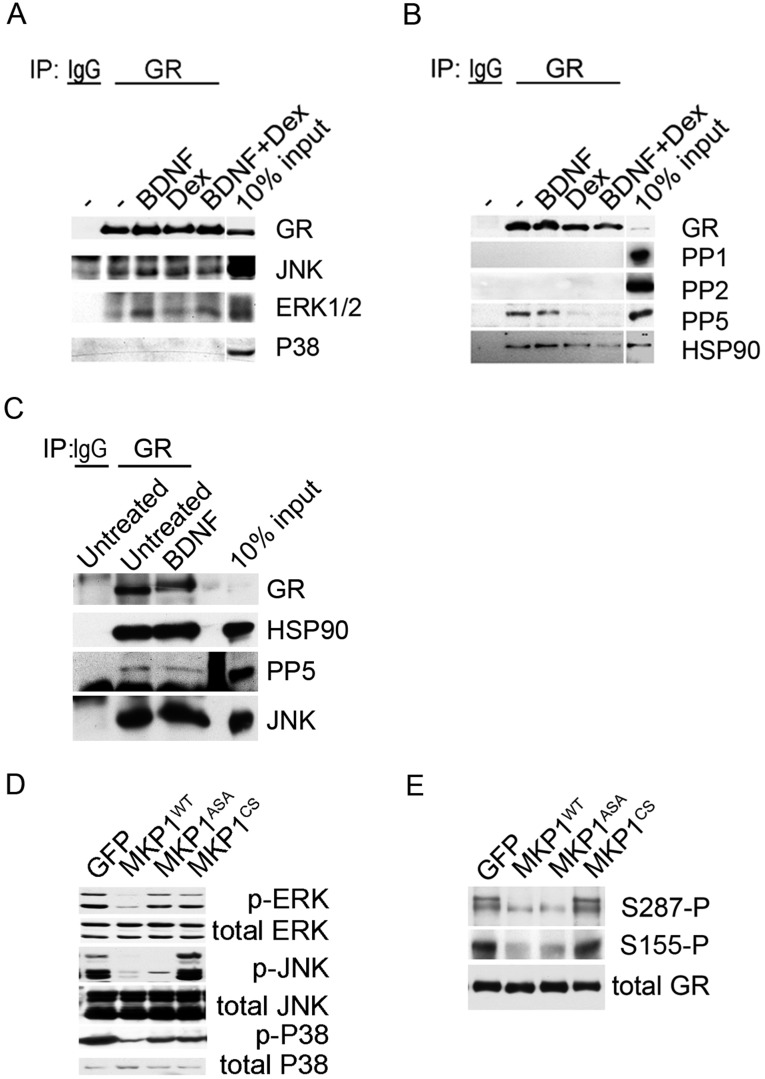
We next tested several kinase inhibitors 1 h before stimulation of primary cortical neurons with BDNF. Inhibitors against TrkB (K252a), ERK (U0126), and JNK (SP600125) reduced GR phosphorylation (Fig. 1 F and G). Coimmunoprecipitation studies in primary neurons confirmed that extracellular regulated kinase (ERK) and Jun N-terminal kinase (JNK) bound to GR (Fig. S6). Inactivation of JNK with a recombinant phosphatase was sufficient to suppressed BDNF-induced GR phosphorylation (Fig. S6) (16). Next, we tested various phosphatase inhibitors. Calyculin A and okadaic acid raised GR phosphorylation of primary neurons contrary to fostriecin that blocks PP2A and PP4. To identify the GR phosphatases, we generated short-hairpin RNA (shRNA) constructs to knockdown PP1 and PP5 in primary neurons (Fig. 1H) (respectively by 64 ± 4%, 58 ± 4% n = 7–9, t test P < 0.0001). Both shRNAs increased GR phosphorylation with no additive effects (Fig. 1I). Coimmunoprecipitation studies in primary neurons revealed that only PP5 bound to GR, and only glucocorticoids detached PP5 from GR complexes (Fig. S6). The results indicate that JNK promoted whereas PP5 reduced GR phosphorylation at BDNF-sensitive sites.
Fig. S6.
Coimmunoprecipitation of GR with MAPK and phosphatases. GR complexes were immunoprecipitated from lysates of cortical neurons stimulated with 50 ng/mL BDNF, 1 μM Dex alone, or in combination using 1 μg of rabbit antibodies against GR or 1 μg of nonimmune rabbit IgG controls. Purification was achieved with protein-A Sepharose by rinsing five times in the lysis buffer. (A) Western blot used to probe the MAPK-specific antibodies. (B) Western blot used to probe the phosphatase specific antibodies. (C) Stimulation of primary cortical neurons with 50 ng/mL BDNF did not affect the constitutive binding of JNK and PP5 to GR complexes by immunoprecipitation. (D) Primary cortical neurons transduced with lentivirus to overexpress MKP1, a promiscuous MAPK phosphatase or MKP1 mutants. MKP1-ASA targeted only JNK and MKP1-CS lacked phosphatase activity. Western blot used to probe the MAPK specific antibodies and validate the efficiency of MKP1 constructs in lysates of neurons treated with 50 ng/mL BDNF for 30 min. (E) Western blot used to probe GR phosphorylation at specific sites as a function of MKP1 construct expression.
The GR-3A Mutant Is Insensitive to BDNF and yet Retains Signaling Attributes for Glucocorticoids.
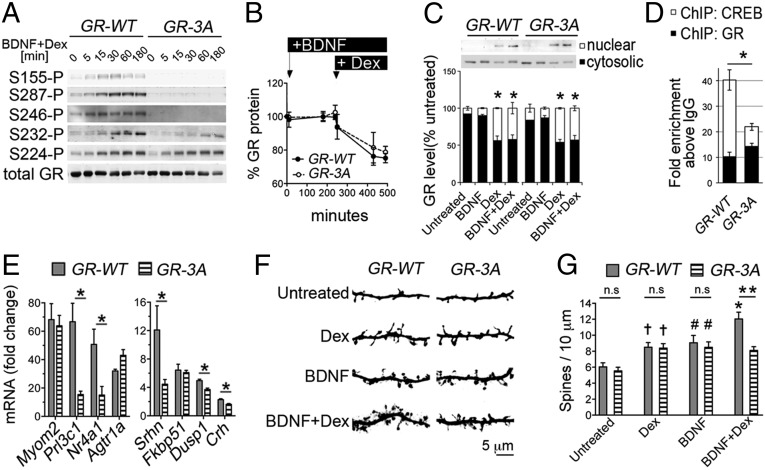
To characterize the role of BDNF-sensitive GR phospho-sites, we mutated S155, S287, and S246 by alanine residues (GR-3A mutant). Despite the phospho-deficiency of the GR-3A mutant, glucocorticoid-induced GR phosphorylation at other sites was preserved (Fig. 2A). Functionally, the GR-3A did not differ from the GR-WT with respect to glucocorticoid-induced GR turnover (Fig. 2B), nuclear import (Fig. 2C), or DNA-binding to the corticotropin releasing hormone (CRH) promoter (Fig. 2D). In contrast, the occupancy of cyclic AMP-responsive element binding (CREB) at this promoter was reduced in neurons expressing the GR-3A mutant (Fig. 2D). This finding is consistent with our previous data at other target promoters coresponsive to BDNF and glucocorticoids, SGK1 and GILZ, where GR binding was not modified by GR phosphorylation, contrary to the recruitment of CREB (12). To test the role of GR phosphorylation on the expression of genes coresponsive to BDNF and glucocorticoids (12), we performed quantitative PCR (qPCR) experiments. The expression of several neuroplasticity genes (e.g., Nr4A1) was markedly reduced in neurons expressing the GR-3A mutant compared with the GR-WT (Fig. 2E).
Fig. 2.
Characterization of the BDNF-insensitive GR-3A mutant in primary cortical neurons. (A) GR phosphorylation induced by 1 μM dexamethasone (dex) + 50 ng/mL BDNF. (B) Levels of GR (mean ± SEM of three experiments) after 50 ng/mL BDNF + 1 μM Dex. (C) Effect of 50 ng/mL BDNF and 1 μM Dex for 30 min on nuclear translocation of GR-WT and GR-3A. Mean ± SEM of three experiments, t test *P < 0.05 Dex vs. untreated. (D) ChIP with antibodies to GR or CREB after 50 ng/mL BDNF + 1 μM Dex for 1 h. Mean ± SEM of three experiments normalized to IgG controls (t test *P < 0.05). (E) qPCR data (mean ± SEM of three experiments, t test *P < 0.05) expressed as fold-change after 50 ng/mL BDNF + 1 μM Dex for 3 h compared with untreated. (F) Effect of GR-3A on the maturation of cortical neurons after 50 ng/mL BDNF, 1 μM Dex for 24 h alone or in combination. (G) Spine density (mean ± SEM of 12 or more neurons per group, t test: CTR vs. Dex †P = 0.0039; CTR vs. BDNF #P = 0.0055; CTR vs. BDNF+Dex *P < 0.0001; WT vs. 3A **P = 0.0004).
This result prompted us to test the role of the GR-3A on the maturation of cortical neurons, given that GR and BDNF are established synaptic modifiers (8). Compared with the GR-WT, we found that cortical neurons expressing the GR-3A for 3 wk in culture featured defects in dendritic spines after costimulation with BDNF and Dex but not after single treatments (Fig. 2 F and G). To address the requirement for the genes dependent on GR phosphorylation, we blocked transcription with actinomycin D during costimulation with BDNF and Dex. This resulted in dendritic growth defects in neurons expressing GR-WT but not GR-3A (WT vs. 3A: 11.07 ± 0.4 and 8.1 ± 0.26 spines per 10 μm, t test P < 0.0001 and after 5 μM actinomycin D: 9.24 ± 0.44 vs. 9.01 ± 0.53, t test P > 0.7, n = 17 or more neurons per group). We conclude that signaling of BDNF and glucocorticoids through the GR-3A mutant resulted in a loss-of-function for dendritic spine growth.
Disruption of GR Phosphorylation at BDNF-Sensitive Sites in Vivo.
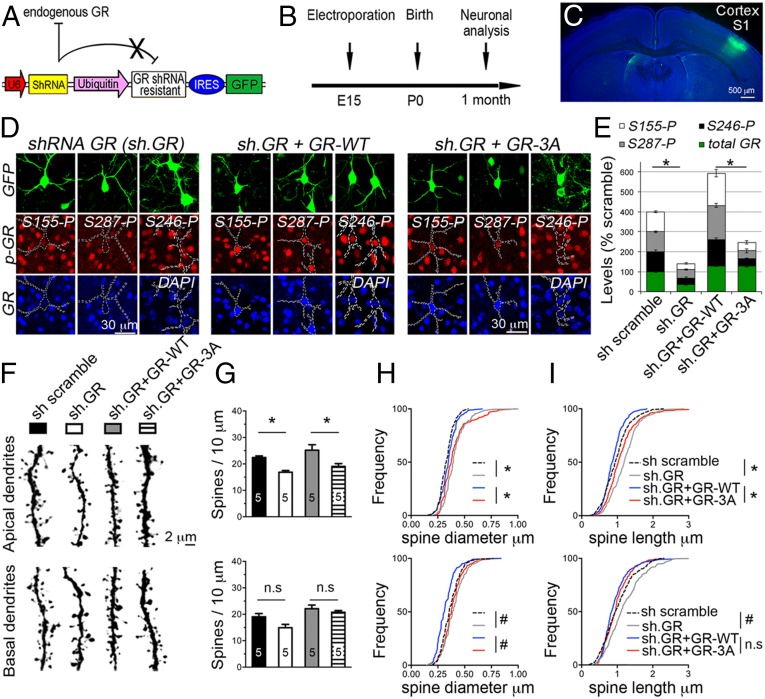
To examine the role of GR phosphorylation in vivo, we substituted endogenous GR with the GR-3A mutant in the sensory cortex after in utero electroporation of the LII/III excitatory neurons in mice (Fig. 3 A–C). We observed robust knockdown of endogenous GR and the expression of the GR-3A mutant in GFP+ cells at 1 mo of age (Fig. 3D). As a result, GR phosphorylation at S155, S246, and S287 residues was robustly reduced in GFP+ cells featuring the knockdown of GR (shRNA GR) or the GR-3A mutant (shRNA GR + GR-3A). In contrast, GFP+ cells expressing the GR-WT (shRNA GR + GR-WT) presented with more GR phosphorylation than GFP+ cells expressing the shRNA control (shRNA scramble) because of the ectopic levels of recombinant GR driven by the ubiquitin promoter (Fig. 3E). This effect was also observed in GR-3A–expressing cells, allowing controlled comparisons of neuronal maturation in vivo (Fig. 3F).
Fig. 3.
The GR-3A mutant impaired the maturation of cortical neurons in vivo. (A) Construct carrying a shRNA against GR, a GFP reporter cassette, and a shRNA-resistant GR cDNA under the control of a weak ubiquitin promoter. (B) Experimental timeline. (C) LII/III excitatory neurons targeted by in utero electroporation. (D) Substitution of endogenous GR by the GR-WT or GR-3A mutant in vivo. (E) Expression data of GR and phospho-isoforms (mean ± SEM of ∼six GFP+ cells) in the sensory cortex (t test, *P < 0.05). (F) Dendrites of LII/III neurons electroporated with the indicated construct. (G) Effect of constructs on spine density (F3, 32 = 14.16, mean ± SEM of five mice per group) at the apical dendrites: shRNA GR, P = 0.0125 and GR-3A, *P = 0.0057 and the basal dendrites: shRNA GR, P = 0.09 and GR-3A, P = 0.87. (H) Cumulative diameter of ∼350 spines in five mice per group. Mean effect of constructs in ∼20 dendrites (F3, 157 = 92.59) at the apical: shRNA GR and GR-3A, *P < 0.0001, and at the basal: shRNA GR and GR-3A, #P < 0.003. (I) Cumulative length of ∼200 spines in five mice per group. Mean effect of constructs in ∼20 dendrites (F3, 161 = 42.36) at the apical: shRNA GR and GR-3A, *P < 0.001 and at the basal shRNA GR, #P < 0.001 and GR-3A, P > 0.7.
We found that the knockdown of GR reduced spine density (Fig. 3G) and increased the mean size of spines (Fig. 3 H and I) at apical dendrites of LII/III excitatory neurons. Restoring the expression of GR reversed this effect (density: P = 0.0002, length: P < 0.0001, diameter: P < 0.0001). In contrast, substituting endogenous GR with the GR-3A mutant recapitulated features of a loss of function at apical tuft dendrites (density: P = 0.0057, length: P < 0.0001, diameter: P < 0.0001), while preserving the basal dendrites (density: P = 0.87, length: P = 0.77, diameter: P < 0.0001). The results suggest that the GR-3A decreases the number of immature thin spines while preserving the larger and mature, mushroom-type spines.
TrkB-Mediated GR Phosphorylation in Vivo and Plasticity to Stress.
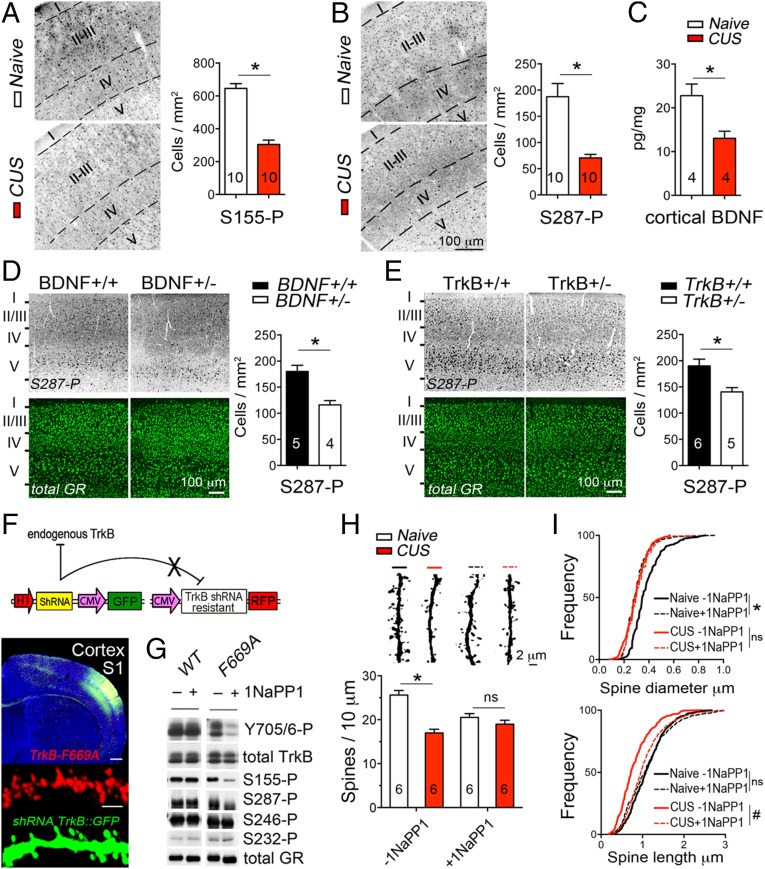
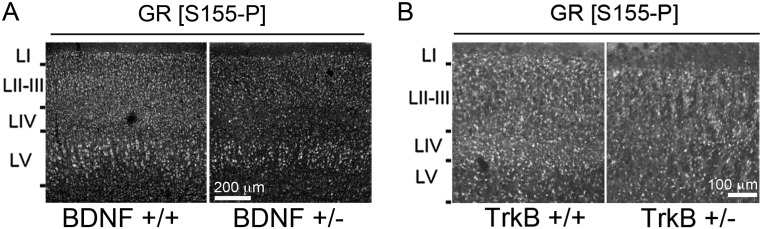
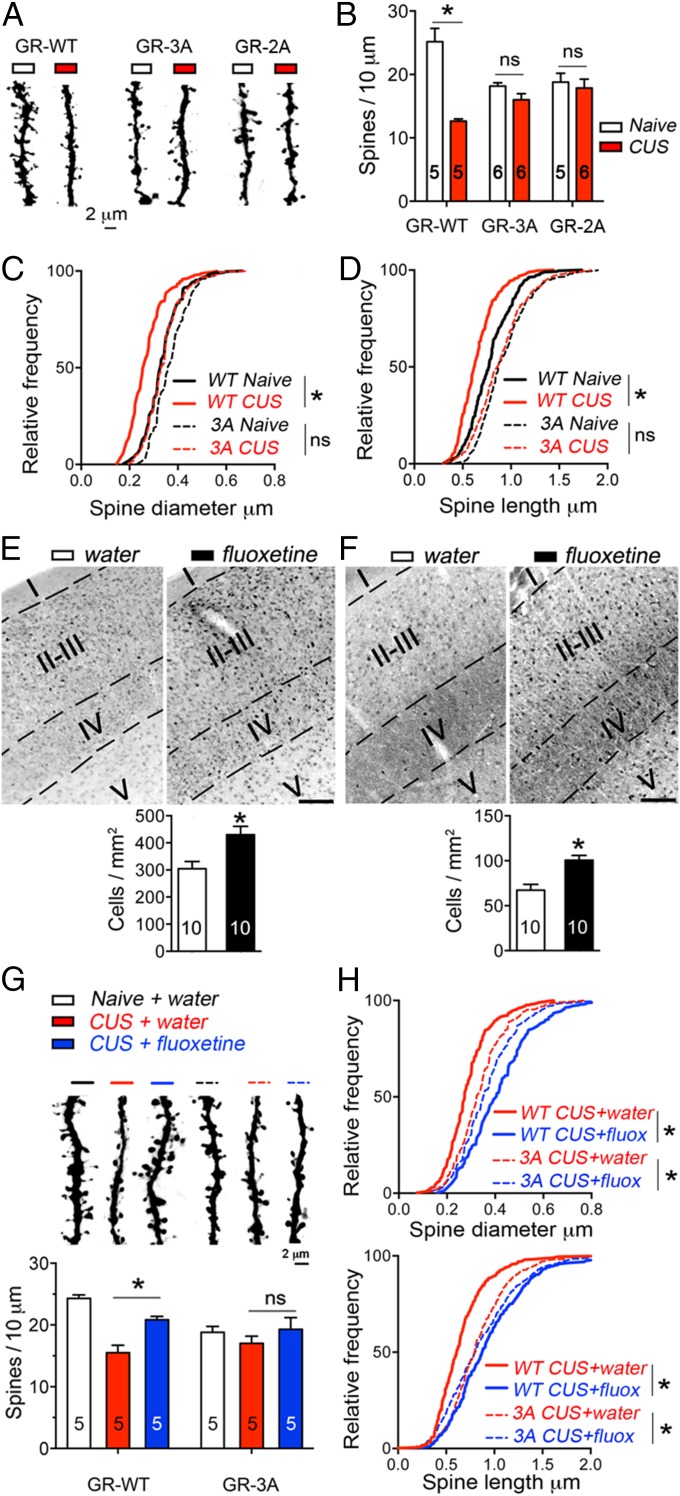
To determine the plasticity of GR phosphorylation upon changes in the endogenous levels of BDNF and glucocorticoids (17), mice were exposed to a chronic unpredictable stress (CUS) that included one daily random stressor for 10 consecutive days from P21 to 1 mo of age. We found that S155-P (Fig. 4A) and S287-P (Fig. 4B) diminished in the cortex S1 of stressed mice compared with naïve controls, which corresponded to less BDNF cortical levels (P = 0.034) (Fig. 4C) and more plasma corticosterone concentration among other hallmarks of CUS (Fig. S7). Similarly, GR phosphorylation was diminished in the cortex S1 of BDNF+/− (Fig. 4D and Fig. S8) and TrkB+/− (Fig. 4E and Fig. S8) mice without altering the levels of endogenous GR.
Fig. 4.
TrkB-mediated GR phosphorylation in vivo and suppression by CUS. (A) S155-P in the cortex S1 LII/III of mice exposed to CUS from P21 to P31 (t test *P < 0.0001, mean ± SEM of 10 mice per group). (B) S287-P in the cortex S1 LII/III of mice exposed to CUS from P21 to P31 (t test *P = 0.0003, mean ± SEM of 10 mice per group). (C) BDNF protein in the cortex S1 by ELISA (t test *P = 0.0346, mean ± SEM of four mice per group). (D) S287-P in the cortex S1 LII/III of BDNF knockout mice (t test *P = 0.004, mean ± SEM of five to four mice per group). (E) S287-P in the cortex S1 LII/III of TrkB knockout mice (t test *P = 0.0137, mean ± SEM of six to five mice per group). (F) LII/III excitatory neurons of cortex S1 targeted by electroporation with the shRNA against TrkB (GFP reporter) and the shRNA-resistant TrkB(F669A) mutant fused to RFP. (G) TrkB(F669A) and GR phosphorylation suppressed by 10 ng/mL 1NaPP1 for 1 h in primary cortical neurons. (H) Effect of stress (F1, 20 = 30.23, mean ± SEM of six mice per group) and interaction with 25 mM 1NaPP1 treatment via the drinking water from P21 to P31 (F1, 20 = 12.19) on the apical spine density: two-way ANOVA post hoc Sidak test −1NaPP1, *P < 0.0001 and +1NaPP1, P = 0.313. (I) Cumulative size of ∼300 apical spines in six mice per group as a function of CUS and 1NaPP1. Two-way ANOVA for the effect of stress on diameter (F1, 20 = 7.41) and length (F1, 20 = 10.08), and interaction with 1NaPP1 on diameter (F1, 20 = 11.84) and length (F1, 20 = 4.19). Post hoc Tukey’s test: #P = 0.041, *P = 0.0019.
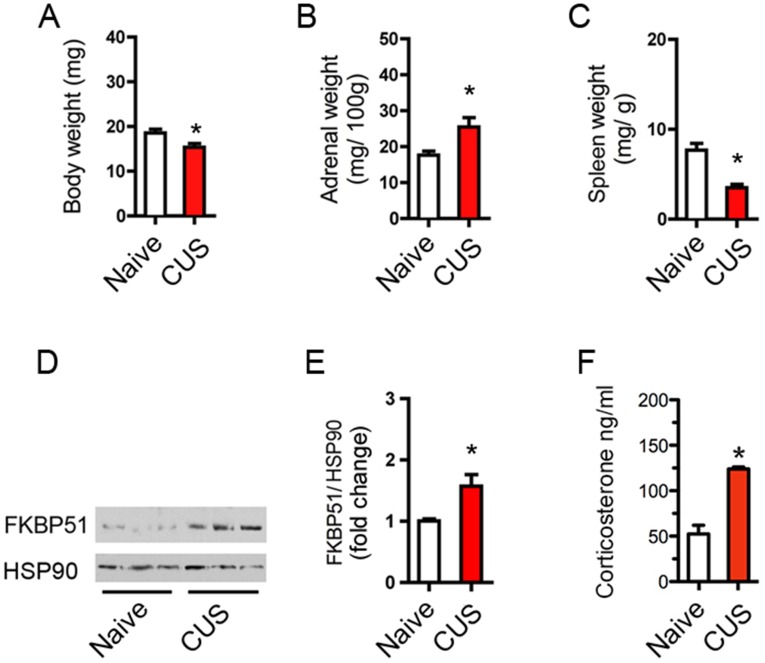
Fig. S7.
Phenotypic analysis of the effects of CUS. (A) Body wet weight is reduced following CUS. Mean ± SEM, n = 5 mice per group, *P = 0.028, t test: vehicle vs. CUS. (B) Adrenal gland wet weight is increased following CUS. Mean ± SEM, n = 6–13 mice per group, *P = 0.046, t test: vehicle vs. CUS. (C) Spleen wet weight is decreased following CUS. Mean ± SEM, n = 3–5 mice group, *P = 0.021, t test: vehicle vs. CUS. (D) Representative FKBP51 and HSP90 Western blots in lysates of the cortex S1 from mice exposed to chronic stress or glucocorticoid administration. Each lane represents individual animals. (E) Quantitative analysis of FKBP51 induction by CUS and glucocorticoids in a larger cohort of animals. Mean ± SEM, n = 6–10 mice per group, *P = 0.018, t test. (F) Plasma corticosterone level following chronic administration or chronic stress. Mean ± SEM. n = 9–11 mice, *P < 0.003, t test compared with vehicle.
Fig. S8.
GR[S155-P] in the cortex S1 of BDNF (A) and TrkB (B) haplo-insufficient mice compared with littermate controls.
To impinge on TrkB-mediated GR phosphorylation in vivo, we used the TrkB(F669A) mutant in combination with 1NaPP1 supplied in the drinking water. To this end, endogenous TrkB was knocked down with a specific shRNA (by 89%, t test P < 0.0001, n = 5) and substituted with the shRNA-resistant TrkB(F669A)-RFP mutant by in utero electroporation of the cortex S1 (Fig. 4F). Importantly, TrkB(F669A) retained full BDNF-signaling capabilities when expressed in cortical neurons, but treatment with 1NaPP1 suppressed its activity and BDNF-induced GR phosphorylation (Fig. 4G). We reasoned that blocking TrkB signaling with 1NaPP1 for 10 consecutive days would recapitulate the effect of GR-3A on dendritic spine formation. Indeed, blocking TrkB reduced the growth of spines at LII/III apical dendrites (density: P = 0.0093, length: P = 0.041, diameter: P = 0.002) while preserving the basal dendrites (density: P = 0.99, length: P = 0.86, diameter: P = 0.66) (Fig. 4 H and I).
We next combined TrkB inactivation with CUS between postnatal day (P) 21 and P31. CUS alone reduced the growth of spines at LII/III apical dendrites (density: P < 0.0001, length: P = 0.0073, diameter: P = 0.0018) while preserving the basal dendrites (density: P = 0.99, length: P = 0.005, diameter: P = 0.83). However, when combined with TrkB deactivation, CUS failed to change the growth of dendritic spines at apical (density: P = 0.67, length: P = 0.85, diameter: P = 0.99) and basal (density: P = 0.89, length: P = 0.99, diameter: P = 0.84) dendrites of LII/III neurons (Fig. 4 H and I). The data indicated that CUS-mediated neuroplasticity corresponded with a reduction of TrkB-mediated GR phosphorylation.
GR-3A Blocked the Neuroplasticity to Stress and Fluoxetine.
To explore the impact of GR phosphorylation on the neuroplasticity to stress, we subjected mice expressing GR-3A to CUS from P21 to P31 (Fig. 5A). We found that CUS produced growth defects of LII/III neurons expressing the GR-WT but no more defects than that already produced by the GR-3A mutant. This was evident on the density (Fig. 5B), diameter (Fig. 5C), and length (Fig. 5D) of apical dendritic spines. Importantly, the GR-3A mutant recapitulated the effects of the GR-2A mutant that preserved S246-P phosphorylation (Fig. 5B and Fig. S9) and the TrkB(F669A) mutant.
Fig. 5.
Priming GR phosphorylation with fluoxetine prevents stress-mediated neuroplasticity. (A) Effect of CUS and GR mutants at LII/III apical dendrites. (B) Effect of CUS (F2, 27 = 26.59, mean ± SEM of five to six mice per group) and interaction with GR mutants (F2, 27 = 13.36) on apical spine density: two-way ANOVA post hoc Sidak test GR-WT, *P < 0.0001, GR-3A, P = 0.5 and GR-2A, P = 0.93. (C) Cumulative diameter of ∼200 spines in five to six mice per group. Two-way ANOVA for effect of CUS (F1, 18 = 38.62) and interaction with GR-3A (F1, 18 = 7.057). Post hoc Sidak test GR-WT, *P < 0.0001. (D) Cumulative length of ∼200 spines in five to six mice per group. Two-way ANOVA for effect of CUS (F1, 18 = 9.621) and interaction with GR-3A (F1, 18 = 2.523). Post hoc Sidak test GR-WT, *P < 0.0077. (E) S155-P in the cortex S1 LII/III of mice exposed to 160 mg/L fluoxetine in the drinking water from P21 to P31 (t test *P = 0.002, mean ± SEM of 10 mice per group). (F) S287-P in the cortex S1 LII/III of mice exposed to 160 mg/L fluoxetine in the drinking water from P21 to P31 with (t test *P = 0.0023, mean ± SEM of 10 mice per group). (G) Interaction of CUS, fluoxetine and GR-3A on apical spines (F2, 24 = 4.73, mean ± SEM of five mice per group). Two-way ANOVA post hoc Tukey’s test indicates an effect of CUS in GR-WT cells, P < 0.0001 and an effect of fluoxetine in GR-WT cells, *P < 0.0083. (H) Cumulative size of ∼250 spines in five mice per group as a function of fluoxetine and GR-3A. Two-way ANOVA for the effect of fluoxetine on diameter (F2, 24 = 29.52, P < 0.0001) and length (F2, 24 = 19.32, P < 0.0001), and interaction with GR-3A on diameter (F2, 24 = 12.56, P = 0.0002) and length (F2, 24 = 8.86, P = 0.0013). (Scale bar, 100 μm.)
Fig. S9.
Substitution of endogenous GR with the GR-2A (S155A/S287A) in vitro. (A) Primary cortical neurons were transduced with lentivirus to express the shRNA against GR together with the cDNA of the GR-2A mutant. Mutation of S155 and S287 to alanine residues decreased GR[S246-P] after stimulation with 1 μM Dex and 50 ng/mL BDNF for the indicated time. (B) Interdependency between GR phospho-sites is evident in the GR-2A mutant. Time course analysis of GR phosphorylation at individual sites in response to cotreatment with 1 μM Dex and 50 ng/mL BDNF. Data (mean ± SEM of three independent experiments at least) are normalized to total GR levels and expressed as fold-change relative to untreated cells. (C) Effect of GR-2A on the maturation of dendritic spine on primary cortical neurons stimulated with 50 ng/mL BDNF, 1 μM Dex for 24 h alone or in combination. GR-2A impeded the growth of cortical neurons when BDNF and Dex stimulations were paired (mean ± SEM of 13 or more neurons per group, t test: untreated vs. Dex †P = 0.0009, untreated vs. BDNF; #P = 0.003, untreated vs. BDNF+Dex; *P = 0.0021, WT vs. 2A **P = 0.0016).
To investigate the role of GR phosphorylation upon neuroplasticity, we investigated the effects of the prototypical antidepressant drug fluoxetine (18). We first tested if a chronic treatment supplied in the drinking water for 10 consecutive days altered GR phosphorylation at BDNF-sensitive sites in the cortex S1. We found that fluoxetine significantly raised S155-P (Fig. 5E) and S287-P (Fig. 5F) in LII/III. We then paired CUS with a prophylactic treatment of fluoxetine in mice electroporated in utero to express the GR-WT or the GR-3A mutant in the cortex S1. We found that fluoxetine prevented CUS-mediated atrophy of apical dendritic spines in GR-WT expressing neurons (Fig. 5 G and H). In contrast, fluoxetine had no effects on GR-3A–expressing cells, suggesting that GR phosphorylation at BDNF-sensitive sites is necessary to counteract the neuroplasticity of CUS and to promote the neuroplasticity of fluoxetine.
Discussion
We discovered posttranslational modifications of GR that provide specific functional attributes when TrkB and GR signaling were paired. Physiological conditions that unpaired BDNF and glucocorticoid signaling like CUS recapitulated the effects of GR-3A, a BDNF-insensitive phospho-deficient GR mutant, as well as TrkB(F669A), a drug-inactivable TrkB mutant. In contrast, priming GR phosphorylation with a chronic treatment of fluoxetine prevented the plasticity of cortical neurons to CUS. Our study indicates that BDNF has permissive roles on GR responses in the cortex, and perhaps in the hippocampus and hypothalamus, where phospho-TrkB and phospho-GR are coexpressed. The coincidence of BDNF and glucocorticoids is necessary to activate genomic GR responses because GR did not translocate into the nucleus of neurons stimulated with BDNF only.
Glucocorticoid-independent GR phosphorylation at S155 was previously identified in response to oxidative stress, but the endogenous molecular trigger was unknown (19). We found that BDNF triggered GR phosphorylation at S155, as well as S287 and S246, via TrkB signaling. However, significant residual levels of S246-P remained after TrkB inhibition, indicating that other pathways, most notably glucocorticoids, phosphorylate S246-P (Fig. S4). Glucocorticoids allowed the detachment of PP5 from GR complexes, permitting GR phosphorylation by upstream kinases. MAPKs were previously characterized as GR kinases (20, 21) because they bind to GR complexes and are activated by BDNF signaling. We speculate that MAPK activation at the time of PP5 deactivation could explain why the paired stimulation of cortical neurons with BDNF and Dex resulted in the prolonged phosphorylation of GR at S287 and S246 compared with individual treatments (Fig. S4). One confound of the GR-3A mutant is that S246-P is lacking when only part of it should be impeded by a reduction of BDNF signaling. To solve this issue, we generated a GR-2A mutant (S155A/S287A). GR-2A recapitulated the effect of TrkB inhibition by reducing S246-P while preserving the other sites (Fig. S9 A and B) and GR-2A mimicked the effects of GR-3A in vitro (Fig. S9C) and in vivo (Fig. 5B), thus confirming that the disruption of GR phosphorylation at BDNF-sensitive sites impairs neuronal plasticity to CUS.
TrkB-mediated GR phosphorylation fosters cofactor recruitment and changes the transcription of specific target genes involved with neuronal plasticity. Numerous neuronal genes respond to BDNF and glucocorticoids (12), and some depend on GR phosphorylation at the three BDNF-sensitive sites. These genes acted on the maturation of cortical neurons when BDNF and GR signaling were paired because dendritic morphogenesis was blocked by the GR-3A or by actinomycin D. We propose that GR phosphorylation at BDNF-sensitive sites could serve as docking sites for specific cofactors like CREB (12). Accordingly, these genes presented with one or more CREB- and GR-responsive elements in their promoters. Alternatively, the epigenetic priming of target loci could explain how the other GR-regulated genes sensitive to BDNF signaling but independent of GR (S155, S287, S246) are regulated (22). Given that BDNF facilitates gene transcription by dismissing histone deacetylase 2 from chromatin (23), reduced BDNF signaling could promote glucocorticoid resistance through changes in histone deacetylase 2-mediated histone acetylation, as suggested in neuropsychiatric stress disorders (24, 25).
Disruption of TrkB signaling or GR phosphorylation recapitulated the atrophy of cortical excitatory neurons mediated by CUS. Similar effects are anticipated in parvalbumin cortical interneurons where GR phosphorylation at BDNF-sensitive sites was also detected. At this stage, it is not understood how disrupted GR phosphorylation impaired the growth of the apical dendrites while preserving the basal. However, similar discrepancies were previously reported in BDNF heterozygous mice subjected to chronic stress (11). In contrast, chronic treatment with fluoxetine, which promotes and requires BDNF/TrkB signaling (18, 26), increased GR phosphorylation and neuronal growth in the cortex. Unfortunately, we could not assess the behavioral effects of disrupted GR phosphorylation at BDNF-sensitive sites because the number of cells targeted by electroporation was low and unilateral. Future use of virus-assisted gene delivery or knockin mouse models will help address this important point. Our findings provide a new framework for understanding how unpaired BDNF and glucocorticoid activities may set the stage for the development of psychiatric disorders comorbid with stress.
Methods
Reagents Ligands.
BDNF (Preprotech), dexamethasone (Sigma). Inhibitors: calyculin A (Cell Signaling), K252a (Calbiochem), roscovitine, H-89, U0126, SP600125, LY294002, KN93 and actinomycin D (Sigma). Antibodies: mouse monoclonal GR BuGr2 (Calbiochem), rabbit polyclonal GR M20 (Santa Cruz Biotechnology), PP1a, PP5 (Cell Signaling), GFAP (Chemicon), parvalbumin and calbindin D28K (Sigma), GFP (Abcam), RFP (Rockland), PP2a, HSP90 (BD Bioscience), TrkB (Millipore), GAPDH (Biodesign). Custom-made rabbit polyclonal p-TrkB (Y816-P) and p-GR (S232-P, S224-P, S246-P, S155-P, and S287-P) were described previously (12, 27).
Animals.
Time-pregnant Sprague-Dawley rats, CD1 mice (Janvier Labs), TrkB knockouts mice, BDNF knockouts and YFP-H mouse line expressing YFP in pyramidal cells predominantly in cortical layer V (Jackson Laboratories) were allowed ad libitum access to food and water and maintained on a 12-h light-dark cycle. Chronic unpredictable stress includes one of the following daily random stressors (wet bedding, no bedding, food deprivation, crowded cage, 2 h or 6 h restraining, forced swim, tail suspension) for 10 consecutive days. All protocols complied with the European Communities Council Directive (86/609/EEC) and approved by the Ministere de la Recherche Française (Agreement no. CEEA-LR-00651.01).
Histochemistry.
Brains perfused with 4% PFA and postfixed 2 h were equilibrated in 30% (wt/vol) sucrose. Free-floating coronal sections rinsed in PBS were blocked in 5% (vol/vol) normal goat serum, PBS, 0.1% triton X-100 for 2 h at 25 °C. Primary antibodies (S287-P 1.5 μg/mL; S155-P 1.5 μg/mL; S246-P 1 μg/mL; GR 1:100; GFP 1:2,000; RFP 1:1,000) were incubated for 2 d. AlexA secondary antibodies (Molecular Probes; 1:1,000) were incubated for 2 h at 25 °C. Epitope unmasking to retrieve S155-P and S287-P consisted in 10 mM Tris, 1 mM EDTA, 0.05% Tween, pH = 9 at 95 °C for 20 min. Fifty micrograms of pTrkB antibodies were labeled with AlexA555 (Molecular Probes) and costained with pGR antibodies.
Dendritic Spine Imaging.
Fluorescence images were taken on a LSM510 laser-scanning confocal microscope (Carl Zeiss) equipped with 63× Plan Neofluor NA1.3 oil-immersion objective and digital zoom 8. Z-stack images were processed using ZEN software and ImageJ (NIH). Laser excitation, fluorescence-emission capture, and pinhole were held constant throughout the study. Dendritic segments included in the analyses met the following criteria: (i) be parallel or at acute angles relative to the coronal surface of sections to allow unambiguous identification of spines; (ii) segments had no overlap with other branches; (iii), dendritic segments from apical tree were imaged within the first 100 μm from the pial surface; and (iv) dendritic segments from the basal tree were captured between ∼50- and 150-μm distance from the soma.
DNA Transfections.
In utero electroporation (30 v, pON 50 ms, pOFF 950 ms, five pulses, 1 μg DNA) was performed at embryonic day 15–15.5 on mouse embryos and newborns developed for 1 mo. In vitro electroporation of plasmids were performed with the AMAXA system. Transfections of primary neurons and 293 cells were performed with Lipofectamine 2000 (Invitrogen).
Statistics.
Optical densities of Western blots bands were measured with NIH imageJ and subtracted of background. About 200 dendritic spines from at least 20–30 dendritic segments were counted per conditions. No more than two dendritic segments per cell were scored and averaged per animal. Pairs of data were analyzed using the Student’s t test and multiple comparison tests with two-way ANOVA, post hoc Tukey’s or Sidak’s tests (GraphPad Prism).
See SI Methods for details of other procedures.
SI Methods
Short-Hairpin Sequence and Mutagenesis.
The scramble and shRNA against mouse/rat GR cloned into the pLentiLox3.7 vector (ATCC) were previously validated (2, 27). To construct the molecular replacement vectors, the sequence containing the U6 promoter and shRNA were excised from the pLL3.7 plasmid and pasted into the MluI site of the FCIV1 lentiviral vector recombinant for either shRNA-resistant GR-WT or mutant. The following mutations were introduced in the GR sequence to create the shRNA-resistant GR: G746C, A749G, A753G, T756C, T759C, A762C, C765T. The ShRNA plasmids against mouse/rat TrkB was obtained from M. Sendtner, Institute for Clinical Neurobiology, Wuerzburg, Germany. The following mutations were introduced in the TrkB sequence to create the shRNA-resistant TrkB: T300C, T301A, C302G, C306A, T307C. Site-directed mutagenesis was performed using Quickchange (Agilent). The following shRNA sequences against phosphatases were cloned into the PLL3.7 plasmid: PP5 5′-aagaagtacatcaaaggttac-3′; PP1α 5′-gcttgttgctggcctataaga-3′; PP1β 5′-gcctatagctgcrattgttga-3′; PP1γ 5′-ggtggttgaagatggatatga-3′; PP2A 5′-tgtgtgacttgctgtggtc-3′. All constructs were verified by sequencing.
Nuclear Fractionation.
To purify nuclear extracts, neurons were rinsed in ice-cold PBS and incubated for 15 min on ice with buffer A (10 mM Hepes-KOH, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT with protease and phosphatase inhibitors) before harvesting. Next, 0.5% Nonidet P-40 was added to the cells for 3 min on ice and lysates were centrifuged for 1 min at 15,000 × g. The resulting supernatant was stored as the cytoplasmic fraction and the pellet was further rinsed in 1 mL of buffer A. The pellet was vortexed for 20 min in 50 mL buffer B (20 mM Hepes-NaOH, pH 7.9, 0.4 mM NaCl, 1 mM EDTA, 1 mM EGTA with protease and phosphatase inhibitors) and cleared by centrifugation for 10 min at 15,000 × g. The resulting supernatant was collected as the nuclear fraction.
ChIP were performed on primary cortical neurons infected with the molecular replacement lentivirus GR-WT or GR-3A. Protein–DNA complexes were cross-linked by incubating cells in 1% formaldehyde for 10 min at 25 °C, followed by incubation in 0.125 M glycine for 5 min to quench the cross-linking reaction. Cells were washed with PBS twice and lysed in Farnham lysis buffer (5 mM Pipes at pH 8.0, 85 mM KCl, 0.5% Nonidet P-40) with added protease inhibitor (Roche). The cell lysate was then centrifuged at 600 × g for 5 min at 4 °C, and the crude nuclear pellets were collected. Chromatin was sonicated using the Bioruptor (Diagenode; Twin UCD-400) into 500-bp fragments. Immunoprecipitation of GR was performed with 6 μg of a GR antibody mixture: MA1-510 (Thermo Scientific), PA1-511A (Thermo Scientific), and H-300 (Santa Cruz Biotechnology) or 3 μg of anti-CREB (48H2, Cell Signaling) or an equivalent amount of rabbit IgG (Sigma-Aldrich) overnight at 4 °C. After incubation with protein A magnetic beads (Invitrogen), the beads were washed with LiCl wash buffer (100 mM Tris at pH 7.5, 500 mM LiCl, 1% Nonidet P-40, 1% sodium deoxycholate) and TE (10 mM Tris⋅HCl at pH 7.5, 0.1 mM Na2EDTA) at 4 °C. Following reversal of cross-linking, recovered DNA was purified using PrepEase DNA Clean-Up Kit (USB). Cycle threshold values were normalized to % input and IgG.
Quantitative Real-Time PCR.
Total RNA from primary neurons was extracted with TRIzol (Invitrogen) and cDNA was synthesized from 1 μg of RNA using the first-strand cDNA Synthesis Kit for Real-Time PCR (USB) and random primer mix (USB). Hot-start SYBR Green Taq Ready Mix (USB) was used in 16-μL reactions containing 1 μL of cDNA, and 1 μL of 5 pmol/μL−1 primer mixture. Primers are as follows: myom2 5′-gcaagtaccggattgagagc-3′ and 5′-atggggattcgttgctactg-3′; prl3c1 5′-gttctcatcgcacccatgta-3′ and 5′-tagaagtggtgtggcaggtg-3′; nr4a1 5′-ggcatggtgaaggaagttgt-3′ and 5′-gggacgtgaggagattggta-3′; agtr1a 5′-gagaggattcgtggcttgag-3′ and 5′-actcagggaatgtggcagag-3′; srgn 5′-gcattgaggagaaaggacca-3′ and 5′-ccggagccagaatagtcatc-3′; FKBP51 5′-GAACCCAATGCTGAGCTTATG-3′ and 5′-ATGTACTTGCCTCCCTTGAAG-3′; Dusp-1 5′-aggacaaccacaaggcagac-3′ and 5′-cacaaacacccttcctccag-3′; crh 5′-agagaaaggggaaaggcaaa-3′ and 5′-gtttaggggcgctcttcttc-3′; RPL19 5′-CACAAGCTGAAGGCAGACAA-3′ and 5′-GCGTGCTTCCTTGGTCTTAG-3′. qPCR was performed with MyiQ single-Color Real-Time PCR detection system (Bio-Rad), followed by melt-curve analysis. Fold-changes in gene expression (vehicle vs. BDNF + Dex stimulation for 3 h) were calculated using ΔΔCt (Ct = cycle number at threshold) analytical method that includes normalization against housekeeping gene RPL19 (encoding 60S ribosomal protein).
Virus.
Viruses were produced 293 FT cells by cotransfecting expression and packaging plasmids (Δ8.9, pCMV-VSVG, FCIV1 for overexpression, and PLL3.7, PLP1, PLP2, PLP-VSVG for knockdown). Media were collected after 48 h and diluted 1:4 with regular culture medium to infect cells at day in vitro (DIV) 0. Lentiviruses to express MKP1 were previously published (16). MKP1-ASA mutant replaced the triplet arginine motif (Arg53-Arg54-Arg55) to the Ala53-Ser54-Ala55 to prevent binding with Erk1/2 and P38 while preserving binding to JNK. The MKP1-CS mutant changed the cysteine 258 to a serine residue that impeded phosphatase activity.
Cell Culture and Lysis.
Primary E18 cortical neurons were prepared from time-pregnant Sprague-Dawley rats, cultured on glass coverslips coated with poly-d-lysine, and maintained in Neurobasal medium containing B27 supplement, 0.5 mM l-glutamine, 5-fluorouridine, and uridine (10 μM each). To reduce baseline GR phosphorylation, neurons were starved from B27 for 5 h. 293FT (Invitrogen) were grown in DMEM containing 10% (vol/vol) FBS plus 200 μg/mL G418. Cells lysates were prepared in 10 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 10% (vol/vol) glycerol, 1% Nonidet P-40, 0.1% SDS plus protease inhibitors, 1 mM Na3VO4, 10 nM calyculin A and 10 mM NAF and cleared (16,000 × g for 10 min). For biochemical analyses, primary cortical cultures were used at DIV7 to -10 and DIV21 for morphometric analysis of dendritic spines. To immunoprecipitate GR with kinases and phosphatases, we added 20 mM NA-molybdate to the lysis buffer because it stabilizes GR-protein complexes.
Cytochemistry.
Cultured cells were fixed in 4% (wt/vol) PFA, 20% (wt/vol) sucrose in PBS for 10 min at 25 °C. After quenching the fixative with 50 mM NH4Cl in PBS, cells were permeabilized with 0.1% triton X-100 in PBS for 3 min, blocked with 5% (vol/vol) goat serum, 2% (vol/vol) BSA and 0.25% fish skin gelatin in TBS for 30 min and then incubated with antibodies (GFP 1:3,000; RFP 1:2,000; secondary antibodies 1:2,000) for 3 h in blocking solution at room temperature. Cells were washed in TBS, 0.25% fish skin gelatin and mounted in mowiol488.
Corticosterone ELISA.
To monitor plasma level of corticosterone by ELISA according to the manufacturer’s instructions (AssayPro), trunk blood was collected in the afternoon in EDTA-coated tubes (BD Vacutainer) after decapitation.
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale Grant AVENIR R12087FS (to F.J.) and National Institutes of Health Grant MH086651 (to M.V.C. and M.J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 15544.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509045112/-/DCSupplemental.
References
- 1.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liston C, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16(6):698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pariante CM. The glucocorticoid receptor: Part of the solution or part of the problem? J Psychopharmacol. 2006;20(4) Suppl:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- 4.Holsboer F, Ising M. Stress hormone regulation: Biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109, C1–11. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- 5.Menke A, et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37(6):1455–1464. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7(9):985–994, 924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- 7.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173–195. doi: 10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revest JM, et al. BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol Psychiatry. 2014;19(9):1001–1009. doi: 10.1038/mp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15(12):1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magariños AM, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21(3):253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert WM, et al. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol Cell Biol. 2013;33(18):3700–3714. doi: 10.1128/MCB.00150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46(1):13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 15.Lai KO, et al. TrkB phosphorylation by Cdk5 is required for activity-dependent structural plasticity and spatial memory. Nat Neurosci. 2012;15(11):1506–1515. doi: 10.1038/nn.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13(11):1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray JD, Milner TA, McEwen BS. Dynamic plasticity: The role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70(5):289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 19.Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011;31(23):4663–4675. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galliher-Beckley AJ, Cidlowski JA. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61(10):979–986. doi: 10.1002/iub.245. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21(3):625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- 22.Gräff J, Tsai LH. Histone acetylation: Molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14(2):97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- 23.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455(7211):411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 24.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida S, et al. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69(2):359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Blugeot A, et al. Vulnerability to depression: From brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31(36):12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA. 2008;105(12):4862–4867. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]