The interaction between neurotrophins and glucocorticoids has been at the center of studies of neuroplasticity in stress-related disorders for several decades (1). The report in PNAS by Arango-Lievano et al. (2) shows that brain-derived neurotrophic factor (BDNF) facilitates glucocorticoid receptor (GR)-mediated signaling and enables glucocorticoids, primed by BDNF, to activate gene transcription and possibly other cellular actions mediated by the GR. As a first step, according to Arango-Lievano et al., glucocorticoids allow the detachment of the protein phosphatase, PP5, from GR complexes, permitting increased GR phosphorylation by MAP kinases. Then, the coincidence of BDNF and glucocorticoids is necessary to activate genomic GR responses because GR is not translocated into the nucleus of neurons stimulated only with BDNF, without the glucocorticoid being present.
The Arango-Lievano et al. (2) paper “closes a loop” that was begun by a previous study by Jeanneteau, Garabedian, and Chao (3), showing that glucocorticoids can selectively activate tropomyosin receptor kinase B (TrkB) after in vivo administration in the brain and in cultures of hippocampal and cortical neurons. This activation did not require increased production of BDNF and was dependent on the genomic action of the GR. Thus, there is a positive feedback loop in which glucocorticoids and BDNF synergistically potentiate each other’s concurrent actions, and Arango-Lievano et al. (2) suggest that failure of this loop underlies loss of plasticity and resilience in mood-related disorders, and possibly also the lack of response to antidepressant medications (Fig. 1). Taken together with other recent work on hormone action, this paper illustrates a number of important emerging themes concerning the positive and modulatory actions of glucocorticoids, as well as other steroid hormones, together with other signaling pathways, on a large number of important cellular processes in neural cells and the capacity of the adult, as well as the developing brain, for adaptive plasticity and resilience in the face of stress.
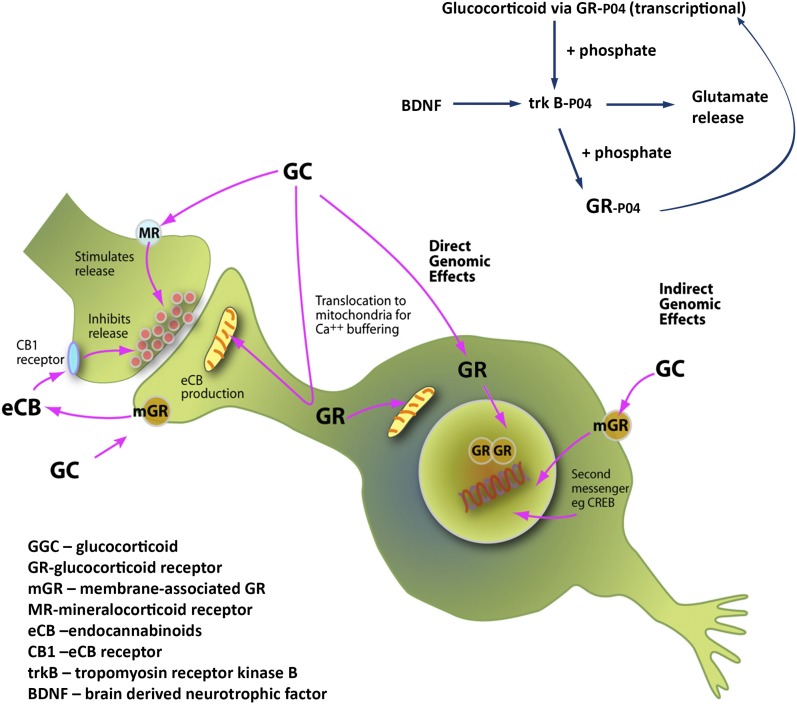
Fig. 1.
Glucocorticoids produce direct and indirect genomic actions as well as nongenomic signaling actions via glucocorticoid (GR) and mineralocorticoid (MR) receptors. These involve not only direct and indirect genomic actions, but also direct stimulation of glutamate release and stimulation of endocannabinoid production, which then feed back on glutamate and GABA release and actions in mitochondria to affect Ca++ buffering and free radical formation (9, 10) (Upper Right). As shown in the article by Arango-Lievano et al. (2), BDNF, in the presence of glucocorticoids, phosphorylates the GR at sites that facilitate its translocation to the cell nucleus for transcriptional actions; this effect is synergistic with the ability of glucocorticoids to activate, via a genomic mechanism, the phosphorylation of the TrkB receptor independently of BDNF, thus creating a positive feedback loop.
The first theme is that we now recognize interconnections among steroid hormone actions on gene expression with other actions of these same hormones, as well as other modulators, that involve cellular signaling pathways at or near the cell surface. In the 1960s, steroid hormone receptors were recognized that regulate gene expression in the cell nucleus (4), and neurotransmitters and neuromodulators were shown to operate at or near the cell surface to regulate signaling pathways in which phosphorylation was a key element (5). However, we know now that nuclear and cellular signaling pathways interact in many ways. For example, dopamine activates progestin receptors in a ligand-independent manner and facilitates estrogen-primed lordosis behavior in the female rat in the absence of progesterone (6). Moreover, estrogen-induced spine synapse formation in female rat hippocampus is mediated in part by estrogen receptors in dendrites via nongenomic signaling involving phosphorylation of LIM kinase 1 and AKT, leading to filopodial formation and the translation of PSD95 mRNA (7, 8).
Indeed, glucocorticoid actions also involve multiple mechanisms, besides direct and indirect regulation of gene expression, from directly stimulating glutamate release to indirectly inhibiting it via stimulation of endocannabinoid secretion and regulation of calcium sequestration and free radical formation via the mitochondria (9, 10) (Fig. 1). Arango-Lievano et al. (2) note in their paper another example of rapid glucocorticoid actions from the work of Liston and Gan, with their colleagues at New York University, on the role of ultradian and circadian variations in glucocorticoids and their role in promoting rapid spine formation in cerebral cortex neurons through a nontranscriptional mechanism, and increased spine elimination through transcriptional mechanisms (11, 12). Arango-Lievano et al. (2) also note the important phenomenon of glucocorticoid resistance (13, 14), wherein they suggest that failure of the BDNF-dependent phosphorylation of the GR may play a role in reducing GR actions at the nuclear level.
A second important theme of the merging of hormone action and cellular signaling are the biphasic effects that are dependent on dose and timing of glucocorticoids. Positive effects of glucocorticoids on cellular function range from enhancement of adaptive immune function by acute stress, to promoting Ca++ buffering by mitochondria, as well as the enhancement of memory related to danger; glucocorticoids also protect against posttraumatic stress disorder-like symptoms, whereas chronic stress and high and chronic doses of glucocorticoids are associated with immune suppression, anti-inflammatory actions, metabolic dysregulation, cognitive impairment, and neuronal atrophy (15–19). The most efficient form of adaptation to acute stress is turning on the hypothalamic-pituitary-adrenal, autonomic, immune, and metabolic responses robustly and efficiently, and turning them off again when the stress is passed (20). Indeed, the ability to mount a successful adrenocorticotropic hormone (ACTH) response to an acute stressor is dependent on a robust circadian variation of ACTH and glucocorticoida, whereas a flat glucocorticoid circadian variation leads to a blunted ACTH stress response (21, 22).
Furthermore, chronic glucocorticoid effects related to BDNF signaling are quite different from acute actions. A study by Numakawa et al. showed that chronic glucocorticoid exposure led to a down-regulation of a BDNF link to glutamate release mediated by phospholipase C (PLC)-γ (23). In particular, Numakawa et al. found that chronic dexamethasone or corticosterone treatment in vitro reduced TrkB–GR interaction, BDNF-stimulated PLC-γ, and BDNF-triggered glutamate release. Moreover, BDNF-dependent binding of PLC-γ to TrkB was diminished by chronic glucocorticoid treatment. These authors concluded that a decrease in TrkB–GR interactions caused by chronic exposure to glucocorticoids results in the suppression of BDNF-mediated neurotransmitter release via a glutamate transporter.
A third theme is that glutamate release and actions, along with glucocorticoids and other interacting mediators, play an essential role in adaptive plasticity of neurons to stressors, where there is resilience in a healthy brain (9, 10). However, a deficiency in BDNF leads to a loss of the normal responsiveness to stressors and to some antidepressant drugs (24, 25). By the same token, depression may be characterized as a loss of resilience to the adaptive effects of stressors, and Arango-Lievano et al. (2) show that fluoxetine significantly raised GR phosphorylation in wild-type mice but failed to do so in mice electroporated in utero to express a GR mutant in the cortex that does not show BDNF-induced GR phosphorylation. Moreover, whereas fluoxetine prevented chronic unpredictable stress-mediated atrophy of apical dendritic spines in GR wild-type–expressing neurons, it had no effect on GR mutant-expressing cells, leading to the conclusion that GR phosphorylation at BDNF-sensitive sites is necessary to counteract the structural remodeling caused by chronic unpredictable stress and to promote the neuroplasticity facilitated by fluoxetine.
Thus, the paper by Arango-Lievano et al. (2) not only further integrates actions of steroid hormones, neurotrophic factors like BDNF, and cellular signaling mechanisms, and shows an important example of the positive, adaptive role of glucocorticoids, it also highlights the capacity of the brain for adaptive plasticity and resilience that is so important in responding to stressors, and which is limited in stress-related psychiatric disorders.
Footnotes
The author declares no conflict of interest.
See companion article on page 15737.
References
- 1.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arango-Lievano M, et al. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc Natl Acad Sci USA. 2015;112:15737–15742. doi: 10.1073/pnas.1509045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA. 2008;105(12):4862–4867. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen E, Jacobson H. Basic guides to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414. [Google Scholar]
- 5.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294(5544):1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 6.Mani SK, Allen JMC, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265(5176):1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 7.Yuen GS, McEwen BS, Akama KT. LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res. 2011;1379:44–52. doi: 10.1016/j.brainres.2010.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23(6):2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA. 2011;108(38):16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liston C, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16(6):698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szappanos A, et al. Tissue-specific glucocorticoid signaling may determine the resistance against glucocorticoids in autoimmune diseases. Curr Med Chem. 2015;22(9):1126–1135. [PubMed] [Google Scholar]
- 14.Quan N, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137(1-2):51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 16.Rao RP, Anilkumar S, McEwen BS, Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72(6):466–475. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zohar J, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21(11):796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Schelling G, Roozendaal B, De Quervain DJ-F. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann N Y Acad Sci. 2004;1032:158–166. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- 19.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells—From barracks to boulevards to battlefields: A tale of three hormones—Curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 21.Akana SF, Jacobson L, Cascio CS, Shinsako J, Dallman MF. Constant corticosterone replacement normalizes basal adrenocorticotropin (ACTH) but permits sustained ACTH hypersecretion after stress in adrenalectomized rats. Endocrinology. 1988;122(4):1337–1342. doi: 10.1210/endo-122-4-1337. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122(4):1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 23.Numakawa T, et al. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci USA. 2009;106(2):647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z-Y, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magariños AM, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21(3):253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]