Significance
Circadian rhythms are common to most eukaryotes, and, in humans, circadian dysfunction is associated with sleep disorders, elevated incidence of cancer, and metabolic abnormalities. The biological oscillators giving rise to fungal and animal clocks share a common regulatory architecture, as well as some highly conserved components, many of which were identified in genetic screens. Here a gene, period-1 (prd-1), previously identified in a genetic screen in the filamentous fungus Neurospora, is shown to encode a protein (an ATP-dependent RNA helicase) that is closely related to yeast and human proteins involved in RNA metabolism and transcription. The cycle length in prd-1 mutants is only abnormal when available glucose is elevated, suggesting that normal clock regulation in response to nutrition is altered.
Keywords: circadian, FRQ, RNA helicase, DDX5, Dbp2p
Abstract
Mutants in the period-1 (prd-1) gene, characterized by a recessive allele, display a reduced growth rate and period lengthening of the developmental cycle controlled by the circadian clock. We refined the genetic location of prd-1 and used whole genome sequencing to find the mutation defining it, confirming the identity of prd-1 by rescuing the mutant circadian phenotype via transformation. PRD-1 is an RNA helicase whose orthologs, DDX5 [DEAD (Asp-Glu-Ala-Asp) Box Helicase 5] and DDX17 in humans and DBP2 (Dead Box Protein 2) in yeast, are implicated in various processes, including transcriptional regulation, elongation, and termination, ribosome biogenesis, and mRNA decay. Although prd-1 mutants display a long period (∼25 h) circadian developmental cycle, they interestingly display a WT period when the core circadian oscillator is tracked using a frq-luciferase transcriptional fusion under conditions of limiting nutritional carbon; the core oscillator in the prd-1 mutant strain runs with a long period under glucose-sufficient conditions. Thus, PRD-1 clearly impacts the circadian oscillator and is not only part of a metabolic oscillator ancillary to the core clock. PRD-1 is an essential protein, and its expression is neither light-regulated nor clock-regulated. However, it is transiently induced by glucose; in the presence of sufficient glucose, PRD-1 is in the nucleus until glucose runs out, which elicits its disappearance from the nucleus. Because circadian period length is carbon concentration-dependent, prd-1 may be formally viewed as a clock mutant with defective nutritional compensation of circadian period length.
The successful dissection of the molecular bases of circadian rhythms by the circadian community over the past three decades has been anchored, in every system from cyanobacteria to mammals, on the products of classical genetic screens for, and analysis of, circadian clock mutants, their molecular cloning, and the conservation of their function. Circadian period or expression mutants have been identified in a variety of organisms, including Neurospora, Drosophila, blowflies, Paramecium, Chlamydomonas, Arabidopsis, Synechococcus, hamsters, mice, and humans (reviewed in refs. 1–3). Among these circadian clock gene mutants, the greatest number in a single system have come from screens in Neurospora, where upwards of a dozen different genes have emerged from unbiased screens for genes informative of the circadian system. The protein products and cellular functions of nearly all of these genes are known, and this knowledge has played a central role in elucidation of the transcription/translation feedback loop model for animal and fungal clocks that guides most research on mammalian clock mechanism (reviewed, e.g., in refs. 4 and 5). Among these Neurospora genes, the product and role of the period-1 (prd-1) gene remains undescribed.
The prd-1 gene [originally called frq-5 (frq, frequency) and later prd] was isolated in a UV-mutagenesis screen for period length mutants (6). On race tubes, the canonical mutant displayed a long (∼25-h) period length in the circadian conidiation rhythm and a growth rate on race tubes about 60% that of WT. Genetic mapping placed it near to the centromere on LGIII (7), proximal to pro-1 and close to acr-2 (acriflavine resistance-2), and thus quite near to the centromere and rendering it difficult to reach via a chromosome walk (8). However, with the well-annotated archival Neurospora genome sequence (9, 10) and the availability of affordable next generation sequencing, the molecular basis of mutations can now be determined by comparing the reference genome with genomic sequences from the appropriate genetic region of strains bearing the mutation (e.g., ref. 11).
We used this approach to determine the identity of prd-1. It encodes an ATP-dependent RNA helicase, a member of a subfamily that is highly conserved from yeast to humans and that is involved in a wide variety of cellular processes, including RNA processing, transcriptional regulation, and transcriptional elongation and termination (reviewed in refs. 12 and 13). Adding to the interest, an ortholog of PRD-1, DDX5 [DEAD (Asp-Glu-Ala-Asp) Box Helicase 5], is found in the complex of proteins constituting the negative arm of the mammalian circadian feedback loop (14).
Results
prd-1 Encodes a Highly Conserved RNA Helicase.
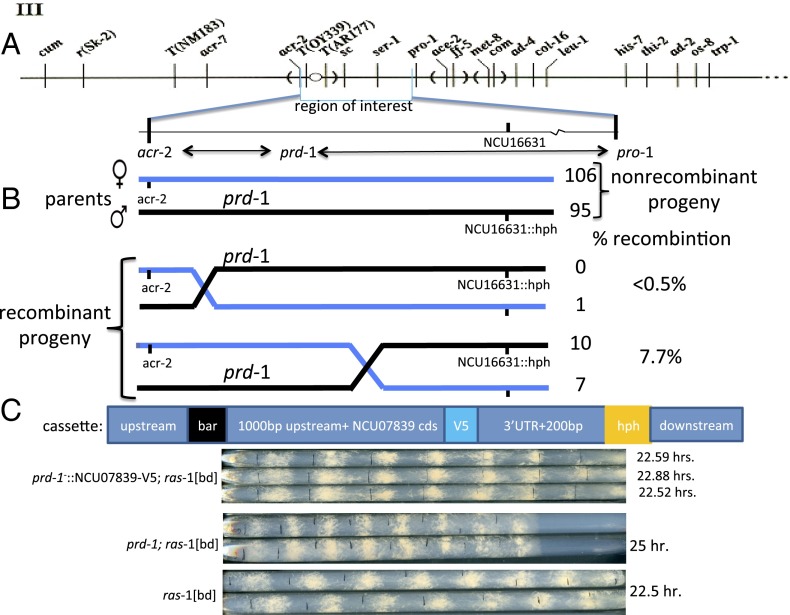
To refine the map location, we crossed prd-1; ras-1[bd] (period-1; Ras-like protein-1 [band allele]) to a hygromycin phosphotransferase (hph)-replacement mutant of gene NCU16631 [∆NCU16631:: hph, generated in the Neurospora genome project (15), chosen here because of its proximity to the reported location of prd-1]. A prd-1; ras-1[bd]; ∆NCU16631:: hph progeny was selected and crossed to acr-2; ras-1[bd], and recombination frequencies (17/219) placed prd-1 7.8 map units from NCU16631 and (1/219) within ∼0.5 map units of acr-2 (Fig. 1A). Genomic DNAs from the seven recombinant prd-1 progeny (i.e., prd-1; acr-2+; NCU16631+) and from one of the recombinant acr-2; ∆NCU16631 progeny (prd-1+; acr-2resistant; ∆NCU16631::hph) were sequenced and analyzed to identify SNPs, insertions, and deletions (Materials and Methods). Eighty-one sequence variants were detected within the genomic region of interest in all seven prd-1 recombinants compared with the WT reference sequence and the single prd-1+ sequenced strain; 79 were intergenic, 1 was in the promoter of a gene already known not to affect circadian period length (pan-3, NCU07836), and 2 G-to-A transitions were found at/near the splice donor site of the third intron in NCU07839. A gene-replacement cassette was constructed (Fig. 1C) containing the WT NCU07839 coding sequence (V5 tagged at the C terminus) flanked by promoter and 3′ UTR sequences and selectable markers, and this cassette was introduced into a prd-1−,ras-1[bd]; Δmus51::barR strain (mus, mutagen sensitive). Primary transformants rendered homokaryotic by crossing to ras-1[bd] displayed a WT period length and growth rate (Fig. 1C), and Western blotting using an anti-V5 antibody confirmed the correct gene replacement and expression of WT NCU7839 in each of the progeny. We conclude that NCU07839 is the prd-1 gene.
Fig. 1.
Genetic mapping and rescue confirming that the prd-1 gene is NCU07839. (A) The genetic map location of prd-1 on linkage group III near to the centromere (31). (B) Genetic outcome of three point mapping cross between parental strains with chromosomes marked blue and black. Numbers of progeny and rates of recombination are noted; no double cross-over events were achieved. (C) Cassette that was transformed into prd-1; ras-1[bd]; mus-51::bar+ background to replace prd-1 with NCU07839, and race tube confirmation of WT period length and phenotype in the transformant compared with prd-1; ras-1[bd] and ras-1[bd] controls. Three independent progeny are shown.
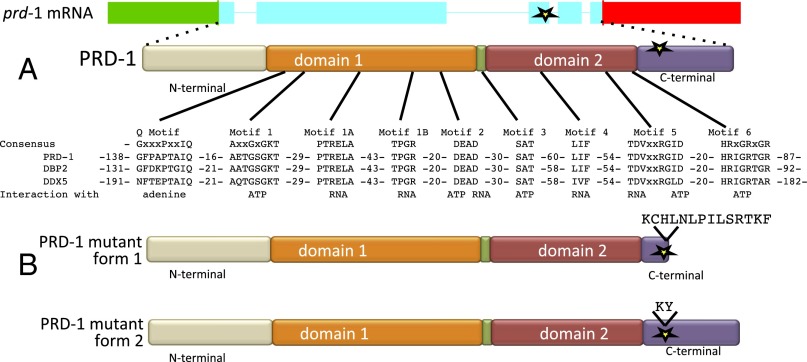
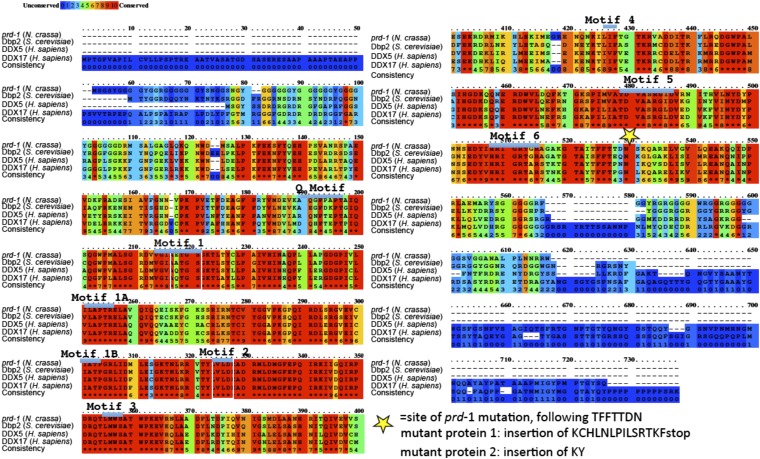
Analysis of cDNAs shows that the prd-1 gene is transcribed with a 704-base 5′ untranslated region followed by a coding region interrupted by four introns and ending with an 882-base 3′ UTR (Fig. 2A). The PRD-1 protein is a DEAD-box protein, bearing all of the conserved hallmarks of a family of proteins characterized by ATPase and helicase activity in vitro, and is an ortholog of Saccharomyces cerevisiae DBP2 (Dead Box Protein 2) (16–18) and two human proteins, DDX5 and DDX17 (59% and 66% identical, respectively; BLAST scores of e-0) (Fig. S1).
Fig. 2.
The prd-1 mutation results in a splicing defect that alters the amino acid sequence in the C-terminal region of a highly conserved DEAD-box helicase. (A) Structure of the prd-1 transcript showing 5′ (green) and 3′ (red) untranslated regions and 5 exons and the PRD-1 protein with conserved domains and motifs marked (32, 33). The location of the mutation in the canonical prd-1 mutant is marked with a star. Image courtesy of the Neurospora crassa Sequencing Project, Broad Institute of Harvard and MIT (www.broadinstitute.org/annotation/genome/neurospora). (B) The two mutant forms of PRD-1 that result from the paired G-to-A mutations in the prd-1 mutant are shown. Form 1 was anticipated and results from loss of splicing of intron 3 and the intron being read through to a STOP codon. Form 2 was unexpected, with just the first six bases into the third intron being included before the remaining sequence is spliced out, resulting in the insertion of two additional amino acids but preservation of the reading frame through the coding region.
Fig. S1.
Conservation and homology of PRD-1 orthologs and effects of the prd-1 mutation on the protein. The alignment was generated using PRALINE (40). Sequence motifs noted in Fig. 1 are marked, and the protein sequence changes resulting from the splicing mutation in the canonical prd-1 mutation are marked.
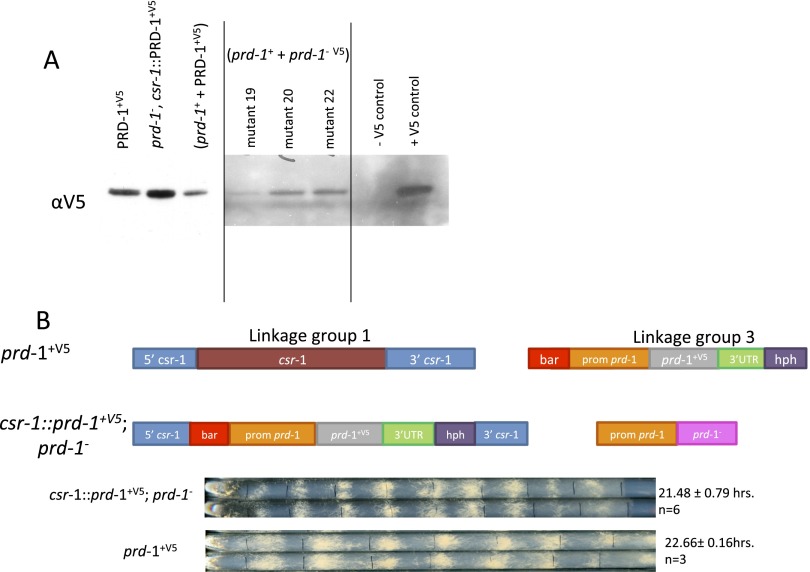
The nature of the two G-to-A mutations defining prd-1 suggested that they might influence splicing. To examine this possibility, cDNAs were generated from three of the prd-1 mutant strains that were sequenced to identify changes in prd-1 transcripts. Strains were grown in Bird medium containing 1.8% (wt/vol) glucose in constant light (LL) at 25 °C, and 24 prd-1 cDNA clones were isolated from each strain. When PCR products encompassing the third intron were sequenced, the strains were consistent with about half (37/69) showing a transcript in which the third intron is not spliced, resulting in a premature stop (TAA) and a truncated PRD-1 protein in which the last 14 amino acids are changed (Fig. 2B and Fig. S1). Interestingly, the remaining half (32/69) simply included the first six bases of the intron before the rest of the intron was spliced out, resulting only in insertion of an additional Lys and Tyr (Fig. 2B and Fig. S1). Additional evidence for the expression of a quasi–full-length PRD-1 protein arose from a strain in which a C-terminal V5 tag was appended to the mutant prd-1 gene. Western blots probed with anti-V5 still detect some full-length protein, indicating the absence of the STOP codon in the misspliced third intron (Fig. S2A). Heterokaryons bearing both mutant and WT PRD-1 display a normal period length but still show a reduced growth rate and slightly altered phase, suggesting that the prd-1 mutation does not result in a complete loss of function mutation (Fig. S2B). Consistent with this hypothesis is our observation that prd-1 gene replacement mutants were never identified either by the Neurospora knockout consortium or in our hands. Taken together, these data strongly suggest that prd-1 is an essential gene.
Fig. S2.
Quasi–full-length PRD-1 protein and incomplete rescue of mutant phenotypes seen in transformants of prd-1 mutants. (A) A C-terminal V5 epitope tag was placed on NCU07839 in the prd-1 mutant gene, and this construct was transformed into a WT strain, generating a heterokaryon. In the middle block of three isolates shown in A, the C-terminal V5 tag on the mutant prd-1 gene and could be translated into protein only if there is read-through of the mutation in intron 3. Slight expression of V5 in mutants 19, 20, and 22 suggests that, even with the mutation, some transcript that is translated through to the original gene stop codon is still being produced. Sequencing confirmed the presence of donor site guanine-to-adenine mutations. (B) WT NC07839-V5 targeted to the cyclosporin locus of the prd-1 original strain shows rescue of period, but only partial rescue of growth phenotype and a phase delay that is not present in WT rescue at the native locus.
PRD-1 Influences the Circadian Oscillator Indirectly.
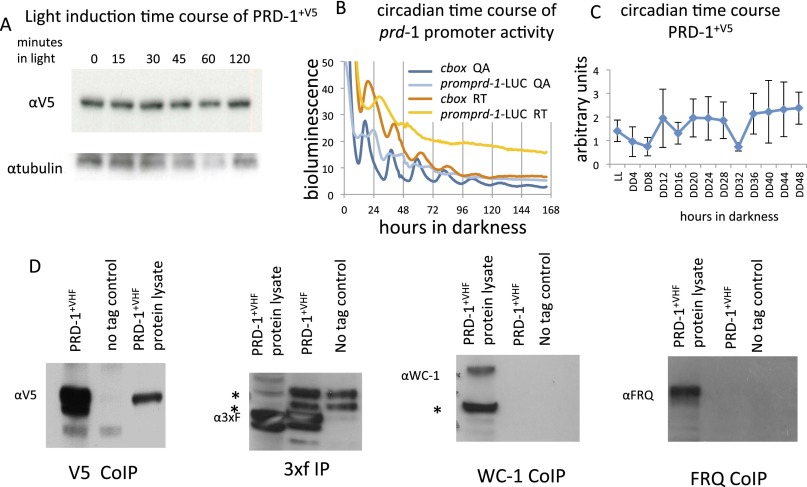
Many genes characterized by mutations that affect the circadian oscillator are themselves clock-regulated or light-regulated, or physically interact with known clock components, and in this way impact the clock or are impacted by the clock in a straightforward manner. To assess light-regulation, strains bearing PRD-1, C-terminally tagged with V5, were grown for 2 d in constant darkness (DD), moved to constant light (LL), and sampled. No change in the level of PRD-1 was detected over 120 min in constant light. (Fig. S3A). When cultures were synchronized on LD12:12 for 2 d before transfer to constant darkness, levels of prd-1 promoter activity did not oscillate (Fig. S3B). Consistent with this observation, when cultures grown in LL were transferred to DD and sampled over a circadian time course covering 48 h, PRD-1 protein fluctuated but did not oscillate (Fig. S3C); both RNA and protein data suggest that the amount of PRD-1 is not clock-controlled. Finally, we checked for interaction between PRD-1 and known components of the positive and negative arms of the circadian feedback loop. Strains bearing PRD-1 C-terminally tagged with V5-10Xhis-3xFLAG (VHF) were grown in LL, and protein extracts were immunoprecipitated using M2 FLAG beads; separate antisera directed against WC-1 (White Collar-1) and FRQ (FREQUENCY) failed to detect any proteins in the immunoprecipitate (Fig. S3D). This observation indicates that there was no strong interaction between the PRD-1 and either the positive arm of the clock, the complex of WC-1 and WC-2, or the negative arm, the complex of FRQ, FRH, and CK1, and further suggests that PRD-1’s influence on period is indirect.
Fig. S3.
PRD-1 is not induced by light up to 120 min, nor does it cycle in a circadian manner. (A) Extracts were collected from a strain bearing WT PRD-1 C-terminally tagged with V5 at various times after a dark-to-light transition. They were Western blotted and probed with anti V5. (B) A construct bearing a transcriptional fusion of the prd-1 promoter driving luciferase was targeted to the csr-1 locus and examined over 7 d in constant darkness. The data show no cycling in two different media types: QA, shown in blue (0.03% glucose, 0.05% arginine, 0.01 M QA) and race tube (RT), shown in red (0.1% glucose, 0.17% arginine). Graph lines indicate the average among all technical replicates (n = 6 for promprd-1, n = 15 for c-box). A single peak is often seen in arrhythmic strains; the csr-1::c-box-LUC–positive control shows an example of the sustained circadian cycling characteristic of rhythmic expression. (C) The strain used in A was grown in constant darkness and harvested every 4 h over 2 d, and the extracts were examined by Western blot. There is no evidence of circadian regulation of PRD-1 content; error bars ± 1 SD. (D) Strains bearing PRD-1 C-terminally tagged with V5-10Xhis-3xFLAG (VHF) were grown in constant light, and protein extracts were immunoprecipitated using M2 FLAG beads. The success of the immunoprecipitation was verified by Western blotting with anti-3xFLAG and anti-V5, but separate antisera directed against WC-1 and FRQ failed to detect these proteins in the immunoprecipitate.
Period Length in the prd-1 Mutant Strain Is Influenced by Carbon Availability.
We routinely screen strains using luciferase (Luc) reporters as well as on race tubes, and, during initial work, knockouts of prd-1-candidate genes were screened using a Luc reporter in 96-well plates. Thus, each candidate bore a gene knockout tagged by hph as well as the reporter, a transcriptional fusion in which a portion of the frq promoter (the clock box or c-box) driving expression of luciferase was inserted into the genome at the csr-1 (cyclosporin resistance-1) locus: c-box-luc reports the level of activation of the frq gene by the white collar complex and thus reports the core circadian oscillator in real time. To our surprise, in this assay, the period lengths of all of the strains, candidate gene knockouts as well as both WT and prd-1 controls, were normal although the prd-1 strains did display a 5-h phase delay (Fig. 3A). After quickly confirming the genotypes of the strains and verifying the characteristic ∼25-h period length of the same prd-1 strains on race tubes, we reasoned that the unexpected WT period might reflect the assay. Unlike race tubes where every minute the culture grows across fresh medium, in 96-well plates, the culture remains on the same growth medium throughout the experiment, in this case, low carbon race tube medium (Materials and Methods); thus, race tubes are reporting the prd-1 period in glucose-sufficient cells whereas 96-well plates could be reporting prd-1 period under glucose starvation. We confirmed this hypothesis in two ways. First, we monitored the clock by sectional analysis on a race tube (Fig. 3 B and C). In these experiments, a prd-1; ras-1[bd]; csr-1::c-box-luc strain was run on race tubes, and bioluminescence was monitored for 6 d (i) over the whole race tube and (ii) just in the region traversed by growth on the first day. It is apparent that the period length differs in the two measurements. In the region traversed during day 1 but monitored for 6 d, the cycle of bioluminescence reports a period length that is initially quite long, but, by 3 d, it has decreased to a WT period of about 22 h whereas, when cycles of bioluminescence are monitored over the whole race tube (chiefly reflecting the growth front which is more metabolically active), the period length remains long, characteristic of the prd-1 strain and corresponding to that reported by the conidiation rhythm. These data are consistent in showing a period length that reflects carbon availability, a possibility directly tested by growing prd-1; ras-1[bd] on race tubes containing different amounts of glucose (Fig. 3D). Plainly, in WT strains, period length is relatively unaffected by the amount of glucose, but, in prd-1, period length increases from a WT period of about 22.5 h at 0 glucose to the full mutant period length of 25 h in 1% glucose (Fig. 3D). Additional experiments showed that it is the level of available carbon, not whether it is fermentable or nonfermentable, that determines period in prd-1 (Fig. S4).
Fig. 3.
In prd-1 mutant strains, the period length increases with the amount of nutritional carbon in the medium. (A) prd-1;ras-1[bd]; csr-1::pfrq-luc run on RT medium (0.17% arg, 0.1% glucose) in 96-well plates; average data are reported for five or six biological replicates, as noted, each with three technical repeats. Period of mutant and WT are not different although phase analysis (Right) reveals a delay of 5.3 h in prd-1 (circles) vs. WT (stars), with a 92% confidence that each like sample is the same (D = 92), and 100% confidence that a difference exists between the samples (M = 100) (based on Hotelling’s test). (B) prd-1;ras-1[bd] csr-1::pfrq-luc strains were run on race tubes with RT medium. Period length is distinctly longer than when analyzed by luciferase in 96-well plates as in A. (C) prd-1;ras-1[bd] csr-1::pfrq-luc on a race tube (0.1% glucose, 0.17% arg). Bioluminescence was monitored either from a small section of the race tube traversed by the growth front during the first day (section A9), or from the entire race tube. (Left) Bioluminescence as a function of time from the two regions. (Right) The circadian period each day over 6 d of growth. The luciferase signal from older tissue in section A9 gives a WT period even though the overall luciferase signal of the strain remains long. (D) prd-1 period length is a function of the amount of glucose in the race tube growth medium. When no glucose is added to the medium, prd-1 runs at a WT period. However, when 0.1% glucose or higher is added in, prd-1 runs at a longer period.
Fig. S4.
The period of prd-1 on various carbon sources, fermentable (glucose and fructose) and nonfermentable (ethanol). In this experiment, the agar used in the race tubes was washed before autoclaving, reducing the amount of any additional carbon in the medium. In race tubes with only Vogel’s medium plus biotin, there is no added glucose or arginine, and cultures are presumably using the agar as a source of carbon. Period lengths are reported as the average ± 1 SD, with only one of the replicates shown.
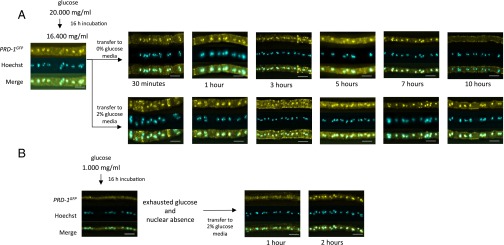
Loss of PRD-1 impacts the mechanism that compensates circadian period length against changes in carbon availability, causing period length to increase as available carbon increases. These data suggested to us that glucose might influence the expression or localization of PRD-1. Additional support for this possibility comes from analysis of the Saccharomyces ortholog of prd-1, the DBP2 gene, by Tran and coworkers, who have found rapid depletion of DBP2 from the yeast nucleus after removal of glucose from cultures (19). To follow expression, cultures of WT and prd-1 were inoculated into Bird medium containing 0.1% glucose and grown 24 h to deplete the glucose. Cultures were then spiked with glucose to reach 2% (wt/vol), and prd-1 RNA was monitored for the subsequent 7 h. WT cultures rapidly displayed an approximately two- to threefold increase in prd-1 mRNA, followed by a gradual decrease to the prior level by 4 h. Strains mutant in prd-1 displayed elevated prd-1 mRNA levels before the addition but showed no subsequent decrease (Fig. 4). These data suggested that, as is the case with Saccharomyces Dbp2p, PRD-1 might decrease within the nucleus upon glucose starvation. To assess this possibility, cultures of PRD-1 C-terminally tagged with GFP were grown overnight in 2% (wt/vol) glucose, transferred to medium containing 0% glucose, and monitored for 10 h by fluorescence microscopy. Fig. 5A plainly shows PRD-1 to be a predominantly nucleus-localized protein in glucose-sufficient cultures, but it gradually disappears from the nucleus upon glucose starvation, unlike Dbp2p, which exits the nucleus within 30 min in 0 glucose; however, PRD-1 requires nearly 10 h to be lost. PRD-1 quickly reappears in the nucleus upon restoration of glucose (Fig. 5B), indicating that PRD-1 localization is a response to glucose and not simply a stress response. Controls (Fig. S5) confirm the specific nature of the fluorescence signals. We note that the human ortholog of PRD-1, p68, bears both nuclear import and export signals that are conserved within PRD-1 and that it also shuttles between the nucleus and cytoplasm (20).
Fig. 4.
The expression of prd-1 is transiently increased in response to glucose and is elevated in prd-1 mutant strains. (Left) Cultures of WT (ras-1[bd]) or prd-1 (prd-1; ras-1[bd]) were grown in Bird medium containing 0.1% glucose for 24 h, and then glucose was added to 2% to half of the cultures. Samples were collected at the times noted, and prd-1 mRNA levels were determined by qRT-PCR (quantitative reverse transcription–polymerase chain reaction) and normalized to the amount of NCU08964, a gene chosen because its level does not change under most growth conditions (34). Error bars ± 1 SD, n = 4 for prd-1+, n = 5 for prd-1−. (Right) When all time points from the Left panel are collapsed across time, it is clear that the amount of prd-1 mRNA is elevated in the prd-1 mutant strain compared with WT; the wide spread of data points in WT with added glucose reflects the transient increase noted in the Left panel. All data are plotted with error bars equal to the 95% confidence interval.
Fig. 5.
PRD-1 is nucleus-localized in glucose sufficient cultures but is lost upon glucose starvation. (A) Mycelia expressing PRD-1GFP were grown in 2% (wt/vol) glucose liquid medium overnight and transferred to liquid medium containing either 2% (wt/vol) or 0% glucose. Mycelia were fixed and stained with AlexaFluor 488-conjugated GFP antibodies at the given time points. PRD-1 is shown in yellow, and nuclei are shown by Hoechst in cyan. All images were collected with a 63× objective. (Scale bars: 10 µm.) Levels of glucose in the medium were confirmed using the Sigma Glucose (GO) Assay; after transfer to 0% glucose medium, average soluble glucose at the five times noted was 0.0128 ± 0.006 mg/mL SEM (n = 5) and was 0.00 at 10 h whereas glucose remained above 16 mg/mL in the glucose replete cultures. (B) Mycelia were grown in 0.1% glucose liquid medium for 16 h overnight; glucose was used so that measured available glucose was 0.000 mg/mL Mycelia were then transferred to flasks containing 2% (wt/vol) glucose and sampled at the times noted.
Fig. S5.
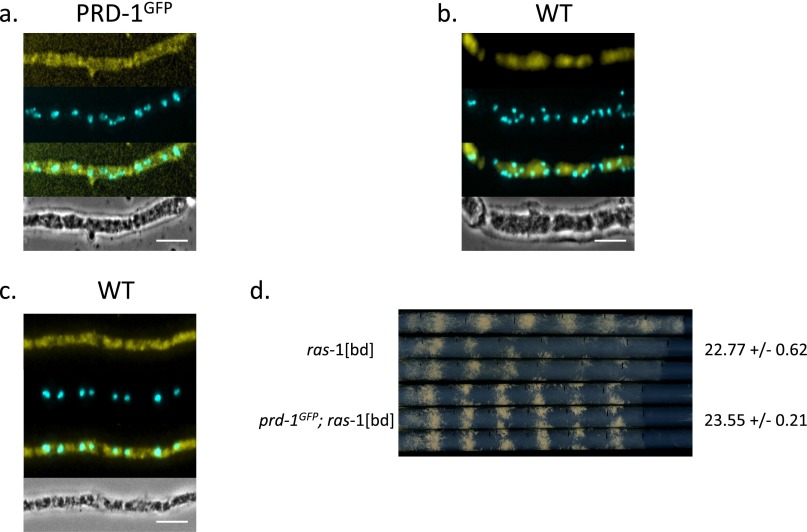
Controls for fixed cell imaging of PRD-1 localization. All hyphae used for microscopy were formaldehyde-fixed, followed by partial enzymatic digestion of the hyphal walls and methanol permeabilization. (A and B) Cells not treated with AlexaFluor 488-conjugated GFP tag antibodies show expected background fluorescence. (C) Cells treated with the AlexaFluor 488-conjugated GFP tag antibody to show cross-reactivity. (D) Race tube analysis to confirm that the GFP tag does not interfere with circadian function in the experimental strain.
Discussion
The clock gene prd-1, originally identified nearly four decades ago, is shown to encode a DEAD-box RNA helicase strongly conserved from fungi to humans. The defining mutation for prd-1 creates a strain that produces both a truncated protein as well as a modified protein containing a two amino acid insertion within a nonconserved region, a change that would not be expected to impact protein function. This observation, combined with our inability to create a gene replacement loss-of-function mutant, suggests that prd-1 is essential and that the defining prd-1 mutant retains a partial function, very likely simply a reduced dosage, that results in the period and phase effects.
In terms of clock genes, prd-1 represents a novel kind of mutation, a conditional mutant whose period lengthening is medium-dependent. In circadian terms, this phenotype presents as a loss of metabolic compensation of the clock, and the effect is opposite from that of csp-1 (conidial separation-1) null mutants in which period shortens with added glucose. The effect of ∆csp-1 is explained via loss of a feedback loop in which elevated expression of the repressor CSP-1 in high glucose counteracts the period-shortening effects of glucose-driven elevated WC-1 expression (21). More generally, temperature and metabolic compensation are defining characteristics of circadian rhythms that ensure the reliability of the time-keeping mechanism, despite changing ambient environmental conditions. Interestingly, prd-1 is reported to have normal temperature compensation (22) so the splice site mutation in prd-1 influences only nutritional compensation; however, prd-1 may not be highly informative in terms of the mechanism of compensation because of its pleiotropic nature. The yeast ortholog of PRD-1, Dbp2p, is a bona fide RNA helicase having documented physical or genetics interactions with 84 unique genes (www.yeastgenome.org/locus/S000005056/overview) and whose mutation affects the expression of nearly 3,000 transcripts (19). DBP2 is not essential in Saccharomyces and has been implicated in a wide variety of functions, ranging from non-sense–mediated decay and rRNA processing (23), to RNA quality control, mRNA decay, mRNP assembly and export of polyadenylated RNA from the nucleus, suppression of transcription from cryptic sites, and promotion of antisense lnc RNAs (16, 17, 24). Although effects of DBP2 mutation are widespread, genes whose expression is affected by loss of DBP2 have a center of gravity within areas relating to carbon utilization and ribosome synthesis: DBP2 seems to promote ribosome biogenesis and suppress components of mitochondrial respiration (19). Yeast prefers to grow via fermentation when glucose is abundant and, in the presence of glucose, Dbp2p localizes within the nucleus and contributes to repression of the respiration genes. Similar to Dbp2p, we found that PRD-1 is nuclear in glucose-replete cells; however, Neurospora is an obligate aerobe unable to grow by fermentation so the mechanism of action of PRD-1 may not to be strictly the same as its ortholog in yeast. In human cells, the orthologs of PRD-1, DDX5, and DDX17 are also nuclear. They are implicated in transcriptional regulation and alternative RNA splicing of proteins involved in redox signaling and are components of the 3′ transcriptional termination complex (25). They also are found in complex with CDK9, the kinase that phosphorylates Ser2 in the heptad repeats of the carboxyl terminal region of the large subunit of RNA Pol II, a modification leading to transcriptional elongation (26). Here again, loss of a protein that broadly affects transcriptional elongation is likely to have pleiotropic effects.
PRD-1 has frequently been mentioned in the context of “FRQ-Less Oscillators” (FLOs) in Neurospora. These FLOs are metabolic oscillators that lack circadian characteristics but that can under some conditions wrest control of development from the circadian system so that the overt appearance of conidiation on race tubes presents as a non-temperature–compensated growth medium-dependent rhythm (27). For instance, prd-1 has been shown to influence membrane fatty acid composition and alter the effects of exogenous fatty acid supplements on certain FLOs (28, 29) and to abolish another FLO that can appear upon starvation for choline (30). Revelation that PRD-1 encodes an RNA helicase implicated in regulating a wide variety of metabolic processes, particularly those related to transcription and energy, may help to explain how the mutation of this gene so often seems to interact with other mutations or metabolic challenges to influence these noncircadian metabolic oscillators.
Perhaps reflecting the myriad possibilities for its action, the exact cause of the carbon source-dependent period lengthening in prd-1 remains obscure. However, there are some clues. Elevated metabolizable carbon in the medium can cause period lengthening if PRD-1 is not available to counteract this lengthening. On the surface, because these period length data were collected by directly monitoring the activity of the white collar complex as evidenced by rhythmic control of the c-box in the frq promoter, and because the rhythmic action of the white collar complex at the c-box is a defining activity within the core of the circadian oscillator, PRD-1 has a role in the circadian clock, whatever other roles it may also have within noncircadian metabolic oscillators (e.g., ref. 28). Based on similarities with its yeast and human orthologs, PRD-1 is likely to act in the nucleus where it is indeed found when available carbon in the medium is high. It is noteworthy that one of the human orthologs of PRD-1, DDX5, has been found in a large nuclear complex with the elements of the negative arm of the circadian oscillator (PER and CRY proteins), as well as the large subunit of RNA pol II and SETX, a helicase that promotes transcriptional termination. Although we failed to find evidence for interaction between PRD-1 and either positive or negative arm circadian complexes in Neurospora, the purification of such complexes is highly condition-dependent so absence of evidence should not be taken as evidence of absence. The carbon-dependent regulation of nuclear localization that is conserved with yeast, combined with the carbon-dependent period lengthening and the roles of orthologs in transcriptional events, suggests that PRD-1 may impact the core oscillator by regulating the expression of components.
Materials and Methods
The prd-1; ras-1[bd] strain 609-26, the original isolate from the Feldman laboratory (6), was used throughout the study. Mutants ∆NCU16631::hph+ [Fungal Genetic Stock Center (FGSC) no. 23078] and acr-2 (FGSC no. 877) were obtained from the FGSC, and crosses were done on Westergaard’s medium containing 1.5% (wt/vol) sucrose by standard methods (www.fgsc.net/Neurospora/NeurosporaProtocolGuide.htm). Resistance screening for hygromycin was performed on solid medium containing 200 μg/mL hygromycin (400051; EMD Millipore). Acriflavin resistance was examined in liquid cultures containing 50 μg/mL acriflavin (A8251; Sigma Aldrich). The prd-1 and ras-1[bd] strains containing the frq promoter driving luciferase (prfrq-luc) targeted to the csr-1 locus were maintained on medium containing 1.5% (wt/vol) sucrose and 2.5 μM cyclosporine A (30024; Sigma). Bird medium (www.fgsc.net/neurosporaprotocols/How%20to%20choose%20and%20prepare%20media.pdf) was used as liquid culture medium for all experiments, with specific glucose concentrations except as noted. Race tube (RT) medium (0.1% glucose, 0.17% arginine) was used for all race tube and 96-well plate experiments unless otherwise noted. QA medium used BIRD salts supplemented with 0.03% glucose, 0.05% arginine, and 0.01 M quinic acid. Strains were maintained on either a complete medium (malt extract, yeast extract, casein hydrolysate) or on a minimal Vogel’s medium [1.5% (wt/vol) sucrose] containing an appropriate drug. In medium containing washed agar, agar was stirred in a large flask with ∼1 L of MilliQ water for ∼15 min. Agar was allowed to settle before decanting off water. This process was repeated four to six times before autoclaving with appropriate medium components.
Additional methods are found in SI Materials and Methods.
Note.
While this manuscript was in preparation, similar data on the effect of glucose on period length were discovered (35).
SI Materials and Methods
Mapping of prd-1.
The prd-1; ras-1[bd] was crossed to ras-1[bd]; ∆NCU16631::hph+, which is located to the left of pro-1, one of the original markers used by Feldman and Atkinson (6). After obtaining prd-1; ras-1[bd]; ∆NCU16631::hph+ triple mutants, roughly 5% of the progeny, one of these triple mutant was then crossed to acr-2 (acriflavine resistance-2). Ascospores from the cross mutant cross were briefly rinsed in a bleach solution [∼10% (vol/vol)] before being spread onto a 4% (wt/vol) agar plate. Spores were then germinated on complete medium for up to a week before being tested for acriflavin resistance and/or hygromycin resistance, and for conidial banding on race tubes. Recombination rates are reported only for the ∼50% of progeny that contained the ras-1[bd] allele that allowed scoring of prd-1.
Generation of prd-1 Candidates for Sequencing.
Progeny from the prd-1; ras-1[bd]; ∆NCU16631::hph+ cross to acr-2; ras-1[bd] were screened for hygromycin resistance and acriflavin resistance. Of the eight ras-1[bd] strains that were resistant to both hygromycin and acriflavin, seven were prd-1− and 1 was prd-1+. Genomic DNA was generated out of these eight strains for sequencing.
DNA purification.
To prepare DNA from the eight samples for sequencing, mycelia were grown in liquid Bird medium for 2 d shaking in a 25° water bath under constant light. Tissue was harvested, and 40-mg samples were ground in liquid nitrogen followed by incubation at 55° for 2 h in 1.2 mL Cell Lysis solution (158908; Qiagen) containing 6 µL of Proteinase K (19133; Qiagen). After the addition of 6 µL of RNase A (19101; Qiagen), the sample was incubated at 37° for 15 min. Finally, 200 µL of Protein Precipitation solution (158912; Qiagen) was added, and the sample was vortexed and incubated on ice for 15 min. After centrifugation, the supernatant was removed and added to isopropanol to precipitate the DNA. The sample was centrifuged, and the resulting pellet was washed with 70% (vol/vol) ETOH and resuspended in DNA hydration solution (158916; Qiagen). DNA purity, quantity, and quality were determined using a NanoDrop spectrophotometer ND-2000 (Thermo Fisher Scientific), Qubit (Life Technologies, Thermo Fisher Scientific), and Fragment Analyzer (Advanced Analytical).
Library preparation.
Using the NEBNext fast DNA fragmentation and library prep kit, 100 ng of DNA was prepared for sequencing by generating adaptor-ligated DNA compatible with ION Torrent chemistry (E6285; NEB). Briefly, DNA was fragmented, adaptors were ligated onto each end, and the resulting library was amplified with eight PCR cycles. After XP bead clean up, libraries were quantified with Qubit, and then the DNA profile was analyzed with the Fragment Analyzer (High Sensitivity NGS kit). The average insert size for the library preparations was 220 bp. Each biological sample was generated with a unique INDEX sequence to allow multiple samples to be pooled together in a single sequencing run. Library preparation was performed in the Dartmouth Genomics and Molecular Biology Shared Resource.
Ion proton sequencing.
The library pool for sequencing was generated by combining 18 pM of each individual library. This mixture was then templated onto the Ion Sphere particles (ISPs) using emulsion PCR within the One Touch 2 (OT2) instrument. Ion PI template OT2 200v3 was used. After this step, sequencing was performed on the Ion Proton sequencer with the Ion PI chip v2, and Ion PI sequencing 200 v3 chemistry. The sequencing run generated over 77 million reads, and 12.8 GB of data. The average read length was 165 bp, and each sample generated between 6.8 and 13 million reads per sample. The sequencing was performed in the Dartmouth Genomics and Molecular Biology Shared Resource.
Next-Generation Sequencing (DNASeq) Analysis.
DNA libraries of seven variant and one normal strain as described above were prepared for whole genome sequencing using ION-Torrent Proton, and Partek Flow (3.0) was used for the following sequencing analysis. Raw sequence reads (fastq format) were first checked for prealignment quality control, and then reads were aligned using the TMAP algorithm (2.9.2) with Neurospora crassa OR74A (NC12) supercontigs as the reference genome. The aligned reads (bam format) were checked for postalignment QC, and then SNV caller (among samples) was used to detect variants jointly for all eight strains. Finally, variant annotator was used to annotate all variants with N. crassa OR74A (NC12) transcripts position file.
Region-Based Susceptible Loci (PRD-1 Gene) Detection.
For the whole genome, Partek Flow found 190,182 possible single nucleotide variants with minimum LOG-ODDS ratio = 5. To identify the prd-1 gene, a candidate region on the left half of supercontig 12.3 (chromosome 3), from 0 to about 2.5 million bases, was targeted for detailed analysis. In this supercontig, 190,182 variants were filtered based on the following criteria: Normal strain must have no variant (negative control) and there should be at least one variant existing among seven mutated strains. After these filtering steps, 8,913 variants were retained for further analysis. Among these 8,913 variants, allele frequency (AF) was calculated for seven mutated strains: for example, if AF equals 1, all seven mutated strains have homozygous variants. There were 81 variants existing in all seven mutated strains. All variants were also stratified by the status of annotation, assuming that it is more likely that annotated variants in the gene-coding regions may have functional effects. A region within the contig between positions 959,500 and 1,040,300 contained a stretch of 10 variant alleles in the mutant strain, including position 960,844, which is a promoter variant in a hypothetical protein, position 1,030,707, which is a variant in exon 1 of the noncoding RNA NCU07836, and position 1,040,300, which is a variant in an intron of NCU7839.
Transformation and Western Blot Protocols.
Methods for transformations into csr-1 and native loci are previously described (15, 36). Transformations into csr-1 were plated on medium containing 2.5 μM cyclosporine whereas transformations targeting the native locus were plated onto medium containing 300 μg/mL hygromycin. Western blot samples were prepared as previously described (37), with protease inhibitor mixture added (78438; Pierce). Primary antibody dilutions were 1/5,000 anti-V5, 1/5,000–1/7,500 anti-tubulin, 1/400 anti-GFP, 1/250 anti-WC-1, or 1/250 anti-FRQ. Secondary antibody dilutions were 1/5,000, and blots were exposed to either West Pico and/or West Femto (34078 and 34095, respectively; Pierce).
Cloning of prd-1 cDNA over Third Intron.
cDNA was generated from three of the prd-1− progeny grown in Bird medium in liquid culture [1.8% (wt/vol) glucose] by methods previously described (38), with minor modifications. Before generation of cDNAs via the superscript III kit from Life Technologies, a DNase I step (Roche) was added to eliminate genomic DNA contamination. Primers that were designed to encompass the third intron of prd-1 were used to generate a product that was cloned into Escherichia coli by blunt end cloning (Thermo Fisher). Colonies were screened by ampicillin resistance and sent in for sequencing.
Luciferase Strain Generation and CCD Analysis.
A construct bearing luciferase driven by the promoter of the frequency gene was transformed in a targeted approach into the csr-1 locus of strains, as described similarly in Hurley et al. (38). Real time CCD recordings were done as described in Hurley et al., or on race tubes in a similar manner. Phase analysis was done using software as described (39).
Light Induction Time Course of PRD-1+V5.
Strains were inoculated onto a Petri dish containing Bird medium and allowed to grow for 24 h in DD conditions. Plugs were then cut and inoculated into 100 mL of Bird medium and allowed to grow in constant darkness (DD) with shaking for an additional 24 h. Samples were then moved to constant light (LL) at 25 °C, and tissue was harvested at LL15, -30, -45, -60, and -120 min, with a control kept in DD. Western blots were run as described above.
Circadian Time Course of PRD-1+V5.
Strains were inoculated into a Petri dish containing Bird medium and allowed to grow for 24 h in LL conditions. Plugs were then cut and inoculated into 50 mL of Bird medium and allowed to grow in LL shaking conditions overnight. Samples were then moved to 25° DD at appropriate intervals to achieve 4-h resolution. Samples were then harvested after 48 h, and a Western blot was performed as described above.
Coimmunoprecipitation of PRD-1+V5-10his-3xF (VHF).
Samples were grown in Bird medium [1.8% (wt/vol) glucose] for 2 d shaking at 25° in LL to achieve ∼10 g of tissue. Next, samples underwent protein extraction as previously described in Transformation and Western Blot Protocols. Then, 250 μL of FLAG M2 beads (M8823; Sigma Aldrich) were then washed 4× in protein extraction buffer (PEB) before adding 50 mg of total protein and incubating for 1–2 h or overnight rotating at 4 °C. Samples were then washed 4× with PEB before eluting from the beads with 100 μg/mL (final concentration) FLAG peptide (F4799; Sigma Aldrich) for 30 min at 4 °C. After centrifugation to clear the extract, the supernatant was added to sample buffer containing DTT and heated at 95° for 5 min. Samples were loaded onto precast gels (ThermoFisher) and Western blots using 1/250 dilutions of anti-WC-1 and anti-FRQ antibodies, and 1/5,000 dilutions of V5 and 3xFLAG (R960-CUS, ThermoFisher and F3165, Sigma Aldrich, respectively) were performed using methods described above.
Glucose Spike in Time Course.
Samples were grown for 2 d (44–48 h) in Bird medium containing 0.1% glucose in Petri dishes at 25 °C LL conditions. Plugs were then cut and inoculated into Bird medium (0.1% glucose) in 50-mL shaking cultures overnight in 25 °C LL. Samples were then either treated with a final concentration of 2% (wt/vol) glucose, or left untreated, with continued shaking at 25 °C LL. Treated samples were collected every hour, with an untreated control collected every 2 h, up to 8 h. Samples then were then Western blotted in the manner described above, or mRNA was extracted as previously described for qPCR using Quantifast SYBR green (204056; Qiagen).
Fluorescence Microscopy.
Mycelial mats of N. crassa were grown from conidia in Petri dishes overnight as described (37, 38). FGSC 2489 was used as WT, and a prd-1GFP strain was used as the experimental strain. Small plugs were cut from the mats, transferred to flasks containing fresh liquid Bird media, and allowed to incubate shaking overnight at 25 °C. The mature hyphae were transferred into liquid Bird medium containing either 1.8% (wt/vol) or 0% glucose. For the glucose recovery experiments, after 10 h of glucose starvation, the 0% glucose media was decanted off and replaced with 2% (wt/vol) glucose media. At the given time points, formaldehyde was added to the flask to a final concentration of 4% (vol/vol), and fixation proceeded for 1 h. Hyphae were washed 3× in PBST (phosphate-buffered saline containing Tween) (0.1% Tween) and mechanically separated. Hyphae were placed on polylysine-coated slides, excess liquid was removed, and the hyphae were allowed to dry. Digestion of the cell walls was done with VinoTaste Pro (Novozymes) in sorbitol buffer, gently shaking at 30 °C until 70–80% of hyphae were phase dark. The slides were washed three times with PBS and blocked with 1× PBS plus 1% wt/vol IgG-free BSA for 1 h. Slides were incubated overnight at 4 °C in 1:3,000 Novex GFP Tag Antibody and Alexa Fluor 488 conjugate in PBS plus BSA. Three washes with PBS plus BSA were followed by a 30-min incubation in 1:500 Hoechst in PBS plus BSA. Two more washes were done with PBS plus BSA, followed by two washes with PBS. Excess moisture was removed, and a drop of Prolong gold mounting medium was placed in the center of the slide. A glass coverslip was placed on top, and weight was applied for 30 min. Images were acquired using a Zeiss AxioImage-M1 upright light microscope (Carl Zeiss) equipped with a Plan-Apochromat 63×/1.4 numerical aperture oil objective and driven by MicroManager. GFP and Hoechst were detected with Chroma Technology filter sets 49002-ET-EGFP(FITC/Cy2) and 49000-ET-DAPI, respectively. Iterative restoration of the images was carried out with Volocity. Final images were prepared with FIJI.
Acknowledgments
We thank Xiangjun Xiao and Christopher I. Amos for help with identification of genetic variants based on genomic sequences, and Joanna Hamilton and the staff of the Genomics Shared Resource at the Geisel School of Medicine for excellent technical support. This work was supported by National Institute of General Medical Sciences, National Institutes of Health Grants GM34985 (to J.C.D.) and GM083336 (to J.J.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521918112/-/DCSupplemental.
References
- 1.Brody S. The Genetics of Circadian Rhythms. In: Goodwin S, Freidman T, Dunlap JC, editors. Advances in Genetics. Vol 74 Elsevier; Boston: 2011. [Google Scholar]
- 2.Dunlap JC. Genetic analysis of circadian clocks. Annu Rev Physiol. 1993;55:683–728. doi: 10.1146/annurev.ph.55.030193.003343. [DOI] [PubMed] [Google Scholar]
- 3.Dunlap JC. Salad days in the rhythms trade. Genetics. 2008;178(1):1–13. doi: 10.1534/genetics.104.86496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown SA, Kowalska E, Dallmann R. (Re)inventing the circadian feedback loop. Dev Cell. 2012;22(3):477–487. doi: 10.1016/j.devcel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman JF, Atkinson CA. Genetic and physiological characteristics of a slow-growing circadian clock mutant of Neurospora crassa. Genetics. 1978;88(2):255–265. doi: 10.1093/genetics/88.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman JF, Gardner GF, Dennison RA. In: Genetic Analysis of the Circadian Clock of Neurospora: Biological Rhythms and Their Central Mechanism. Suda M, editor. Elsevier; Amsterdam: 1979. pp. 57–66. [Google Scholar]
- 8.Davis CR. 1993. The cloning and characterization of genes involved in amino acid metabolism in Neurospora crassa. PhD dissertation (Dartmouth College, Hanover, NH)
- 9.Dunlap JC, et al. Enabling a community to dissect an organism: Overview of the Neurospora functional genomics project. Adv Genet. 2007;57:49–96. doi: 10.1016/S0065-2660(06)57002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galagan JE, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422(6934):859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 11.McCluskey K, et al. Rediscovery by whole genome sequencing: Classical mutations and genome polymorphisms in Neurospora crassa. G3 (Bethesda) 2011;1(4):303–316. doi: 10.1534/g3.111.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller-Pace FV. DExD/H box RNA helicases: Multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006;34(15):4206–4215. doi: 10.1093/nar/gkl460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller-Pace FV. The DEAD box proteins DDX5 (p68) and DDX17 (p72): Multi-tasking transcriptional regulators. Biochim Biophys Acta. 2013;1829(8):756–763. doi: 10.1016/j.bbagrm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337(6094):599–602. doi: 10.1126/science.1221592. [DOI] [PubMed] [Google Scholar]
- 15.Colot H, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci USA. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cloutier SC, Ma WK, Nguyen LT, Tran EJ. The DEAD-box RNA helicase Dbp2 connects RNA quality control with repression of aberrant transcription. J Biol Chem. 2012;287(31):26155–26166. doi: 10.1074/jbc.M112.383075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma WK, Cloutier SC, Tran EJ. The DEAD-box protein Dbp2 functions with the RNA-binding protein Yra1 to promote mRNP assembly. J Mol Biol. 2013;425(20):3824–3838. doi: 10.1016/j.jmb.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalev N, Barajas D, Nagy PD. Similar roles for yeast Dbp2 and Arabidopsis RH20 DEAD-box RNA helicases to Ded1 helicase in tombusvirus plus-strand synthesis. Virology. 2012;432(2):470–484. doi: 10.1016/j.virol.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Beck ZT, et al. Regulation of glucose-dependent gene expression by the RNA helicase Dbp2 in Saccharomyces cerevisiae. Genetics. 2014;198(3):1001–1014. doi: 10.1534/genetics.114.170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Gao X, Huang Y, Yang J, Liu ZR. P68 RNA helicase is a nucleocytoplasmic shuttling protein. Cell Res. 2009;19(12):1388–1400. doi: 10.1038/cr.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancar G, Sancar C, Brunner M. Metabolic compensation of the Neurospora clock by a glucose-dependent feedback of the circadian repressor CSP1 on the core oscillator. Genes Dev. 2012;26(21):2435–2442. doi: 10.1101/gad.199547.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner GF, Feldman JF. Temperature compensation of circadian period length in clock mutants of Neurospora crassa. Plant Physiol. 1981;68(6):1244–1248. doi: 10.1104/pp.68.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond AT, Mangus DA, He F, Jacobson A. Absence of Dbp2p alters both nonsense-mediated mRNA decay and rRNA processing. Mol Cell Biol. 2001;21(21):7366–7379. doi: 10.1128/MCB.21.21.7366-7379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloutier SC, Wang S, Ma WK, Petell CJ, Tran EJ. Long noncoding RNAs promote transcriptional poising of inducible genes. PLoS Biol. 2013;11(11):e1001715. doi: 10.1371/journal.pbio.1001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, et al. Molecular architecture of the human pre-mRNA 3′ processing complex. Mol Cell. 2009;33(3):365–376. doi: 10.1016/j.molcel.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, et al. Systematic determination of human cyclin dependent kinase (CDK)-9 interactome identifies novel functions in RNA splicing mediated by the DEAD Box (DDX)-5/17 RNA helicases. Mol Cell Proteomics. 2015;14(10):2701–2721. doi: 10.1074/mcp.M115.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi M, Larrondo LF, Loros JJ, Dunlap JC. A developmental cycle masks output from the circadian oscillator under conditions of choline deficiency in Neurospora. Proc Natl Acad Sci USA. 2007;104(50):20102–20107. doi: 10.1073/pnas.0706631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Lakin-Thomas P. Effects of prd circadian clock mutations on FRQ-less rhythms in Neurospora. J Biol Rhythms. 2010;25(2):71–80. doi: 10.1177/0748730409360889. [DOI] [PubMed] [Google Scholar]
- 29.Coté GG, Brody S. Circadian rhythms in Neurospora crassa: Membrane composition of a mutant defective in temperature compensation. Biochim Biophys Acta. 1987;898(1):23–36. doi: 10.1016/0005-2736(87)90106-4. [DOI] [PubMed] [Google Scholar]
- 30.Lakin-Thomas PL, Brody S. Circadian rhythms in Neurospora crassa: Interactions between clock mutations. Genetics. 1985;109(1):49–66. doi: 10.1093/genetics/109.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins D, Radford A, Sachs M. The Neurospora Compendium. Academic; San Diego: 2001. [Google Scholar]
- 32.Rocak S, Linder P. DEAD-box proteins: The driving forces behind RNA metabolism. Nat Rev Mol Cell Biol. 2004;5(3):232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 33.Banroques J, Tanner NK. Bioinformatics and biochemical methods to study the structural and functional elements of DEAD-Box RNA helicases. In: Boudvillain M, editor. RNA Remodeling Proteins: Methods and Protocols. Springer Humana; New York: 2015. pp. 165–181. [DOI] [PubMed] [Google Scholar]
- 34.Hurley JH, et al. A tool set for the genome-wide analysis of Neurospora crassa by RT-PCR. G3 (Bethesda) 2015;5(10):2043–2049. doi: 10.1534/g3.115.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starkey N, McDonald J, Lee K. PERIOD-1 is a component of the Neurospora metabolic oscillator. Lett. Gen. Microb. 2013;1:13–15. [Google Scholar]
- 36.Collopy PD, et al. High-throughput construction of gene deletion cassettes for generation of Neurospora crassa knockout strains. Methods Mol Biol. 2010;638:33–40. doi: 10.1007/978-1-60761-611-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CH, DeMay BS, Gladfelter AS, Dunlap JC, Loros JJ. Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora. Proc Natl Acad Sci USA. 2010;107(38):16715–16720. doi: 10.1073/pnas.1011190107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley JM, et al. Analysis of clock-regulated genes in Neurospora reveals widespread posttranscriptional control of metabolic potential. Proc Natl Acad Sci USA. 2014;111(48):16995–17002. doi: 10.1073/pnas.1418963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 2002;3:1. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simossis VA, Heringa J. 2005. PRALINE: A multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res 33(Web Server issue):W289–W294.