Significance
There is great interest in elucidating the diverse roles of microbes in biology, in general and, specifically, in mediating animal communication. We demonstrate that the gut bacterial community plays a pivotal role in production of aggregation pheromones in the German cockroach. The feces of normal and gut bacteria-inoculated cockroaches emits highly attractive volatile carboxylic acids (VCAs) that elicit aggregation whereas bacteria-free feces contains few VCAs and is relatively unattractive. VCAs may reflect the gut microbiota and, in turn, the cockroach's local environment, explaining why divergent chemical structures have been proposed as aggregation pheromones. This new insight emphasizes the importance of gut microbes in insect–insect communication and highlights the plasticity of the chemistry and function of fecal aggregation pheromones.
Keywords: gut bacteria, aggregation, pheromone, cockroach, communication
Abstract
Aggregation of the German cockroach, Blattella germanica, is regulated by fecal aggregation agents (pheromones), including volatile carboxylic acids (VCAs). We demonstrate that the gut microbial community contributes to production of these semiochemicals. Chemical analysis of the fecal extract of B. germanica revealed 40 VCAs. Feces from axenic cockroaches (no microorganisms in the alimentary tract) lacked 12 major fecal VCAs, and 24 of the remaining compounds were represented at extremely low amounts. Olfactory and aggregation bioassays demonstrated that nymphs strongly preferred the extract of control feces over the fecal extract of axenic cockroaches. Additionally, nymphs preferred a synthetic blend of 6 fecal VCAs over a solvent control or a previously identified VCA blend. To test whether gut bacteria contribute to the production of fecal aggregation agents, fecal aerobic bacteria were cultured, isolated, and identified. Inoculation of axenic cockroaches with individual bacterial taxa significantly rescued the aggregation response to the fecal extract, and inoculation with a mix of six bacterial isolates was more effective than with single isolates. The results indicate that the commensal gut microbiota contributes to production of VCAs that act as fecal aggregation agents and that cockroaches discriminate among the complex odors that emanate from a diverse microbial community. Our results highlight the pivotal role of gut bacteria in mediating insect–insect communication. Moreover, because the gut microbial community reflects the local environment, local plasticity in fecal aggregation pheromones enables colony-specific odors and fidelity to persistent aggregation sites.
Diverse microbial communities inhabit the alimentary tract and other tissues of many insect species. Their effects on the host vary, ranging from facultative provision of essential nutrients to stimulation of the immune system and exclusion of pathogenic microbes (1–6). Insect-symbiotic associations, some obligatory, are common, where hosts are nutritionally and immunologically dependent on their symbiotic microbes: Buchnera in aphids (7), nitrogen-fixing bacteria in termites (8), Blattabacterium in cockroaches (e.g., ref. 9), lactic acid bacteria in honey bees (10) and Wolbachia, which affects sex determination (11), immune function (e.g., ref. 12) and nutrition (13) in many insect species. The alimentary tract, and especially the hindgut of many (possibly all) insects, is persistently colonized by opportunistic, facultative, and commensal microbiota largely structured by exogenous (diet and local environment) and endogenous (gut environment) factors. The commensal gut microbiota can modulate various aspects of insect biology, including behavior (e.g., refs. 14–16), host–parasite and host–pathogen interactions (e.g., refs. 2 and 4), and various life history traits (1, 17).
The German cockroach, Blattella germanica is a major pest of the built environment, where it can acquire and transmit pathogens, contaminate food, and produce allergenic proteins that cause human morbidity (18, 19). The German cockroach lives in aggregations (20), and contact with conspecifics accelerates nymphal development (21) and reproductive maturation in both sexes (22, 23). Younger nymphs benefit from coprophagy in aggregations (24), and gregarious behavior may also facilitate mate location, predator avoidance, thermoregulation, and prevention of water loss. Fidelity to the resting/aggregation site may also facilitate group foraging in the rapidly changing human environment. Aggregation behavior is mediated by at least two types of chemical cues: endogenous compounds produced by the insect and compounds contained in feces. Cuticular hydrocarbons facilitate aggregations (25), and salivary compounds contribute to dissolution of aggregations (26); both are examples of endogenous signals. Feces-associated compounds function as powerful attractants and arrestants in all life stages of the German cockroach (27, 28).
Identification of the fecal aggregation pheromones of cockroaches has been fraught with controversy. Candidate pheromones are thought to be endogenously produced by rectal pads (29), with arrestment agents, including blattellastanoside A and B (30) and volatile carboxylic acids (VCAs) (31, 32), and attractants, including ammonia, alkyl amines, amino alcohols, alcohols (33), and VCAs (31, 32). However, the chemical profiles of aggregation-inducing agents vary greatly among reports. The structures of blattellastanosides may be an artifact of chemical isolation and fractionation (34). Some compounds are inconsistently detected in feces, and behavioral responses to them range from attraction to neutral to avoidance (32, 35). More than 150 compounds, including 57 carboxylic acids, have been identified from feces of the German cockroach (31). Because methylation decreased the aggregation response (31), a mix of VCAs was considered the likely aggregation stimuli (32).
Symbiotic and commensal bacteria modulate the production of sex pheromones in grass grub beetles (36) and Drosophila (15) and the aggregation pheromone in locusts (37). We hypothesized that the fecal VCAs that mediate aggregation in the German cockroach originate from the bacterial community in the feces, and, because gut-associated bacteria are acquired from the environment, we posited that both the VCA profiles and the behavioral responses to them depend on environmental conditions. Our behavioral assays and chemical analysis revealed that the feces of axenically reared cockroaches (no microorganisms in the alimentary tract) contained many fewer VCAs and failed to elicit aggregation behavior. Inoculation with fecal aerobic bacteria rescued the aggregation activity of fecal extracts of axenic cockroaches. A synthetic blend of VCAs was an effective aggregation stimulus for German cockroaches. We propose that gut bacteria impact the production of fecal VCAs as aggregation agents and that cockroaches use fecal VCAs from commensal microbes as aggregation cues that reflect their colony odor.
Results
Environmental Microbes Contribute to Feces-Associated Aggregation Agents.
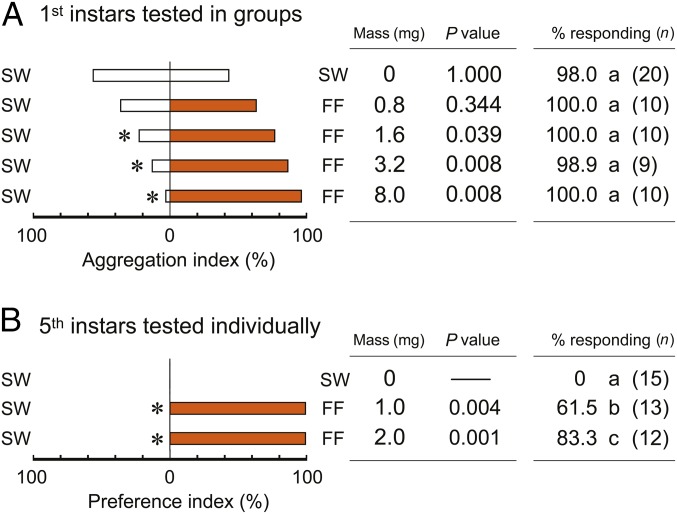
We used first and fifth instar nymphs in behavioral assays because they are highly motivated to aggregate during the photophase. First instar nymphs sheltered equally under two water-treated filter papers in a Petri dish, but these stimuli failed to elicit upwind orientation in older nymphs in an olfactometer (Fig. 1). Both young and older nymphs were significantly attracted to, and arrested in a dose-dependent manner by, extracts of adult female feces and in orientation assays that required walking toward the stimulus and choosing between it and sterile water (solvent). Water extracts of feces stimulated significantly more fifth instar nymphs to walk upwind and to prefer the extract over the sterile water (Fig. 1B).
Fig. 1.
Orientation to and aggregation of nymphs on fecal extracts. (A) Aggregation preference of first instar nymphs under filter paper shelters in two-choice bioassays. Groups of 10 first instars (n = number of groups) were tested in Petri dish assays with sterile water (SW) and female feces (FF) at different concentrations of feces extract (feces mass extracted in mg per filter paper). Aggregation index is the percentage of first instars that chose each of the filter papers. Asterisks indicate a significant preference for feces extract (sign test, P < 0.05). The total percentages of nymphs that sheltered under both stimuli were compared by Tukey’s WSD, and different letters denote significant differences (P < 0.05). (B) Orientation and choice assays of individual fifth instar nymphs (n = total individuals) to treated filter papers in two-choice olfactometer bioassays. Preference index is the percentage of nymphs that chose each of the filter papers. Asterisks indicate a significant preference for feces extract (exact binominal test, P < 0.05). Statistical analyses could not be conducted for SW vs. SW because none of the nymphs responded. In both A and B, the total percentage of nymphs that responded to both stimuli were compared by Tukey’s WSD, and different letters indicate significant differences among the treatments (P < 0.05).
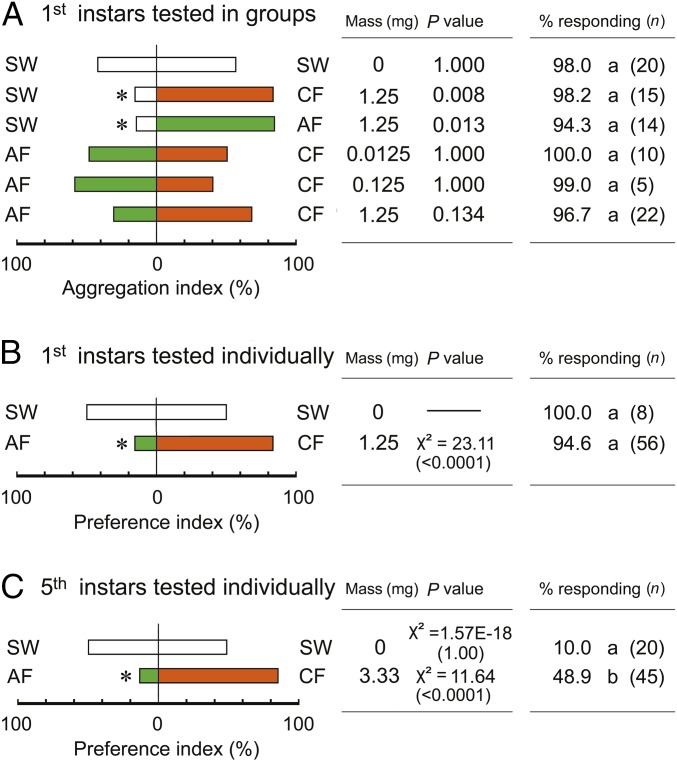
To test whether the aggregation response was influenced by bacteria associated with feces, we generated sterile and control (nonsterile) feces (Table S1). Egg cases were (i) surface-sterilized and maintained under axenic conditions with sterilized cockroach feces, food, and water (axenic group) or (ii) maintained with nonsterile feces and food and water (control group). The sterilization procedure eliminated environmental bacteria but not vertically transmitted endosymbiotic (intracellular) bacteria in the fat body. In the second procedure, cockroaches could ingest environmental bacteria, including the fecal bacteria excreted by their colony members. Because both groups reached the adult stage approximately at the same time, it seemed that environmentally acquired microbes did not contribute significantly to cockroach nutrition and development. In two-choice sheltering assays, first instar nymphs sheltered under extracts of control feces (CF) and axenic feces (AF) more than in shelters treated with sterile water (Fig. 2A). However, when given a choice between the control and axenic feces, they significantly preferred the extract of control feces (Fig. 2 A and B), and they responded to this extract faster than to the axenic feces (Fig. S1). Fifth instar nymphs also preferred the control over the axenic feces in olfactometer tests (Fig. 2C), and odors of control feces stimulated significantly more nymphs to respond than odors emanating from axenic feces (Fig. S2). These results indicate that attractants and arrestants that mediate aggregation in the German cockroach are associated with fecal microbes and that the feces of axenically reared cockroaches is much less attractive than feces of cockroaches with an active gut microbial community.
Table S1.
Treatment groups generated from the original cockroach colony
| Treatment group* | Egg cases† | Feces‡ | Bacterial inoculation§ | Food and water | Shelter and cage | Behavioral Test¶ | GC-MS# |
| Control|| | N-St** | N-St | None | N-St | N-St | Done | Done |
| Axenic | St†† | St | None | St | St | Done | Done |
| In-Mix6 | St | St | Six bacteria species | St | St | Done | |
| In-1 | St | St | Enterococcus avium | St | St | Done | |
| In-2 | St | St | Weissella cibaria | St | St | Done |
Each group originated from egg cases from the original cockroach colony. The control nonsterile group was treated as the original colony. The axenic groups were generated by surface-sterilizing egg cases and rearing cockroaches under sterile conditions. Three axenic groups were inoculated with bacteria during the first instar stage.
Treatment groups were generated from the same cockroach colony.
Three egg cases (∼40 eggs in each) were used to develop each treatment group.
First instar nymphs received feces from the original colony in addition to food.
The control and axenic groups did not receive any bacteria.
First and fifth instar nymphs from the colony were tested with fecal extracts from each treatment group.
Volatile carboxylic acids of feces were compared between the control and axenic groups. Feces of In-Mix6, In-1, and In-2 were not analyzed.
Control groups were treated as the original colony.
N-St, nonsterile.
St, sterile.
Fig. 2.
Microbes contribute to orientation and aggregation responses to feces extracts. (A) Groups of 20–30 first instar nymphs (n = number of groups) were tested in two-choice sheltering assays in Petri dishes with sterile water (SW) and fecal extracts of control (CF) and axenic (AF) cockroaches at different concentrations of feces extract (feces mass extracted in mg per filter paper). Asterisks represent significant differences in aggregation index (sign test, P < 0.05). (B) To eliminate the effect of social interactions of nymphs, individual first instar nymphs (n = total individuals) were tested in the same sheltering assays, with the asterisk denoting significant differences in their sheltering preference (χ2 test, P < 0.05). (C) Fifth instar nymphs were tested in two-choice olfactometer assays, as in Fig. 1B, and significant preferences are denoted by asterisk (χ2 test, P < 0.05). Statistical analyses could not be conducted for SW vs. SW because only 2 of 20 nymphs responded. In A–C, the total percentages of nymphs that responded to both stimuli were compared by Tukey’s WSD, and different letters represent significant differences among treatments (P < 0.05).
Fig. S1.
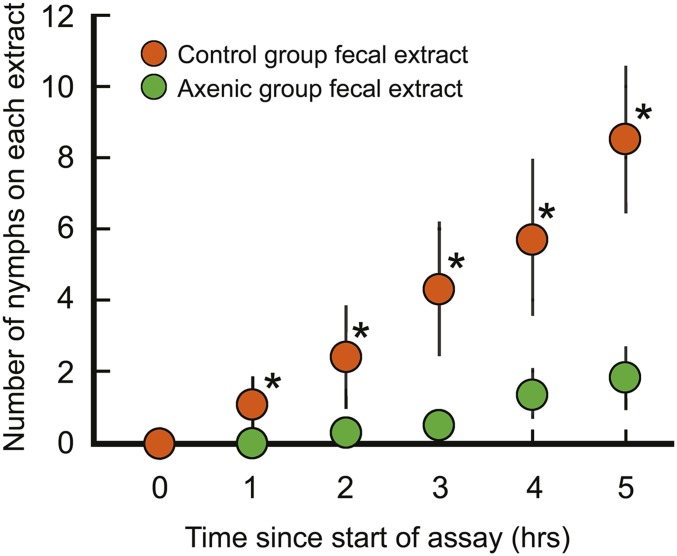
Time course of aggregation behavior of first instar nymphs. Shown is the time course of nymphs sheltering under filter papers treated with extracts of control nonsterile feces and axenic sterile feces. Each filter paper was treated with the extract of 1.25 mg of feces. Mean ± SE are shown of 15 trials with 30 nymphs per trial. Within each trial, the 30 nymphs were introduced individually over the course of 10–15 min in the scotophase. Asterisks indicate a significant difference between the control and axenic extracts (χ2 test, P < 0.05).
Fig. S2.
Microbes contribute to orientation responses of fifth instar nymphs to feces extracts. Individuals (n total) were tested in no-choice olfactometer assays with sterile water (SW), control feces (CF), and axenic feces (AF). Different letters represent significant differences in percentage of nymphs responding of the total assayed (Tukey’s WSD, P < 0.05).
VCAs in Feces Act as Aggregation Agents.
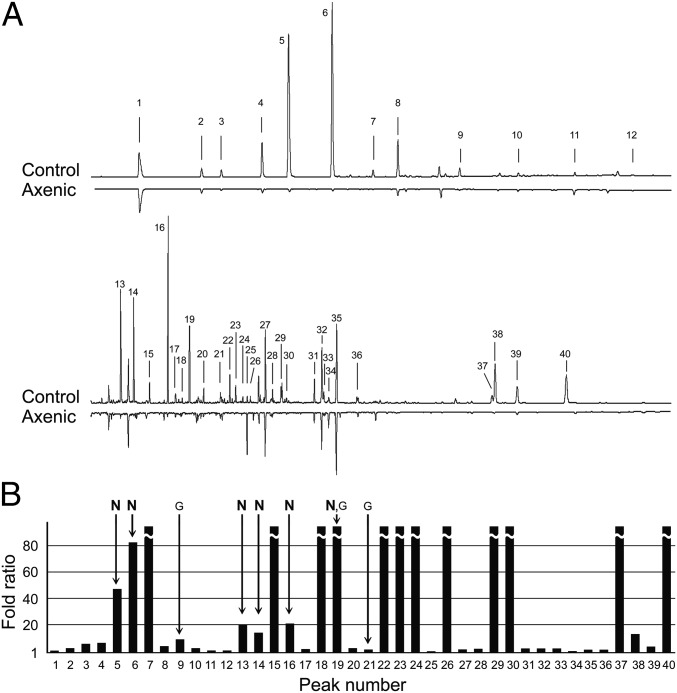
To explore candidate compounds in feces that mediate aggregation behavior, we analyzed VCAs by gas chromatography-flame ionization detection (GC-FID) and GC-MS. VCAs have been suggested as aggregation pheromones in the German cockroach (e.g., refs. 31 and 32), but results have been disparate and inconclusive. Of the 40 VCAs identified in control nonsterile feces, 31 were found in lower amounts (>twofold difference) or were not detected at all in the axenic group; 15 of these compounds occurred at >20-fold higher amounts in nonsterile feces (Fig. 3 and Table S2). Only three related compounds, decanoic acid, 4-methoxybenzoic acid, and 3,4-dimethoxybenzoic acid, were found in considerably larger amounts in sterile feces (<onefold difference) (Table S2).
Fig. 3.
Carboxylic acids extracted from control nonsterile and axenic feces. (A) Gas chromatograms of the control and axenic extracts. Chemical names and percentage composition of each total extract are listed in Table S2. The control group had 65 μg of VCAs per 100 mg of feces, which was fourfold the VCA content of the axenic group. (B) Fold ratio (FR) is the peak area of each compound in the control group divided by its peak area in the axenic group. FR = 1 indicates no effect of the treatment whereas FR > 1 indicates that the axenic condition decreased a particular compound relative to the control extract. Arrows indicate the compounds that were included in the synthetic VCA mixtures for Fig. 4. G, Mix-G (32); N, Mix-NCSU. Three compounds from Mix-G were not detected in our chromatograms (Tables S2 and S3).
Table S2.
Forty carboxylic acids identified in the fecal extracts of the control and axenic groups
| Peak* | Compound IUPAC name (common name) | Control group | Axenic group | ||||
| Rt† | Area§ | RA¶ | Area§ | RA¶ | FR‡ | ||
| Identified as free acids | |||||||
| 1 | Acetic acid | 9.66 | 7.52 | 4.04 | 7.50 | 17.76 | 1.0 |
| 2 | Propanoic acid (propionic) | 10.52 | 1.82 | 0.98 | 0.89 | 2.11 | 2.0 |
| 3 | 2-Methylpropanoic acid (isobutyric) | 10.79 | 1.42 | 0.76 | 0.27 | 0.64 | 5.3 |
| 4 | Butanoic acid (butyric) | 11.35 | 6.48 | 3.49 | 0.99 | 2.34 | 6.5 |
| 5# | 3-Methylbutanoic acid (isovaleric) | 11.71 | 31.89 | 17.15 | 0.68 | 1.61 | 46.9 |
| 6# | Pentanoic acid (valeric) | 12.31 | 31.48 | 16.93 | 0.38 | 0.90 | 82.8 |
| 7 | 4-Methylpentanoic acid (isocaproic) | 12.87 | 1.27 | 0.68 | ND|| | 0.00 | Inf.** |
| 8 | Hexanoic acid (caproic) | 13.22 | 6.42 | 3.45 | 1.62 | 3.84 | 4.0 |
| 9†† | Heptanoic acid (oenanthic) | 14.06 | 2.11 | 1.13 | 0.22 | 0.52 | 9.6 |
| 10 | Octanoic acid (caprylic) | 14.87 | 1.36 | 0.73 | 0.52 | 1.23 | 2.6 |
| 11 | Nonanoic acid (pelargonic) | 15.65 | 1.22 | 0.66 | 1.15 | 2.72 | 1.1 |
| 12 | Decanoic acid (capric) | 16.44 | 0.41 | 0.22 | 0.46 | 1.09 | 0.9 |
| Identified as methyl esters | |||||||
| 13# | Butanedioic acid (succinic) | 11.02 | 9.90 | 5.32 | 0.50 | 1.18 | 19.8 |
| 14# | Benzoic acid | 11.47 | 7.16 | 3.85 | 0.50 | 1.18 | 14.3 |
| 15 | Pentanedioic acid (glutaric) | 12.01 | 1.41 | 0.76 | ND | 0.00 | Inf. |
| 16# | Phenylacetic acid | 12.65 | 11.66 | 6.27 | 0.56 | 1.33 | 20.8 |
| 17 | 2-Hydroxybenzoic acid (salicylic) | 12.91 | 0.78 | 0.42 | 0.53 | 1.26 | 1.5 |
| 18 | 3-Methylhexanedioic acid | 13.14 | 0.45 | 0.24 | ND | 0.00 | Inf. |
| 19#,†† | 3-Phenylpropanoic acid (hydrocinnamic) | 13.38 | 5.04 | 2.71 | ND | 0.00 | Inf. |
| 20 | Heptanedioic acid (pimelic) | 13.88 | 1.01 | 0.54 | 0.51 | 1.21 | 2.0 |
| 21†† | Tetradecanoic acid (myristic) | 14.46 | 1.64 | 0.88 | 1.21 | 2.87 | 1.4 |
| 22 | 3-Methoxybenzoic acid (m-methylsalicylic) | 14.77 | 1.98 | 1.06 | ND | 0.00 | Inf. |
| 23 | 12-Methyltetradecanoic acid (12-methylmyristic) | 14.98 | 1.62 | 0.87 | ND | 0.00 | Inf. |
| 24 | Pentadecanoic acid | 15.22 | 0.54 | 0.29 | ND | 0.00 | Inf. |
| 25 | 4-Methoxybenzoic acid (p-anisic) | 15.37 | 0.50 | 0.27 | 3.60 | 8.53 | 0.1 |
| 26 | Nonanedioic acid (azelaic) | 15.48 | 0.49 | 0.26 | ND | 0.00 | Inf. |
| 27 | Hexadecanoic acid (palmitic) | 16.00 | 5.68 | 3.06 | 4.68 | 11.08 | 1.2 |
| 28 | (9Z)-Hexadec-9-enoic acid (palmitoleic) | 16.25 | 0.99 | 0.53 | 0.48 | 1.14 | 2.1 |
| 29 | 2-Aminobenzoic acid (anthranilic) | 16.57 | 3.05 | 1.64 | ND | 0.00 | Inf. |
| 30 | Heptadecanoic acid (margaric) | 16.78 | 0.35 | 0.19 | ND | 0.00 | Inf. |
| 31 | Octadecanoic acid (stearic) | 17.69 | 2.18 | 1.17 | 0.89 | 2.11 | 2.4 |
| 32 | (9Z)-Octadecenoic acid (oleic) | 17.94 | 5.77 | 3.10 | 2.89 | 6.85 | 2.0 |
| 33 | (9E)-Octadecenoic acid (elaidic) | 18.00 | 1.16 | 0.62 | 0.48 | 1.14 | 2.4 |
| 34 | 3,4-Dimethoxybenzoic acid (veratric) | 18.18 | 0.92 | 0.49 | 2.76 | 6.54 | 0.3 |
| 35 | (9Z,12Z)-9,12-Octadecadienoic acid (linoleic) | 18.45 | 8.02 | 4.31 | 5.96 | 14.12 | 1.3 |
| 36 | (9Z,12Z,15Z)-9,12,15-Octadecatrienoic acid (linolenic) | 19.15 | 0.67 | 0.36 | 0.52 | 1.23 | 1.3 |
| 37 | 3-Hydroxybezoic acid (m-salicylic) | 23.80 | 1.50 | 0.81 | ND | 0.00 | Inf. |
| 38 | 4-Methoxyphenylacetic acid (homoanisic) | 23.90 | 7.45 | 4.01 | 0.58 | 1.37 | 12.8 |
| 39 | 4-Hydroxyphenylacetic acid | 24.66 | 3.59 | 1.93 | 0.89 | 2.11 | 4.0 |
| 40 | 4-(4-Hydroxyphenyl)butyric acid | 26.36 | 7.01 | 3.77 | ND | 0.00 | Inf. |
| Total area of all FID peaks | 185.92 | 42.22 | |||||
Compounds corresponding to peak numbers 1–12 were identified as underivatized free acids. Compounds corresponding to peak numbers 13–40 were identified as derivatized methyl esters. The 100 mg of control cockroach feces extract contain ∼65 µg of acids. IUPAC, International Union of Pure and Applied Chemistry.
Peak numbers correspond to Fig. 3.
RT, retention time, min.
FR, fold ratio [(peak area of control group)/(peak area of axenic group)].
Peak area.
RA, relative peak area, % = (each peak area/total area of all FID peaks) × 100.
Bolded names indicate compounds used in Mix-NCSU.
ND, not detected.
Inf., infinite (the compound was detected in the control group extract but not in the axenic extract).
Compounds used in Mix-G (32). Three components of this mix (cyclohexanecarboxylic acid, 3-phenyllactic acid, and decanedioic acid) were not detected in either axenic or control feces. 3-Phenylpropanoic acid (no. 19) was common to both mixes.
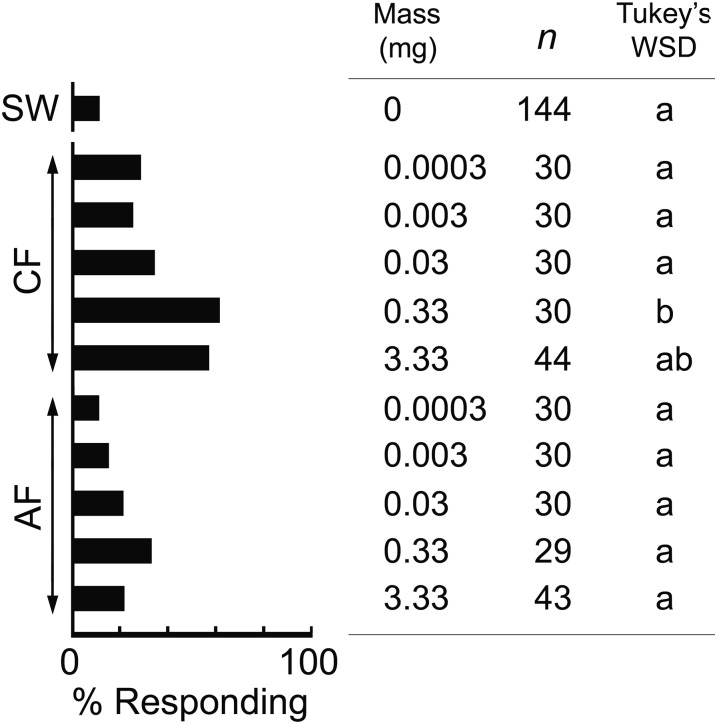
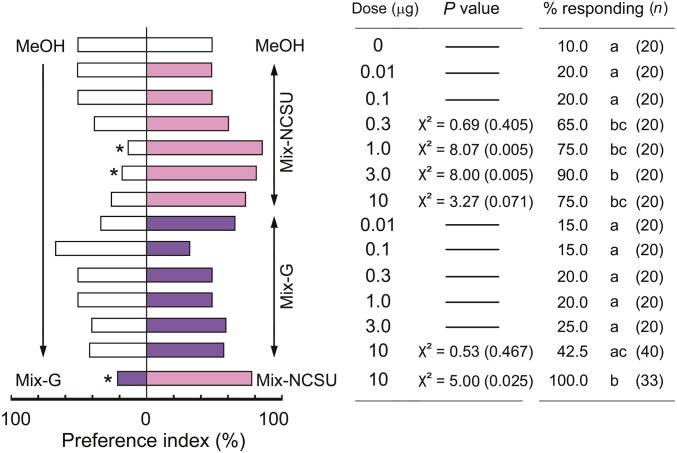
We conjectured that the most underrepresented VCAs in axenic feces contribute most as aggregation agents. A blend of six major compounds (Tables S2 and S3 and Fig. 3), designated Mix-NCSU, was significantly preferred over the solvent vehicle control and increased the number of nymphs that responded in a dose-dependent manner (Fig. 4). We also prepared Mix-G, a previously reported effective blend of six fecal VCAs (32) (Tables S2 and S3 and Fig. 3). Notably, three of these compounds were not detected in our control group, heptanoic acid and tetradecanoic acid occurred in low amounts, and only 3-phenylpropanoic acid was well represented in both blends (Tables S2 and S3 and Fig. 3). At the highest dose (10 µg) Mix-G stimulated nymphs to walk upwind in the olfactometer, but they had no preference for this blend at any dose (Fig. 4). In a direct comparison of the two six-compound blends, 100% of the nymphs responded with a significant preference for Mix-NCSU.
Table S3.
Two VCA blends as candidate aggregation pheromones
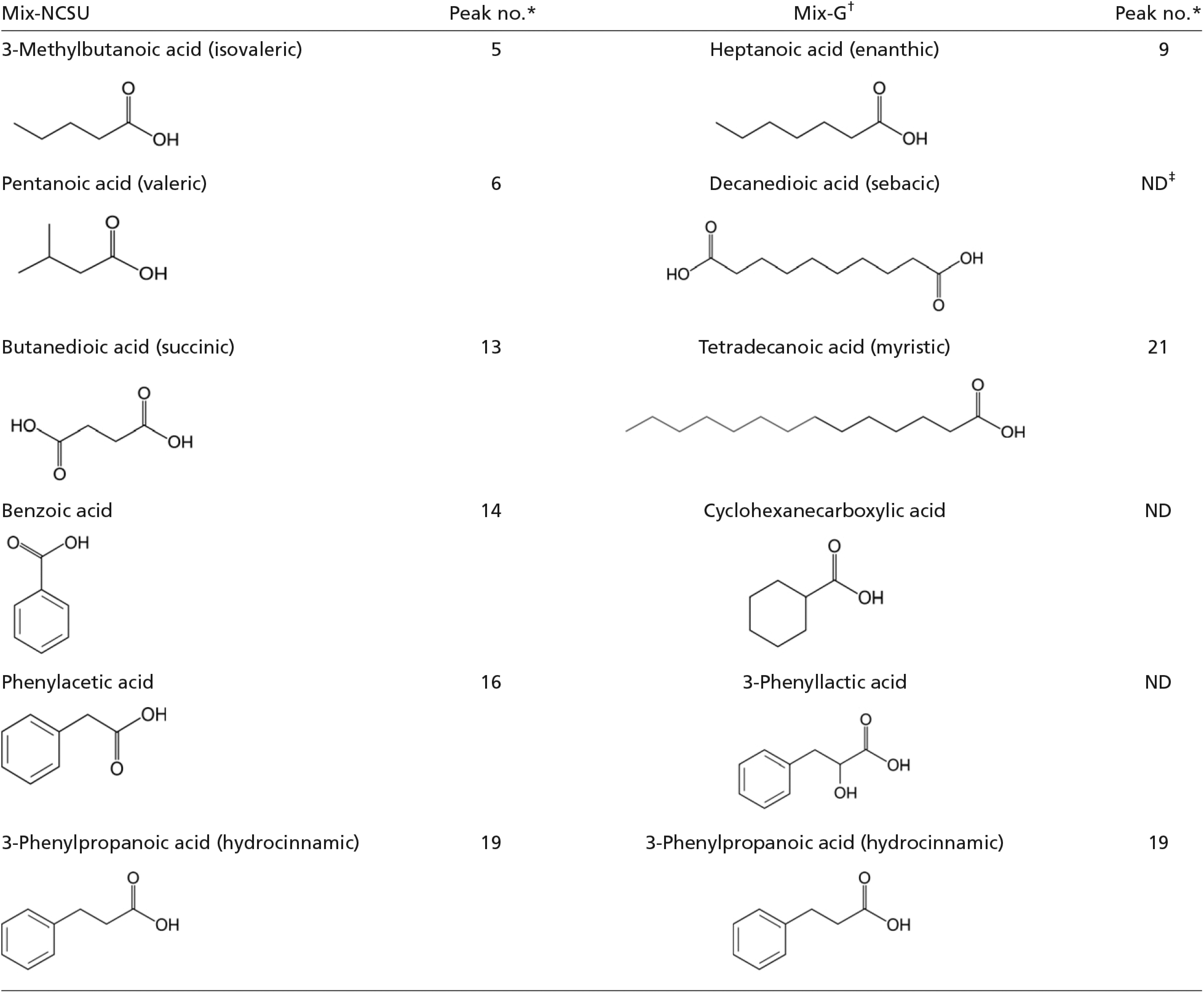 |
Fig. 4.
Orientation responses of fifth instar nymphs to synthetic mixtures of carboxylic acids. Orientation assays were conducted in two-choice olfactometers with individual nymphs (n = total). The significance of the preference index was tested with χ2 tests (asterisks, P < 0.05). Statistical analyses could not be conducted for some assays because few nymphs responded. The percentage of tested nymphs that responded to both stimuli is also shown, and different letters indicate significant differences among treatments (Tukey’s WSD, P < 0.05). Synthetic Mix-G was prepared according to ref. 32. Mix-NCSU consisted of six major VCAs in the control group that showed >10× abundance (fold-ratio) relative to axenic feces (Table S2 and Fig. 3).
Inoculation of Axenic Feces with Bacterial Isolates from Control Feces Rescues the Aggregation Response.
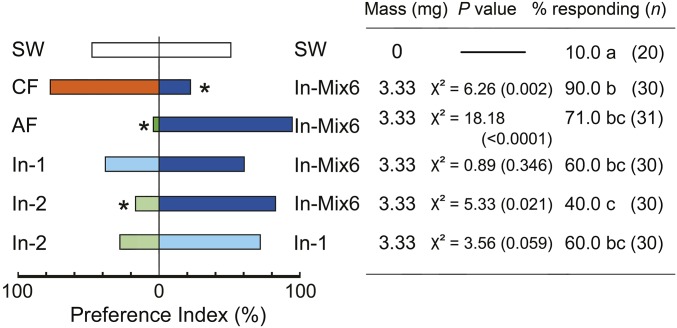
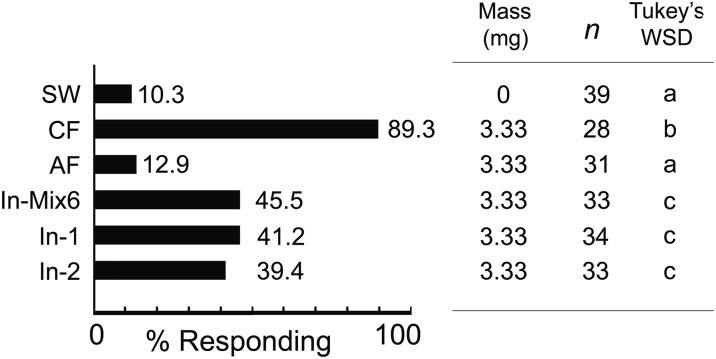
Because fecal VCAs are common metabolites of gut microbial communities (e.g., refs. 38 and 39), we hypothesized that bacterial inoculation of sterile feces would rescue the aggregation response. Bacteria from feces of the control colony were cultured and identified by PCR amplification and sequencing of 16S rDNA (Table S4). Although aerobic bacteria were found in the control feces, none were detected in the feces of the axenic cockroaches. The concentration of bacteria (cfu per mg) was 7.87 ± 0.38 × 106 on trypticase soy agar (TSA), 2.56 ± 0.21 × 103 on MacConkey agar (MAC), and 5.15 ± 0.17 × 106 on modified Enterococcus agar (mENT). We detected six bacterial species in four genera (Enterococcus, Weissella, Pseudomonas, and Acinetobacter), all of which represent environmental and commensal bacteria (Table S4). Interestingly, the bacterial community was dominated by Enterococcus avium, and no typical enteric bacteria (Enterobacteriaceae) were detected. The low cfu count on MacConkey agar represented Pseudomonas and Acinetobacter sp. In two-choice olfactometer assays, 90% of the nymphs were stimulated to walk upwind, and they significantly preferred the control nonsterile feces extracts (CF) over the fecal extracts of cockroaches inoculated with a mix of six bacterial isolates (In-Mix6) (Fig. 5). This mix, however, effectively rescued the aggregation response in comparison with the extract from axenic feces. Generally, the In-Mix6 feces was preferred by nymphs over the single inoculates, including In-1 (E. avium) and In-2 (Weissella cibaria). Feces containing E. avium was more attractive to nymphs than feces containing W. cibaria (Fig. 5). Similarly, in no-choice olfactometer bioassays, the highest responses were to the control feces, and the lowest to the axenic feces (Fig. S3). All three bacterial inoculations significantly increased the numbers of responding nymphs, but not to the level of response to the control feces. These results suggest that various bacterial species contribute to the production of fecal aggregation agents, but multiple species in the gut additively or synergistically contribute to the potency of cockroach aggregation agents under natural conditions.
Table S4.
Bacterial isolates from control cockroach feces
| Isolate | Identification | Length, bp | Identity, % |
| In-1 | Enterococcus avium | 791 | 99 |
| In-2 | Weissella cibaria | 793 | 100 |
| In-3 | Pseudomonas japonica | 772 | 99 |
| In-4 | Pseudomonas monteilii | 768 | 99 |
| In-5 | Acinetobacter pittii | 767 | 99 |
| In-6 | Acinetobacter sp. | 765 | 96 |
Fig. 5.
Differential orientation of fifth instar nymphs toward fecal extracts of bacteria-inoculated cockroaches. Individuals (n total) were tested with sterile water (SW), extracts of control feces (CF), axenic feces (AF), and feces from bacteria-inoculated axenic groups (In-Mix6, In-1, and In-2). The bacterial isolates are listed in Table S4. The preference index was derived from orientation assays in two-choice olfactometers and was statistically tested with a χ2 test (asterisks, P < 0.05). The percentages of nymphs that responded to either stimulus in the choice tests are shown, and different letters indicate significant differences among treatments (Tukey’s WSD, P < 0.05). The results indicate that environmental bacteria contribute to the production of fecal aggregation agents and that multiple bacteria species are involved in producing attractants.
Fig. S3.
Differential responses of fifth instar nymphs toward fecal extracts from axenic nymphs inoculated with various bacterial isolates. Fifth instar nymphs (n total) were tested in no-choice olfactometers with sterile water (SW), extracts of control feces (CF), axenic feces (AF), and feces from bacteria-inoculated axenic groups (In-Mix6, In-1, and In-2). The bacterial isolates are listed in Table S4. Responding (%) is the percentage of tested nymphs that were stimulated to orient upwind and shelter on the filter paper. Different letters indicate significant differences among treatments (Tukey’s WSD, P < 0.05).
Discussion
VCAs Act as Fecal Aggregation Agents.
The excreta of animals contain blends of species-typical compounds (e.g., refs. 40 and 41) and compounds that reflect the health (e.g., ref. 42), habitat, and diet (1, 43, 44) of animals. Subsets of these chemicals can serve as conspecific signals demarcating territories (16) or as aggregation signals, as observed in bark beetles (45), locusts (37), bed bugs (46), firebrats (47), and cockroaches. All life stages of the German cockroach are attracted to conspecific feces, and various chemical constituents of feces have been implicated as aggregation pheromones. In this study, we demonstrated that fecal VCAs act as cockroach aggregation pheromones and that many of the bioactive VCAs are likely of microbial origin.
Various VCAs have been considered cockroach semiochemicals in several contexts, including as aggregation agents (31, 32), repellents (35), necromones (48), and food attractants (49). Broad disparities among studies as to which chemicals guide aggregation behavior in cockroaches suggest that aggregation agents likely operate differently from sex pheromones. Species specificity is encoded in sex pheromones by chemical components and their overall blend ratio. Cockroach fecal VCAs that serve as aggregation agents, on the other hand, seem much less species-specific (e.g., ref. 50), and variation in their quality and quantity may depend to a large extent on environmental factors such as food quality, ingested microbes, and the cockroach strain. Thus, some VCAs were found in relatively large quantities in our investigation (e.g., butanedioic acid) but were not detected in other studies. Conversely, some carboxylic acids [e.g., 2-hydroxypropanoic acid (lactic acid)] were previously described as prominent aggregation agents (51) but were not detected in our GC-MS analysis.
In support of the idea that the composition of aggregation signals is plastic was the observation that synthetic blends consisting of equal amounts of VCAs (i.e., Mix-NCSU and Mix-G) elicited aggregation activity in cockroaches regardless of the blend ratios. Nevertheless, cockroaches in our assays preferred a mix of six VCAs that reflected abundant compounds in their own colony feces over six VCAs (Mix-G) reported from another cockroach colony (32). Although it still remains unclear whether the variation in VCAs indicates that VCAs act as pheromones with intraspecific variation or as signature mixtures, as in ant colony odors (16), these results strongly suggest that the fecal VCAs act not only as aggregation agents, but also as a means for cockroaches to discriminate their own colony from other colonies that emit unfamiliar VCAs.
Gut Bacterial Community Influences Fecal VCA Profiles.
We hypothesized that VCA emissions are related to the gut/feces microbiota and that across-colony variation in VCA profiles could be the result of differences in environmentally acquired gut microbial communities. Because elimination of horizontally acquired gut microbes did not affect the period of nymphal development, these microbes do not seem to have major short-term effects on nutrition or performance (the sterilization procedure did not eliminate vertically transmitted endosymbiotic bacteria). However, elimination of gut microbes dramatically diminished the attractiveness of their feces in aggregation bioassays, and the feces of axenically reared cockroaches had much lower amounts of VCAs. Moreover, inoculation of axenic cockroaches with bacteria isolated from cockroach feces rescued the attractiveness of feces in aggregation bioassays, and this response intensified with the complexity of the bacterial inoculate: A mix of six bacterial isolates was significantly more attractive than single species inoculates. Cockroach feces likely contains other bacteria that did not grow aerobically and on TSA. This observation reflects the fact that fresh cockroach feces stimulated aggregation significantly more than fresh axenic feces inoculated with the mixture of six bacterial isolates.
At least three nonmutually exclusive mechanisms could function in the bacteria-dependent production of VCAs: Commensal bacteria may stimulate the cockroach to produce VCAs, they may function within a consortium of other microorganisms that produce VCAs, or the gut bacteria unaided produce the VCAs either before or after feces is excreted. These diverse mechanisms of semiochemical production have not been uncoupled in insects (52). Some bacteria (e.g., Pantoea agglomerans and other Enterobacteriaceae) have been shown to produce guaiacol as an aggregation pheromone component of the locust Schistocerca gregaria (37), and metabolites associated with Enterobacter cloacae and the fungus Mycotypha microspora stimulate aggregation behavior in the firebrat Thermobia domestica (47). Because cockroach feces contains highly volatile constituents yet it remains attractive to nymphs long after it is excreted, it is likely that bacteria in feces continue to produce VCAs. The bacterial isolates tested in our study, including E. avium, W. cibaria, Pseudomonas sp., and Acinetobacter sp., are tolerant of a wide range of environmental conditions and would be capable of continued metabolism in the excreted feces. The isolates that we tested individually, E. avium and W. cibaria, have fermentative metabolism and are capable of producing various VCAs (53, 54). We therefore suggest that the VCAs that serve as aggregation agents in the German cockroach are not endogenously produced by the cockroach but rather are produced by commensal gut bacteria obtained from the environment.
Recently, the gut microbiota of the German cockroach has been characterized (55). The embryo contained only vertically transmitted maternal Blattabacterium (Bacteroidetes) endosymbionts and no gut bacteria, but diverse environmental bacteria colonized the gut of first instar nymphs and the bacterial load increased 100-fold in the second instar. The bacterial community remained largely stable through the rest of nymphal development and in the adult male although gradual changes in the microbial diversity resulted in significant differences between nymphs and adult males (55). Together with our results and extensive evidence of coprophagy, especially in neonates (24), the German cockroach most likely acquires bacteria through its diet and by ingesting conspecific feces in aggregations. The omnivorous and coprophagous habits of cockroaches are expected to generate highly variable gut microbiomes that emit highly variable blends of fecal VCAs. Within aggregations, however, it is possible that coprophagy promotes a preference for VCAs produced by the local gut bacterial community and thus may drive preference and fidelity to specific aggregation sites. It is not surprising, therefore, that nymphs in our study were attracted more to VCAs from their own colony (Mix-NCSU) than to VCAs that had been reported as aggregation agents in another colony (Mix-G). This difference in odor preference suggests that nymphs may discriminate familiar from unfamiliar odors, as do members of social insect colonies.
Information Content of Aggregation Cues.
Aggregation stimuli guide the accumulation and persistence of individuals at a fixed location, and semiochemicals that influence aggregation can operate differently in different ecological and evolutionary contexts (16). Aggregation signals are pheromonal in nature and mediate communication between proper signalers and proper responders. Aggregation cues, on the other hand, may be used by conspecifics to eavesdrop on inadvertently emitted cues for their own benefit. Gregarious and social insects, for example ants, use both aggregation cues (e.g., colony odor, signature mixtures) and signals (e.g., queen pheromones) in different contexts (16). Our study suggests that cockroach fecal VCA profiles act as highly variable signature mixtures because they are produced by a locally determined and highly variable gut microbial community. This variability of a signature mixture is in stark contrast to the much less variable species-specific pheromones, such as queen pheromones in ants and sex pheromones in moths. The VCAs might provide the German cockroach with explicit information about the physiological status of the colony (health, infections, demography) and environmental conditions, such as population density. Moreover, when these emissions are associated with feces, they can convey information about food quality and the associated microbes. Young nymphs are especially dependent on such information because they need to colonize their gut with beneficial microbes and they are more susceptible to abiotic and biotic hazards, such as desiccation and predation.
This study tested only a mixture of six VCAs selected by their relative representation in normal and axenically produced feces. Interestingly, the axenically produced feces did not completely lose its attractiveness to first instar nymphs in aggregation bioassays (Fig. 2). Therefore, we cannot exclude the possibilities that some VCAs are produced by the cockroach itself or by nonbacterial gut microbes that were not eliminated by our sterilization procedures. Moreover, other fecal components, such as by-products of nitrogen metabolism, have been suggested as aggregation pheromones (33), and it is possible that ammonia and related compounds convey reliable information about nitrogen availability. Proteins are a limited resource for cockroaches, and this lineage of insects evolved diverse and highly efficient strategies to acquire, preserve, and recycle nitrogen (56). Members of the gut microbiota likely play prominent roles in producing and emitting these cues as well.
To define the function of fecal VCAs and the contribution of the gut microbial community, future work will need to determine whether the six VCAs of Mix-NCSU are necessary and sufficient and in what ratios. Comparative studies of fecal VCAs of cockroach colonies with different gut microbiota will reveal whether fecal odors mediate colony and site recognition and fidelity.
Materials and Methods
Insects.
A laboratory strain of B. germanica (Orlando Normal, collected in a Florida apartment >60 y ago; American Cyanamid) was reared on water and food pellets (Purina No. 5001 Rodent Diet; PMI Nutrition International) at 27 ± 1 °C, 40–70% relative humidity, and light:dark = 12:12 photoperiod.
Fecal Materials from Control and Axenic Cockroaches.
For the control groups of cockroaches, three egg cases (each containing about 40 eggs) were separated, and the nymphs that emerged received 100 mg of feces from the original colony, in addition to food and water. For the axenic groups of cockroaches, the surface of three egg cases was sterilized with 0.5% sodium hypochlorite for 1 min and 70% (vol/vol) ethanol for 1 min and then rinsed three times with sterile water. The nymphs that emerged were reared on sterile (autoclaved) food, water, and 100 mg of autoclaved feces in sterilized cages with double filters to prevent contamination from air. For the axenic bacteria-inoculated groups, axenically reared first instars were exposed to sterile food inoculated with individual or a mix of bacterial isolates in PBS. Three inoculated groups were prepared using six bacterial isolates from the original colony (Table S4): A mix of six isolates (In-Mix6), E. avium (In-1) and W. cibaria (In-2). The inoculated nymphs were reared under axenic conditions. Feces from each colony was aseptically collected within 4 d of emergence of the first adult females and mixed in sterile water for species identification, cfu determinations, GC-MS analysis, and behavioral tests.
Chemical Analysis of VCAs in Feces.
See SI Materials and Methods for information relating to these methodologies.
Bacterial Count and Identification.
Ten milligrams of feces were homogenized in 10 mL of PBS, serially diluted, and spread-plated in triplicate on trypticase soy agar (TSA), MacConkey agar (MAC), and modified Enterococcus agar (mENT). TSA was incubated in 26 °C for 72 h, MAC in 37 °C for 24 h, and mENT in 37 °C for 48 h. Colony forming units (cfu) were counted and calculated per mg of fresh feces. Morphologically different colonies were isolated on TSA and identified by amplification and sequencing of ∼800 bp of the 16S rRNA gene with universal eubacterial primers 8F (5′-AGAGTTTGATCC TGGCTCAG-3′) and 806R (5′- CTACCAGGGTATCTAAT-3′) following standard protocols. Sequences were manually edited in CodonCode Aligner (version 1.3.4) (CodonCode Corporation) and identified by a basic local alignment search tool (BLAST) search of the GenBank database.
Aggregation Bioassays.
Test samples.
Feces of each cockroach group were extracted in sterile water (200 mg/mL) with vortexing for 3 min and centrifuged (11,750 × g, 10 min), and the fresh supernatant was used in behavioral assays. Synthetic mixtures of VCAs for Fig. 4 were prepared as follows. Mix-G was prepared according to ref. 32: cyclohexanecarboxylic acid, 3-phenyllactic acid, decanedioic acid (sebacic acid), heptanoic acid (oenanthic acid), tetradecanoic acid (myristic acid), and 3-phenylpropanoic acid (3-phenylpropionic acid). Mix-NCSU (3-methylbutanoic acid, pentanoic acid, butanedioic acid, benzoic acid, phenylacetic acid, and 3-phenylpropanoic acid) was prepared by comparing the VCA peaks of control and axenic fecal extracts. A fold-ratio (FR) was calculated (Table S2 and Fig. 3) as the Peak area of each compound in the control group/peak area of each compound in the axenic group. Mixtures of VCAs for behavioral tests contained equal amounts of each VCA dissolved in methanol (total 10 mg/mL).
Two-choice sheltering bioassays with first instar nymphs.
The aggregation responses of nymphs to test samples were assayed in 15 cm × 2.5 cm Petri dishes. Two pieces of tent-shaped filter papers (FP1 and FP2; 2.5 × 2.5 cm) were each impregnated with 50 µL of a test sample. First instar nymphs (10–30 per assay) were introduced in the center of the Petri dish between the two tents. The distribution of nymphs between the two filter papers was noted during the photophase 24 h later. We derived an aggregation index (AI) for each test sample: AIFP1 = (FP1mean)/(FP1mean + FP2mean) and AIFP2 = (FP2mean)/(FP1mean + FP2mean) where FP1mean and FP2mean are the averages of nymphs on FP1 and FP2, respectively. When the nymphs chose both filter papers equally, AIFP1 and AIFP2 are each 50%. When all tested nymphs chose either FP1 or FP2, the AI is either 100% or 0%. Differences in aggregation between FP1 and FP2 were tested using the Sign-test (P < 0.05). The percentages of nymphs that responded were compared with Tukey’s wholly significant difference (WSD) (P < 0.05). No-choice sheltering assays have limited resolution because nymphs have a high propensity to shelter during the photophase even under clean filter papers.
Two-choice and no-choice olfactometer bioassays with fifth instar nymphs.
Preferences of individual fifth instar nymphs for test samples were tested with straight-tube olfactometers, as described in ref. 57. SI Materials and Methods has additional information relating to these methodologies. To evaluate orientation and preference of nymphs to each stimulus independently of other stimuli, no-choice assays were carried out with a single test sample. The percentages of responders were compared using Tukey’s WSD (P < 0.05). In all bioassays, nymphs were used only once.
SI Materials and Methods
Chemical Analysis of VCAs in Control and Axenic Feces.
For the determination of VCAs, diethyl ether solutions of axenic and control feces were individually prepared. Feces (100 mg each) was soaked in 1 mL of diethyl ether for 1 h in a 2-mL glass vial. The yellow supernatants were decanted into new vials, and the diethyl ether solutions were extracted with a 5% NaOH aqueous solution and shaken vigorously (3× 1 mL). The water layers were separated and combined, and the NaOH aqueous solutions were then acidified to pH 1 with 2 M HCl. The acidified solutions were extracted with diethyl ether (3× 1 mL). The combined diethyl ether solutions were dried over Na2SO4 and concentrated to ∼100 µL. Each of the diethyl ether extracts of the axenic and control feces was further divided into two equal samples. The first sample (50 µL) was analyzed directly as free acids (peaks 1–12), and the second sample (50 µL) was derivatized with 10 drops of diazomethane and analyzed as methyl esters (peaks 13–40).
Quantitative analysis was conducted by GC-FID (5890; Hewlett Packard), and qualitative analysis was conducted by GC coupled with an Agilent 5973 mass selective detector using the same DB-WAXetr columns (J&W Scientific Inc.) (60 m × 0.25 mm i.d., 0.25 μm film thickness; temperature programmed at 50 °C for 2 min, then to 250 °C at 25 °C/min and held for 15 min) in splitless mode with hydrogen (FID) or helium (MS) as carrier gas and linear velocity of 36 cm/s. A 70-eV electron beam was used for MS sample ionization. Mass spectra and retention times were compared with authentic carboxylic acids.
Two-Choice and No-Choice Olfactometer Bioassays with Fifth Instar Nymphs.
xEach olfactometer consisted of a Plexiglas tube (54.5 cm long, 3.2 cm i.d.) with a 15-cm-long divider sealed vertically in the upwind end. A tube cage (15 cm long, 3.2 cm i.d.) with a swivel metal screen gate was used to introduce a single nymph into the downwind end of the olfactometer. Each olfactometer was connected to a vacuum pump that provided a linear air velocity of 25 cm/s through the tube; the tubes were exhausted outside the building. Fluorescent lights covered with red photographic safety filters placed 60 cm below and above the olfactometers facilitated observation in the dark. A single fifth instar nymph was allowed to acclimate to the airflow for 5 min, and only quiescent insects were used in the assays. Filter papers (FP1 and FP2, 1 × 3 cm) were impregnated with 20 μL of solvent or test sample and placed in the upwind end of the olfactometer. The positions of the two treatments were randomly switched between the left and right sides. When a nymph was quiescent, the gate was opened carefully, filter paper stimuli were introduced at the upwind end of the olfactometer, and the nymph’s response was noted by visual observation. A positive response was scored when the insect approached the filter paper within 3 min and remained on the filter paper for 2 min. A preference index was calculated for fifth instars using the same approach as for the first instar aggregation index. Responses to FP1 and FP2 were compared by χ2 test (P < 0.05).
The same apparatus was used in no-choice assays, but with only one stimulus.
Acknowledgments
We thank Rick Santangelo for maintaining the insect colonies. This investigation was supported in part by US Department of Housing and Urban Development Healthy Homes Program Awards NCHHU0001-11 and NCHHU0017-13, Alfred P. Sloan Foundation Award 2013-5-35 MBE, National Science Foundation Award IOS-1456973, and the Blanton J. Whitmire endowment at North Carolina State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504031112/-/DCSupplemental.
References
- 1.Douglas AE. Multiorganismal insects: Diversity and function of resident microorganisms. Annu Rev Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011;27(11):514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikuchi Y, et al. Symbiont-mediated insecticide resistance. Proc Natl Acad Sci USA. 2012;109(22):8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boissière A, et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012;8(5):e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frago E, Dicke M, Godfray HCJ. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol Evol. 2012;27(12):705–711. doi: 10.1016/j.tree.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Chu CC, Spencer JL, Curzi MJ, Zavala JA, Seufferheld MJ. Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc Natl Acad Sci USA. 2013;110(29):11917–11922. doi: 10.1073/pnas.1301886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas AE. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol. 1998;43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 2014;12(3):168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- 9.Sabree ZL, Kambhampati S, Moran NA. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc Natl Acad Sci USA. 2009;106(46):19521–19526. doi: 10.1073/pnas.0907504106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vásquez A, et al. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS One. 2012;7(3):e33188. doi: 10.1371/journal.pone.0033188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6(10):741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 12.Martinez J, et al. Symbionts commonly provide broad spectrum resistance to viruses in insects: A comparative analysis of Wolbachia strains. PLoS Pathog. 2014;10(9):e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikoh N, et al. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc Natl Acad Sci USA. 2014;111(28):10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. Animal behavior and the microbiome. Science. 2012;338(6104):198–199. doi: 10.1126/science.1227412. [DOI] [PubMed] [Google Scholar]
- 15.Sharon G, et al. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA. 2010;107(46):20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyatt TD. Pheromones and Animal Behavior: Chemical Signals and Signatures. 2nd Ed Cambridge Univ Press; Cambridge, UK: 2014. [Google Scholar]
- 17.Ferrari J, Vavre F. Bacterial symbionts in insects or the story of communities affecting communities. Philos Trans R Soc Lond B Biol Sci. 2011;366(1569):1389–1400. doi: 10.1098/rstb.2010.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gore JC, Schal C. Cockroach allergen biology and mitigation in the indoor environment. Annu Rev Entomol. 2007;52:439–463. doi: 10.1146/annurev.ento.52.110405.091313. [DOI] [PubMed] [Google Scholar]
- 19.Schal C, Hamilton RL. Integrated suppression of synanthropic cockroaches. Annu Rev Entomol. 1990;35:521–551. doi: 10.1146/annurev.en.35.010190.002513. [DOI] [PubMed] [Google Scholar]
- 20.Rust MK, Owens JM, Reierson DA. Understanding and Controlling the German Cockroach. Oxford Univ Press; New York: 1995. [Google Scholar]
- 21.Willis ER, Riser GR, Roth LM. Observations on reproduction and development in cockroaches. Ann Entomol Soc Am. 1958;51(1):53–69. [Google Scholar]
- 22.Uzsák A, Schal C. Differential physiological responses of the German cockroach to social interactions during the ovarian cycle. J Exp Biol. 2012;215(Pt 17):3037–3044. doi: 10.1242/jeb.069997. [DOI] [PubMed] [Google Scholar]
- 23.Uzsak A, Schal C. Social interaction facilitates reproduction in male German cockroaches, Blatttella germanica. Anim Behav. 2013;85(6):1501–1509. [Google Scholar]
- 24.Kopanic RJ, Holbrook GL, Sevala V, Schal C. An adaptive benefit of facultative coprophagy in the German cockroach Blattella germanica. Ecol Entomol. 2001;26(2):154–162. [Google Scholar]
- 25.Sreng L, Cloarec A, Rivault C. Cuticular extracts inducing aggregation in the German cockroach, Blattella germanica (L.) J Insect Physiol. 1998;44(10):909–918. doi: 10.1016/s0022-1910(98)00062-6. [DOI] [PubMed] [Google Scholar]
- 26.Ross MH, Tignor KR. Response of German cockroaches to a dispersant emitted by adult females. Entomol Exp Appl. 1985;39(1):15–20. [Google Scholar]
- 27.Ishii S, Kuwahara Y. Aggregation of German cockroach (Blattella germanica) nymphs. Experientia. 1968;24(1):88–89. doi: 10.1007/BF02136814. [DOI] [PubMed] [Google Scholar]
- 28.Sakuma M, Fukami H. Dose response relations in taxes of nymphs of the German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae) to their aggregation pheromone. Appl Entomol Zool (Jpn) 1990;25(1):9–16. [Google Scholar]
- 29.Ishii S. An aggregation pheromone of the German cockroach Blattella germanica L. (Orthoptera: Blattellidae). I. Site of the pheromone production. Appl Entomol Zool (Jpn) 1967;2:203–217. [Google Scholar]
- 30.Sakuma M, Fukami H. Aggregation arrestant pheromone of the German cockroach,Blattella germanica (L.) (Dictyoptera: Blattellidae): Isolation and structure elucidation of blattellastanoside-A and -B. J Chem Ecol. 1993;19(11):2521–2541. doi: 10.1007/BF00980688. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs MEA, Franke S, Francke W. Carboxylic acids in the feces of Blattella germanica (L) and their possible role as part of the aggregation pheromone. Zeitschrift Fur Angewandte Entomologie-Journal of Applied Entomology. 1985;99(5):499–503. [Google Scholar]
- 32.Scherkenbeck J, et al. Aggregation agents in German cockroach Blattella germanica: Examination of efficacy. J Chem Ecol. 1999;25(5):1105–1119. [Google Scholar]
- 33.Sakuma M, Fukami H, Kuwahara Y. Attractiveness of alkylamines and aminoalcohols related to the aggregation attractant pheromone of the German cockroach, Blattella germanica (L) (Dictyoptera: Blattellidae) Appl Entomol Zool (Jpn) 1997;32(1):197–205. [Google Scholar]
- 34.Mori K, Fukamatsu K, Kido K. Synthesis of chlorinated steroids related to the structures proposed for Blattellastanosides A and B, the aggregation pheromone of the German cockroach, Blattella germanica L. Liebigs Ann Chem. 1993;6:657–663. [Google Scholar]
- 35.McFarlane JE. Repellent effect of volatile fatty acids of frass on larvae of german cockroach,Blattella germanica (L.) (Dictyoptera: Blattellidae) J Chem Ecol. 1984;10(11):1617–1622. doi: 10.1007/BF00988429. [DOI] [PubMed] [Google Scholar]
- 36.Hoyt CP, Osborne GO, Mulcock AP. Production of an insect sex attractant by symbiotic bacteria. Nature. 1971;230(5294):472–473. doi: 10.1038/230472a0. [DOI] [PubMed] [Google Scholar]
- 37.Dillon R, Charnley K. Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res Microbiol. 2002;153(8):503–509. doi: 10.1016/s0923-2508(02)01361-x. [DOI] [PubMed] [Google Scholar]
- 38.Topping DL. Short-chain fatty acids produced by intestinal bacteria. Asia Pac J Clin Nutr. 1996;5(1):15–19. [PubMed] [Google Scholar]
- 39.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 40.Wood JR, et al. High-resolution coproecology: Using coprolites to reconstruct the habits and habitats of New Zealand’s extinct upland moa (Megalapteryx didinus) PLoS One. 2012;7(6):e40025. doi: 10.1371/journal.pone.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bon C, et al. Coprolites as a source of information on the genome and diet of the cave hyena. Proc Biol Sci. 2012;279(1739):2825–2830. doi: 10.1098/rspb.2012.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garner CE, et al. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007;21(8):1675–1688. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- 43.McFarlane JE, Alli I. Volatile fatty acids of frass of certain omnivorous insects. J Chem Ecol. 1985;11(1):59–63. doi: 10.1007/BF00987605. [DOI] [PubMed] [Google Scholar]
- 44.Pascher M, Hellweg P, Khol-Parisini A, Zentek J. Effects of a probiotic Lactobacillus acidophilus strain on feed tolerance in dogs with non-specific dietary sensitivity. Arch Anim Nutr. 2008;62(2):107–116. doi: 10.1080/17450390801892583. [DOI] [PubMed] [Google Scholar]
- 45.Wermelinger B. Ecology and management of the spruce bark beetle Ips typographus: A review of recent research. For Ecol Manage. 2004;202(1-3):67–82. [Google Scholar]
- 46.Gries R, et al. Bed bug aggregation pheromone finally identified. Angew Chem Int Ed Engl. 2015;54(4):1135–1138. doi: 10.1002/anie.201409890. [DOI] [PubMed] [Google Scholar]
- 47.Woodbury N, Gries G. Firebrats, Thermobia domestica, aggregate in response to the microbes Enterobacter cloacae and Mycotypha microspora. Entomol Exp Appl. 2013;147(2):154–159. [Google Scholar]
- 48.Rollo CD, Czyzewska E, Borden JH. Fatty acid necromones for cockroaches. Naturwissenschaften. 1994;81(9):409–410. [Google Scholar]
- 49.Tsuji H. Attractive and feeding stimulative effect of some fatty acids and related compounds on three species of cockroaches. Jap J Sanit Zool. 1966;17:89–97. [Google Scholar]
- 50.Rust MK, Appel AG. Intraspecific and interspecific aggregation in some nymphal blattellid cockroaches (Dictyoptera, Blattellidae) Ann Entomol Soc Am. 1985;78(1):107–110. [Google Scholar]
- 51.McFarlane JE, Alli I. Aggregation of larvae ofBlattella germanica (L.) by lactic acid present in excreta. J Chem Ecol. 1986;12(6):1369–1375. doi: 10.1007/BF01012356. [DOI] [PubMed] [Google Scholar]
- 52.Douglas AE, Dobson AJ. New synthesis: Animal communication mediated by microbes: fact or fantasy? J Chem Ecol. 2013;39(9):1149. doi: 10.1007/s10886-013-0343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huycke MM. 2002. Physiology of enterococci.The Enterococci: Pathogenesis, Molecular Biology and Antibiotic Resistance, eds Gilmore MS, et al. (ASM, Washington, DC), pp 133–177.
- 54.Björkroth KJ, et al. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int J Syst Evol Microbiol. 2002;52(Pt 1):141–148. doi: 10.1099/00207713-52-1-141. [DOI] [PubMed] [Google Scholar]
- 55.Carrasco P, et al. Succession of the gut microbiota in the cockroach Blattella germanica. Int Microbiol. 2014;17(2):99–109. doi: 10.2436/20.1501.01.212. [DOI] [PubMed] [Google Scholar]
- 56.Mullins DE. Physiology of environmental adaptations and resource acquisition in cockroaches. Annu Rev Entomol. 2015;60:473–492. doi: 10.1146/annurev-ento-011613-162036. [DOI] [PubMed] [Google Scholar]
- 57.Nalyanya G, Schal C. Evaluation of attractants for monitoring populations of the German cockroach (Dictyoptera: Blattellidae) J Econ Entomol. 2001;94(1):208–214. doi: 10.1603/0022-0493-94.1.208. [DOI] [PubMed] [Google Scholar]