Significance
Ionotropic glutamate AMPA receptors (AMPARs) play a fundamental role in normal function and plasticity of the brain, and they are also involved in many brain disorders. Despite the central role of AMPARs in neurobiology, the modulation of synaptic AMPA responses by endogenous modulators remains not well understood. Here, in three synapses found in two different brain areas, we provide the first evidence, to our knowledge, that endogenous zinc is coreleased with glutamate and modulates the strength of synaptic AMPAR responses. Because in many neocortical areas more than 50% of excitatory presynaptic terminals contain zinc within their glutamatergic vesicles, our findings establish zinc as a general neuromodulator that allows for fine-tuning and plasticity of glutamatergic fast synaptic transmission in the brain.
Keywords: AMPA receptors, zinc, ZnT3, synaptic plasticity, auditory
Abstract
The vast amount of fast excitatory neurotransmission in the mammalian central nervous system is mediated by AMPA-subtype glutamate receptors (AMPARs). As a result, AMPAR-mediated synaptic transmission is implicated in nearly all aspects of brain development, function, and plasticity. Despite the central role of AMPARs in neurobiology, the fine-tuning of synaptic AMPA responses by endogenous modulators remains poorly understood. Here we provide evidence that endogenous zinc, released by single presynaptic action potentials, inhibits synaptic AMPA currents in the dorsal cochlear nucleus (DCN) and hippocampus. Exposure to loud sound reduces presynaptic zinc levels in the DCN and abolishes zinc inhibition, implicating zinc in experience-dependent AMPAR synaptic plasticity. Our results establish zinc as an activity-dependent, endogenous modulator of AMPARs that tunes fast excitatory neurotransmission and plasticity in glutamatergic synapses.
The development, function, and experience-dependent plasticity of the mammalian brain depend on the refined neuronal interactions that occur in synapses. In the majority of excitatory synapses, the release of the neurotransmitter glutamate from presynaptic neurons opens transmembrane ion channels in postsynaptic neurons, the ionotropic glutamate receptors, thereby generating the flow of excitatory signaling in the brain. As a result, these receptors play a fundamental role in normal function and development of the brain, and they are also involved in many brain disorders (1).
The ionotropic glutamate receptor family consists of AMPA, kainate, and NMDA receptors (NMDARs). Although kainate receptor-mediated excitatory postsynaptic responses occur in a few central synapses (2), AMPA receptors (AMPARs) and NMDARs are localized in the postsynaptic density of the vast majority of glutamatergic synapses in the brain, mediating most of excitatory neurotransmission (1). NMDAR function is regulated by a wide spectrum of endogenous allosteric neuromodulators that fine-tune synaptic responses (3–5); however, much less is known about endogenous AMPAR neuromodulators [(1, 5), but see refs. 6 and 7]. Recent structural studies revealed that the amino terminal domain (ATD) and ligand-binding domain (LBD) are tightly packed in NMDARs but not AMPARs (8–10). These structural differences explain some of the functional differences in allosteric modulation between AMPARs and NMDARs, such as why the ATD of NMDARs, unlike that of AMPARs, modulates function and contains numerous binding sites for allosteric regulators. Nonetheless, given the importance of fine-tuning both synaptic AMPAR and NMDAR responses for brain function, it is puzzling that there is not much evidence for endogenous, extracellular AMPAR modulation. The discovery and establishment of endogenous AMPAR modulators is crucial both for understanding ionotropic glutamate receptor signaling and for developing therapeutic agents for the treatment of AMPAR-related disorders, such as depression, cognitive dysfunctions associated with Alzheimer’s disease, and schizophrenia (1, 11).
Free, or readily chelatable, zinc is an endogenous modulator of synaptic and extrasynaptic NMDARs (12–15). Free zinc is stored in glutamatergic vesicles in many excitatory synapses in the cerebral cortex, limbic, and brainstem nuclei (16). In some brain areas, such as in the hippocampus, 50% of boutons synapsing onto CA1 neurons and all mossy fibers synapsing onto CA3 neurons contain synaptic free zinc (17). Whereas earlier studies demonstrated that exogenous zinc inhibits AMPARs (18–21), more recent work suggests that endogenously released synaptic zinc does not modulate AMPARs in central synapses (14, 22). This conclusion was derived from the inability to efficiently chelate and quantify synaptic zinc with the zinc-selective chelators and probes used (15), in apparent support of the hypothesized low levels of released zinc during synaptic stimulation (14).
Recent work in our laboratories used new chemical tools that allowed us to intercept and visualize mobile zinc efficiently (15). These studies revealed modulation of extrasynaptic NMDARs by zinc and led us to reinvestigate whether synaptically released zinc might be an endogenous modulator of AMPARs as well. In the present study, we applied these same tools in electrophysiological, laser-based glutamate uncaging and in imaging experiments using wild type and genetically modified mice that lack synaptic zinc.
Results
ZnT3-Dependent Synaptic Zinc Inhibits AMPAR EPSCs in Dorsal Cochlear Nucleus Synapses.
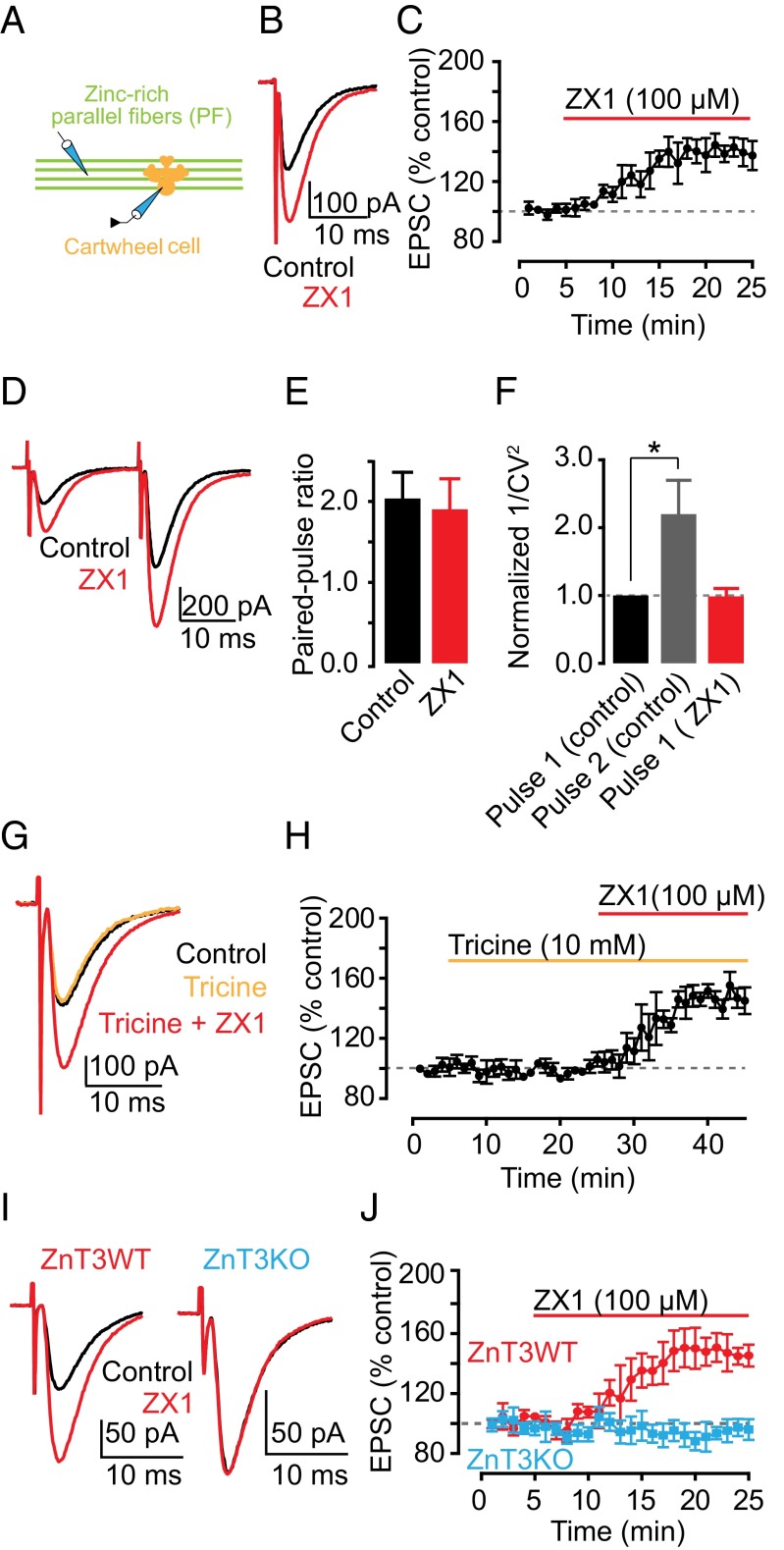
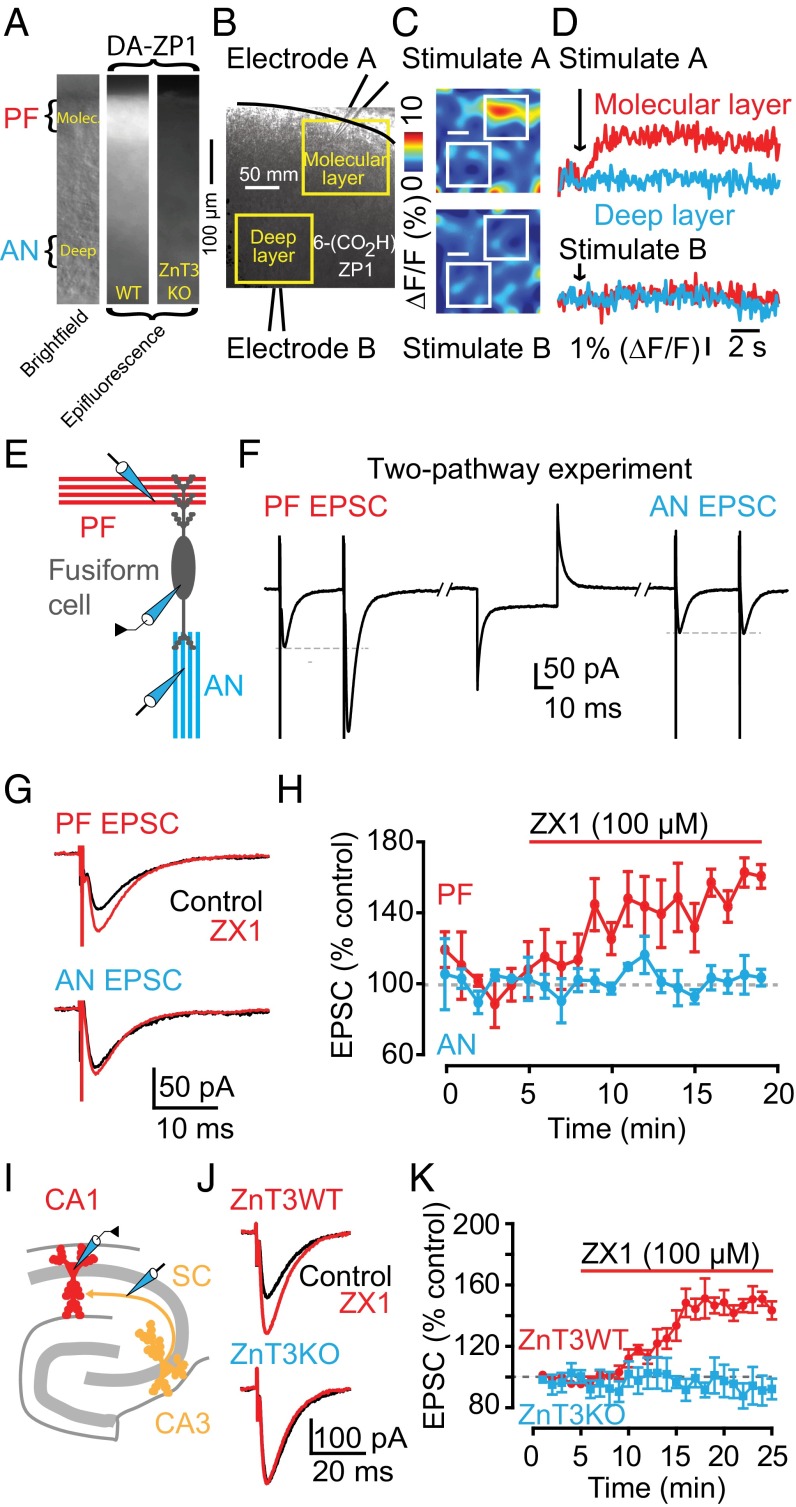
First, we explored the effect of synaptic zinc on AMPAR-mediated excitatory postsynaptic currents (EPSCs) in the dorsal cochlear nucleus (DCN), a zinc-rich auditory brainstem nucleus. The DCN is a cerebellum-like structure (23), where glutamatergic parallel fibers (PFs) are zinc-rich (24) and innervate interneurons and principal neurons, cartwheel cells (CWCs), and fusiform cells (FCs), respectively. Bath application of 100 µM ZX1, an extracellular fast, high-affinity zinc chelator (13, 15), potentiated cartwheel cell AMPAR EPSCs evoked by a single PF stimulus (PF EPSCs) (Fig. 1 A–C). This finding suggested, for the first time to our knowledge in a mammalian synapse, that endogenous zinc inhibits AMPAR EPSCs.
Fig. 1.
Synaptic ZnT3-dependent zinc inhibits AMPAR EPSCs in DCN parallel fiber synapses via a postsynaptic mechanism. (A) Schematic of the experimental setup for electrophysiological experiments in cartwheel cells. In this figure, AMPAR EPSCs were recorded from cartwheel cells and evoked by parallel fiber stimulation (PF EPSCs). (B) Representative PF EPSCs before and after ZX1 application. (C) Time course of PF EPSC amplitude before and after ZX1 application (PF EPSC amplitude, 15–20 min after ZX1 application: 140.22 ± 7.66% of baseline, n = 7, P < 0.01). (D) Representative PF EPSCs in response to two stimuli 20 ms apart: before and after ZX1 application. (E) Summary graph of paired-pulse ratio (n = 7, P = 0.36 for control vs. ZX1). (F) Summary graph of normalized 1/CV2 (n = 7, P = 0.01 for second pulse vs. first pulse; n = 7, P = 0.95 for control first pulse vs. ZX1 first pulse). (G) Representative PF EPSCs in control, after tricine, and after tricine and ZX1 application. (H) Time course of PF AMPAR EPSC amplitude before, after tricine, and after tricine and ZX1 application (PF EPSC amplitude: 15–20 min after tricine application: 100.07 ± 3.35% of baseline, n = 5, P = 0.97; 15–20 min after tricine and ZX1 application: 146.51 ± 7.20% of baseline, n = 5, P < 0.01). (I) Representative PF EPSCs from ZnT3WT and ZnT3KO mice before and after ZX1 application. (J) Time course of PF EPSC amplitude from ZnT3WT and ZnT3KO mice before and after ZX1 application (PF EPSC amplitude, 15–20 min after ZX1 application: ZnT3WT: 147.02 ± 8.82% of baseline, n = 5, P < 0.01; ZnT3KO: 94.69 ± 6.85% of baseline, n = 5, P = 0.16; ZnT3WT vs. ZnT3KO: P < 0.01). Values represent mean ± SEM. For details of statistical tests and detailed values shown in main figures, see SI Materials and Methods.
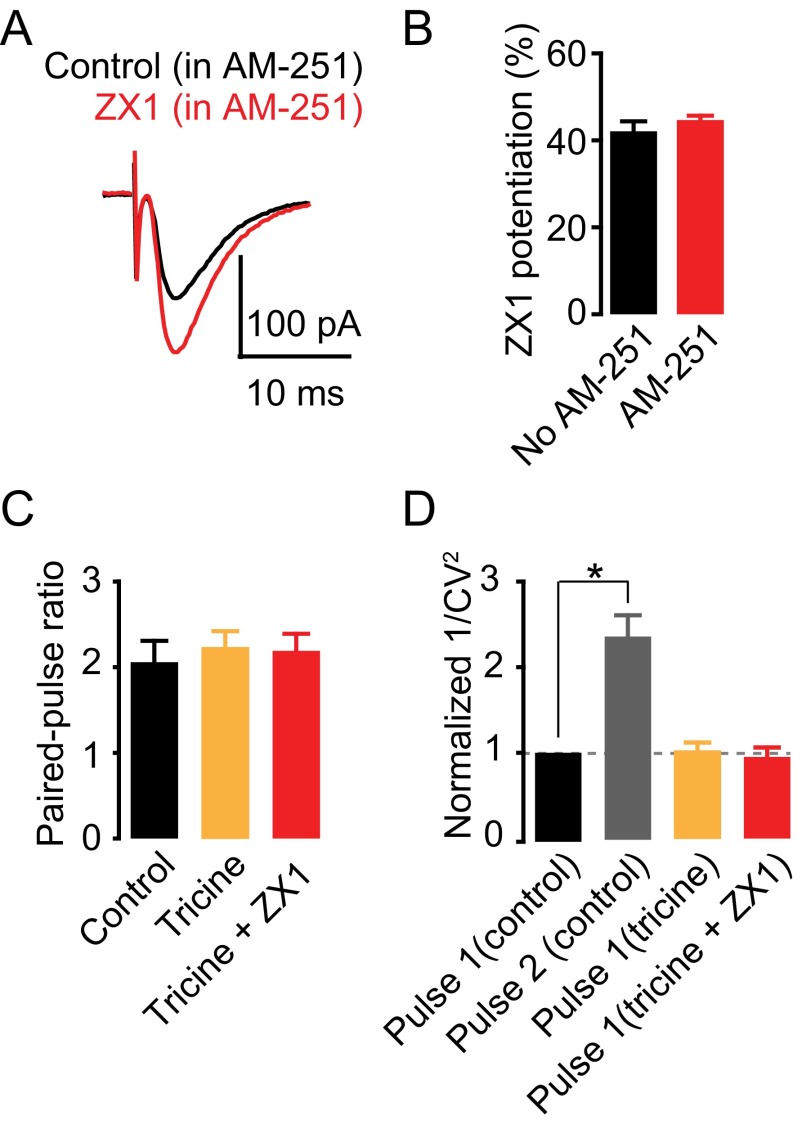
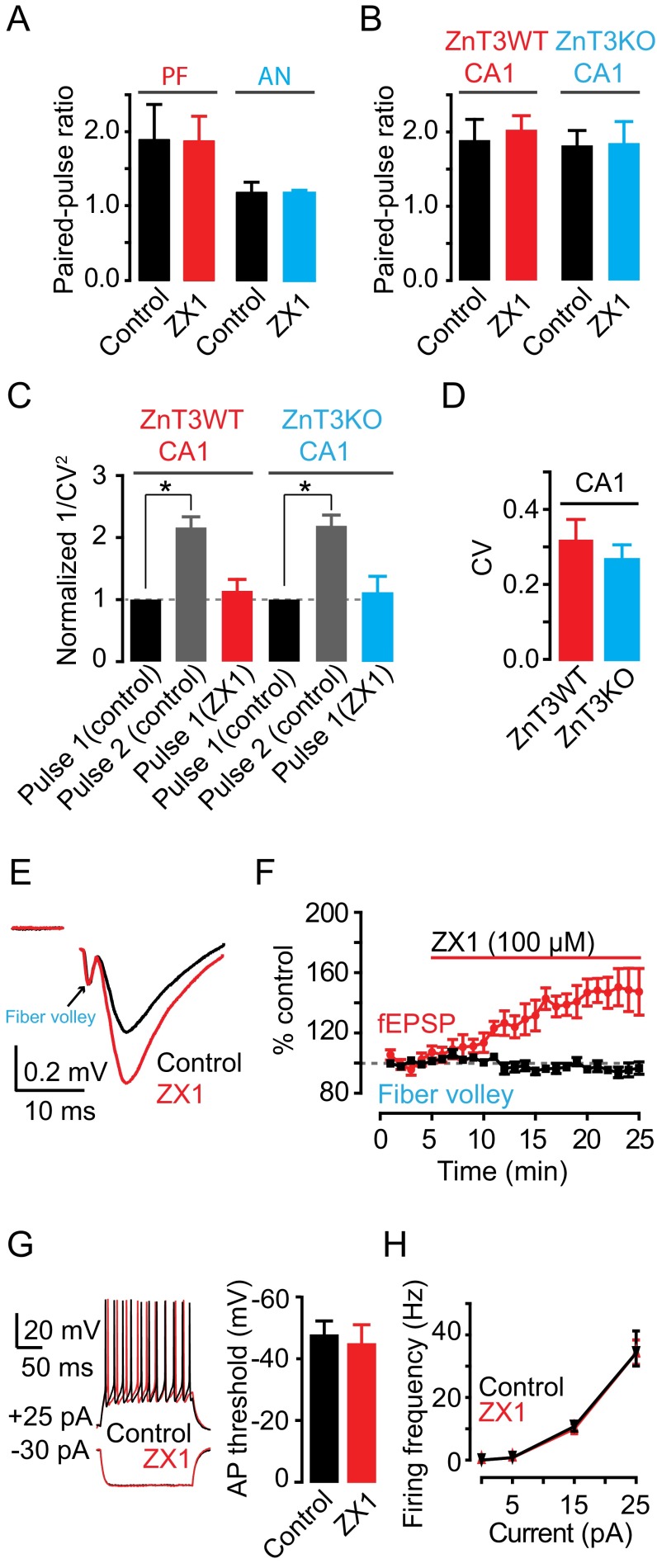
To determine whether the effects of synaptic zinc on PF EPSCs were mediated by presynaptic mechanisms, we used paired-pulse ratio (PPR) and coefficient of variation (CV) analysis, two assays that are sensitive to changes in presynaptic probability of neurotransmitter release [Pr (25, 26)]. PPR, the ratio of the amplitude of the second EPSC to the first EPSC evoked by two stimuli applied in rapid succession (Fig. 1D), depends on Pr. High Pr synapses show paired-pulse depression, whereas low Pr synapses show paired-pulse facilitation. CV, which is the standard deviation (SD) of the EPSC amplitudes normalized to the mean amplitude, varies inversely with quantal content. The inverse square, 1/CV2, is directly proportional to quantal content, where quantal content = n Pr, n being the number of release sites. ZX1 did not alter either PPR or 1/CV2 of PF EPSCs, indicating a lack of contribution of presynaptic mechanisms in the zinc-mediated depression in PF EPSCs (Fig. 1 E and F). As expected, 1/CV2 of the second pulse in control conditions was increased, consistent with increased Pr of the second stimulus (Fig. 1F). Previous studies showed that endogenous zinc inhibits Pr via endocannabinoid signaling in DCN synapses (22). However, this effect required prolonged high frequency presynaptic stimulation. To further interrogate the lack of presynaptic effects of zinc on PF EPSCs, we examined the influence of ZX1 in the presence of 1 μM AM-251, a cannabinoid receptor (CB1R) antagonist, which blocks endocannabinoid signaling. ZX1 enhanced PF EPSCs in the presence of AM-251 to an extent similar to that as in the absence of AM-251 (Fig. S1 A and B). Taken together, these results are consistent with the notion that endogenous zinc inhibits AMPAR EPSCs evoked by a single PF stimulus via a postsynaptic mechanism.
Fig. S1.
(A) Representative PF AMPAR EPSCs before and after ZX1 application in the presence of 1 μM of AM-251. (B) Summary of PF AMPAR EPSC amplitude,15–20 min after ZX1 application in the following: control: 140.22 ± 7.66% of baseline; AM-251: 148.97 ± 3.35% of baseline, n = 5, P = 0.32, Student unpaired t test. (C and D) Summary graph of PPR (C) and normalized 1/CV2 (D) of CWC PF EPSCs. (PPR: control: 2.06 ± 0.25; tricine: 2.23 ± 0.19; ZX1: 2.19 ± 0.20; control vs. tricine: P = 0.89, n = 5; control vs. ZX1: P = 0.68, n = 5; tricine vs. ZX1: P = 0.91, n = 5; 1/CV2 normalized to control first pulse: control second pulse: 2.53 ± 0.24, P < 0.01, n = 5; tricine first pulse: 1.02 ± 0.10, P = 0.99, n = 5; ZX1 first pulse: 0.95 ± 0.11, P = 0.74, n = 5, one-way ANOVA, post hoc Tukey.) Values represent mean ± SEM.
At hippocampal mossy-fiber (MF) to CA3 zinc-rich synapses, the use of either tricine or CaEDTA, two of the most widely used extracellular zinc chelators, did not reveal any effects on either AMPAR or NMDAR EPSCs (13, 14). CaEDTA is a slow chelator and is therefore not expected to intercept fast synaptic zinc transients (12, 13, 15). Studies using tricine, a commonly used chelator for studying the role of synaptic zinc (27), did not reveal any effect of synaptic zinc on AMPAR EPSCs, either in MF synapses onto CA3 neurons or in PF synapses onto DCN fusiform cells (14, 22). Here, by using ZX1, an extracellular zinc chelator with a second-order rate constant for binding zinc that is 200-fold higher than those for tricine and CaEDTA (15), we used the most efficient chelator for studying the effect of synaptic zinc on AMPAR neurotransmission. Indeed, bath application of 10 mM tricine did not affect PF EPSCs in CWCs (Fig. 1 G and H). However, subsequent addition of 100 µM ZX1 potentiated PF EPSCs, without affecting either PPR or CV (Fig. 1 G and H and Fig. S1 C and D). Together, these results indicate that ZX1, unlike tricine, can unmask the inhibitory effect of synaptically released zinc on baseline AMPAR synaptic transmission.
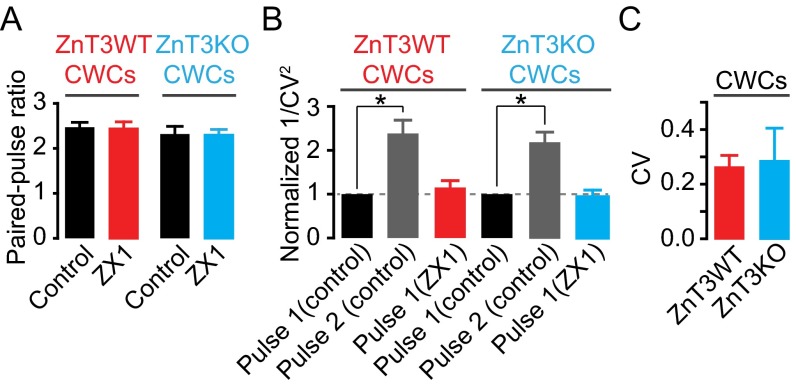
Because zinc transporter 3 (ZnT3) is the protein that loads zinc in presynaptic glutamatergic vesicles, we hypothesized that mice lacking this transporter (ZnT3KO mice), and thereby synaptic zinc (28), would not exhibit zinc-mediated modulation of AMPAR EPSCs. Consistent with this hypothesis, ZX1 potentiated PF EPSCs in ZnT3 WT mice (ZnT3WT) without affecting either PPR or CV, but did not affect PF EPSCs in ZnT3KO mice (Fig. 1 I and J and Fig. S2 A and B). PF quantal release properties (22), PPR, CV, and kinetics of postsynaptic AMPAR responses were not different between ZnT3WT and ZnT3KO mice, indicating that ZnT3KO mice have similar presynaptic properties and postsynaptic AMPARs (Fig. S2 A–C and Table S1). The lack of effect of ZX1 on PF EPSCS in ZnT3KO mice is therefore not a consequence either of changes in basal synaptic properties or changes in AMPAR composition between WT and KO mice. Moreover, these results show that the effects of ZX1 on PF EPSCs in WT mice are not due to nonspecific effects of ZX1 on synaptic AMPARs. We conclude that synaptic release of ZnT3-dependent vesicular zinc mediates the inhibition of PF EPSCs.
Fig. S2.
(A–C) Summary graph of PPR (A), normalized 1/CV2 (B), and CV (C) of CWC PF EPSCs from ZnT3WT and ZnT3KO mice (PPR: ZnT3WT: Control: 2.47 ± 0.11; ZX1: 2.43 ± 0.16; P = 0.75, n = 5; ZnT3KO: Control: 2.32 ± 0.17; ZX1: 2.29 ± 0.13; P = 0.78, n = 5, Student paired t test; Control PPR: ZnT3WT vs. ZnT3KO; P = 0.48, n = 5, Student unpaired t test; 1/CV2 normalized and compared with control first pulse: ZnT3WT: control second pulse: 2.35 ± 0.32, P < 0.01, n = 5; ZX1 first pulse: 1.15 ± 0.15, P = 0.99, n = 5; ZnT3KO: control second pulse: 2.20 ± 0.23, P < 0.01, n = 5; ZX1 first pulse: 0.93 ± 0.15, P = 0.99, n = 5, one-way ANOVA, post hoc Tukey; CV of pulse 1: ZnT3WT: 0.26 ± 0.04; ZnT3KO: 0.28 ± 0.12; P = 0.87, Student unpaired t test). Values represent mean ± SEM.
Table S1.
Summary of mean rise times and decay time constants of EPSCs from CWCs, CA1 neurons, and FCs
| Experimental condition | ZnT3WT | ZnT3KO | ||
| Rise time (ms) | Decay time constant (ms) | Rise time (ms) | Decay time constant (ms) | |
| Control (CWCs) | 0.95 ± 0.12 | 4.75 ± 0.47 | 1.05 ± 0.08 | 5.12 ± 0.58 |
| ZX1 (CWCs) | 1.00 ± 0.13 | 5.05 ± 0.60 | 1.10 ± 0.11 | 5.17 ± 0.89 |
| Control (CA1) | 1.54 ± 0.23 | 9.53 ± 0.87 | 1.59 ± 0.30 | 10.05 ± 0.93 |
| ZX1 (CA1) | 1.64 ± 0.26 | 10.23 ± 1.53 | 1.68 ± 0.25 | 10.58 ± 1.12 |
| ICR sham-exposed | ICR noise-exposed | |||
| Control (FCs) | 0.76 ± 0.15 | 3.96 ± 0.27 | 0.67 ± 0.09 | 3.89 ± 0.25 |
| ZX1 (FCs) | 0.89 ± 0.10 | 4.09 ± 0.39 | 0.74 ± 0.12 | 4.02 ± 0.36 |
Mean rise times and decay time constants of CWCs and CA1 EPSCs before and after ZX1 application were not statistically different either within or across genotypes (ZnT3WT and ZnT3KO). Mean rise times and decay time constants before and after ZX1 application were not statistically different either within or across sham- and noise-exposed mice. Detailed values for n and P and statistical tests: CWCs and CA1 EPSCs: ZnT3WT, CWCs: rise times: control vs. ZX1, P = 0.23, n = 5; decay time constants: control vs. ZX1, P = 0.79, n = 5; ZnT3WT, CA1: rise times: control vs. ZX1, P = 0.83, n = 5; decay time constants: control vs. ZX1, P = 0.18, n = 5; ZnT3KO, CWCs; rise times: control vs. ZX1, P = 0.77, n = 5; decay time constants: control vs. ZX1, P = 0.91, n = 5; ZnT3KO CA1; rise times: control vs. ZX1, P = 0.80, n = 5; decay time constants: control vs. ZX1, P = 0.96, n = 5; Student paired t test; ZnT3WT vs. ZnT3KO: control CWCs rise times, P = 0.46, n = 5; control CWCs decay time constants, P = 0.63, n = 5; ZX1 CWCs rise times, P = 0.57, n = 5; ZX1 decay time constants, P = 0.91, n = 5; control CA1 rise times, P = 0.89, n = 5; control CA1 decay time constants, P = 0.69, n = 5; ZX1 CA1 rise times, P = 0.91, n = 5; ZX1 decay time constants, P = 0.85, n = 5; Student unpaired t test. FC EPSCs: Sham-exposed, FCs: rise times: control vs. ZX1, P = 0.15, n = 5; decay time constants: control vs. ZX1, P = 0.80, n = 5; noise-exposed, FCs: rise times: control vs. ZX1, P = 0.26, n = 5; decay time constants: control vs. ZX1. P = 0.90, n = 5; Student paired t test; sham-exposed vs. noise-exposed: control FCs rise times, P = 0.62, n = 5; control FCs decay time constants, P = 0.85, n = 5; ZX1 FCs rise times, P = 0.36, n = 5; ZX1 FCs decay time constants, P = 0.89, n = 5; Student unpaired t test.
Evoked Action Potential Driven Release of Zinc from Presynaptic Terminals Mediates AMPAR EPSC Inhibition.
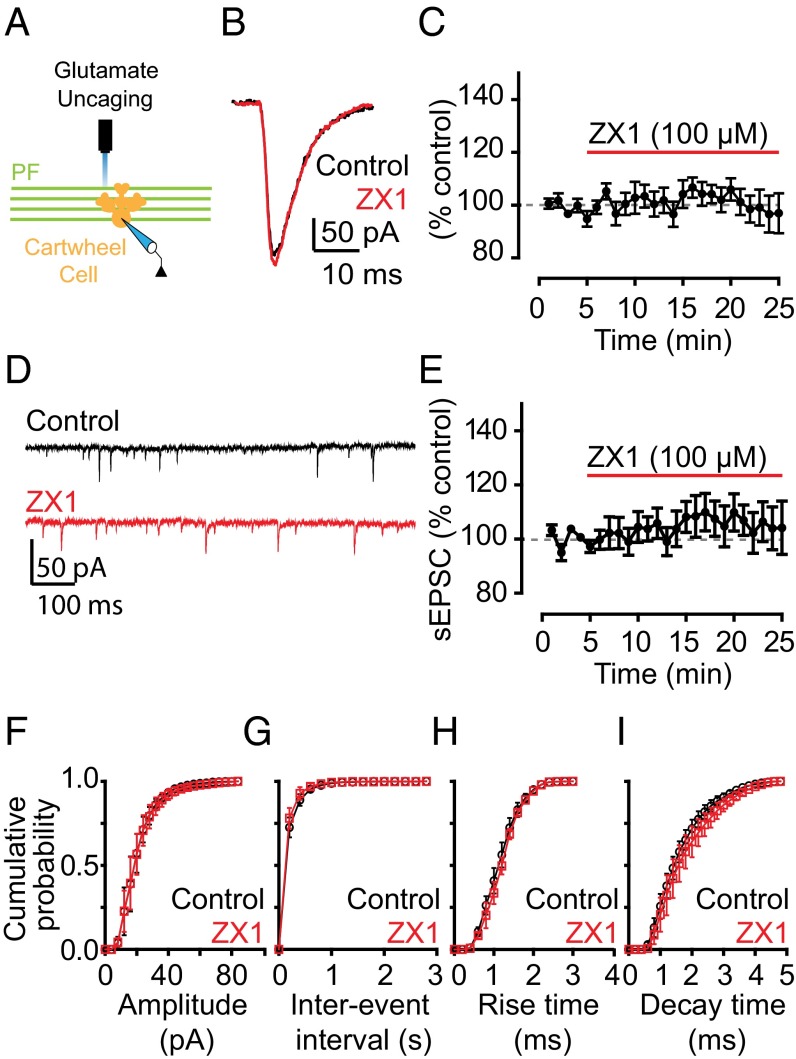
ZX1 potentiation of PF EPSCs is consistent with the hypothesis that chelation of stimulus-driven, synaptically released zinc removes AMPAR inhibition by the metal ion. Alternatively, there might be a tonic level of ZnT3-dependent zinc, arising from spontaneous release of zinc from presynaptic vesicles, which inhibits AMPARs but is independent of synaptic stimulation. Low nanomolar tonic zinc levels in DCN brain slices inhibit extrasynaptic NMDARs and potentiate glycine receptors (15, 29). To determine whether tonic zinc modulates AMPAR currents, we used glutamate uncaging to activate AMPARs and bypass synaptic stimulation. When we uncaged glutamate onto the dendritic arbor of CWCs in the molecular layer (Fig. 2A), we evoked pharmacologically isolated AMPAR currents that were not potentiated by the addition of ZX1. This finding indicates that tonic zinc does not modulate AMPARs and is consistent with the nanomolar zinc affinity of NMDARs containing NR2A subunits (30) and glycine receptors containing the α1 subunit (31), compared with the lower zinc affinity of AMPARs (18, 19). Moreover, ZX1 application did not affect the amplitude, frequency, or kinetics of spontaneous EPSCs (sEPSCs; Fig. 2 D–I), which are elicited by random, nonevoked firing of presynaptic granule cells, indicating that inhibition of PF EPSCs by zinc requires evoked, action potential-driven release of zinc from presynaptic vesicles.
Fig. 2.
Inhibition of AMPAR EPSCs by synaptic zinc is dependent on evoked, action potential-driven release of zinc from presynaptic terminals. (A) Schematic of the location of glutamate uncaging. (B) Representative AMPAR currents in response to glutamate uncaging before and after ZX1 application. (C) Time course of amplitude of AMPAR uncaging currents before and after ZX1 application (AMPAR current amplitude, 15–20 min after ZX1 application: 98.46 ± 6.27% of baseline, n = 5, P = 0.84). (D) Representative traces of spontaneous AMPAR EPSCs (sEPSCs) before and ZX1 application. (E) Time course of the mean sEPSC amplitude before and after ZX1 application (mean sEPSC amplitude, 15–20 min after ZX1 application: 104.34 ± 8.78% of baseline, n = 5, P = 0.56). (F–I) Cumulative probability plot of sEPSC amplitude (F), frequency (G), rise time (H), and decay time (I) before and after ZX1 application (mean sEPSC amplitude: n = 5, P = 0.24 for control vs. ZX1; mean sEPSC frequency: n = 5, P = 0.72 for control vs. ZX1; mean sEPSC rise time: n = 5, P = 0.25 for control vs. ZX1; mean sEPSC decay time: n = 5, P = 0.10 for control vs. ZX1).
Zinc Inhibition of AMPAR EPSCs Is Input Specific in DCN Synapses.
The DCN is a laminar structure with layers that contain glutamatergic PF zinc-rich terminals synapsing onto different neurons, as well as layers that harbor zinc-lacking glutamatergic terminals. In particular, fusiform cells receive zinc-rich PF input at their apical dendrites in the molecular layer, and zinc-lacking auditory nerve (AN) input at their basal dendrites in the deep layer (Fig. 3E) (32). Moreover, fusiform cells express AMPARs containing GluA2-3 subunits in their apical dendrites, whereas cartwheel cells express GluA1-3 subunits (33). To determine whether zinc modulation of PF EPSCs is input-specific in fusiform cells and whether zinc modulates PF EPSCs in another synapse with different AMPAR composition, we took advantage of this anatomical and functional segregation of synaptic inputs in the DCN. First, we demonstrated that the DCN molecular layer is zinc-rich by using a cell-permeable, acetylated zinc fluorescent sensor diacetylated Zinpyr-1 (DA-ZP1) (34). Consistent with previous anatomical studies (32), our imaging experiments revealed a zinc-specific fluorescent signal that is ZnT3 dependent and specific to the molecular layer of the DCN (Fig. 3A). Next, we used an extracellular fluorescent sensor, ZP1-6COOH, to determine whether we could observe input-specific zinc release. We performed two-pathway imaging experiments in the same slice, which showed that PF stimulation in the molecular layer generated a fluorescent response, indicating zinc release, whereas stimulation of AN fibers in the deep layer did not generate any zinc fluorescence signal (Fig. 3 B–D). To test for input-specific inhibition of PF EPSCs by zinc, we used two-pathway electrophysiological experiments to record PF EPSCs and AN EPSCs from the same fusiform cell (Fig. 3 E and F). Note that PF EPSCs, but not AN EPSCs, showed paired-pulse facilitation, further confirming our ability to stimulate two independent, anatomically and functionally distinct inputs (Fig. 3F and Fig. S3A). Application of ZX1 potentiated only PF EPSCs without affecting PPR, but left AN EPSCs unaffected (Fig. 3 G and H and Fig. S3A). These results show that zinc-mediated modulation of AMPAR EPSCs in fusiform cells is input-specific, occurring only at glutamatergic synapses that contain zinc, and is mediated by postsynaptic mechanisms. Moreover, these results indicate that zinc modulates synapses with AMPARs containing GluA1-3 subunits.
Fig. 3.
Zinc-mediated inhibition of AMPAR EPSCs is input specific in DCN synapses and occurs in hippocampal synapses. (A, Left) Brightfield image of a DCN slice showing the molecular and deep layer of the DCN where parallel fiber (PF) and auditory nerve (AN) inputs reside, respectively. (Center) DA-ZP1, a cell-permeable fluorescent zinc sensor reveals zinc-mediated fluorescence in the molecular but not deep layer of a DCN slice from a WT mouse. (Right) Absence of DA-ZP1 fluorescence in a DCN slice from a ZnT3KO mouse. (B) Illustration of two-pathway imaging experiments with stimulating electrodes placed in the molecular and deep layer of the DCN. (C) In response to a 100-Hz, 1-s stimulation in the molecular layer, ZP1-6COOH, a membrane-impermeable fluorescent zinc sensor reveals evoked zinc signals in the molecular but not in the deep layer of the DCN. No fluorescence is evoked by identical electrical stimulation in the deep layer. (D) Representative ZP1-6COOH fluorescent responses in response to a 100-Hz, 1-s electrical stimulation. (E) Schematic of the experimental setup for two-pathway electrophysiological experiments in fusiform cells. (F) Representative traces from two-pathway experiment showing, in response to paired-pulse stimulation, PF EPSCs, and AN EPSCs recorded from the same fusiform cell. (G) Representative PF and AN EPSCs, recorded from the same fusiform cell as shown in F, before and after ZX1 application. (H) Time course of PF and AN EPSC amplitude before and after ZX1 application (AMPAR EPSC amplitude, 10–15 min after ZX1 application: PF EPSC: 151.09 ± 7.05% of baseline, n = 3, P < 0.01; AN EPSC: 100.01 ± 1.66% of baseline, n = 3, P = 0.79; PF EPSC vs. AN EPSC: P < 0.01). (I) Schematic of the experimental setup for experiments in the hippocampus, including stimulation of Schaffer collaterals (SC) and recording from CA1 neurons (J) Representative SC CA1 EPSCs from ZnT3WT and ZnT3KO mice before and after ZX1 application. (K) Time course of ZnT3WT and ZnT3KO SC CA1 EPSC amplitude before and after ZX1 application (AMPAR EPSC amplitude, 15–20 min after ZX1 application: ZnT3WT: 146.71 ± 5.66% of baseline; n = 5, P < 0.01; ZnT3nKO: 92.23 ± 9.20% of baseline; n = 5, P = 0.18; ZnT3WT vs. ZnT3KO: P < 0.01).
Fig. S3.
(A) Summary graph of PPR of fusiform cell PF and AN EPSCs from two-pathway experiments. (PF EPSCs: control: 1.88 ± 0.475; ZX1: 1.85 ± 0.334, P = 0.97, n = 3; AN EPSCs: control: 1.18 ± 0.13; ZX1: 1.17 ± 0.03, P = 0.94, n = 3, Student paired t test.) (B–D) Summary graph of PPR (B), normalized 1/CV2 (C), and CV (D) of SC EPSCS from CA1 hippocampal neurons form ZnT3WT and ZnT3KO mice (PPR: ZnT3WT: control: 1.89 ± 0.28; ZX1: 2.03 ± 0.19, P = 0.18, n = 5; ZnT3KO: control: 1.82 ± 0.20; ZX1: 1.85 ± 0.29, P = 0.35, n = 5, Student paired t test; Control PPR: ZnT3WT vs. ZnT3KO; P = 0.84, n = 5, Student unpaired t test; 1/CV2 normalized and compared with control first pulse: ZnT3WT: control second pulse: 2.19 ± 0.17, P < 0.01, n = 5; ZX1 first pulse: 1.11 ± 0.26, P = 0.97, n = 5; ZnT3KO: control second pulse: 2.16 ± 0.17, P < 0.01, n = 5; ZX1 first pulse: 1.14 ± 0.18, P = 0.79, n = 5, one-way ANOVA, post hoc Tukey; CV of pulse 1: ZnT3WT: 0.32 ± 0.08; ZnT3KO: 0.27 ± 0.07; P = 0.65, Student unpaired t test). (E) Representative hippocampal fEPSPs recorded in stratum radiatum before and after ZX1 application. (F) Time course of initial slope of fEPSPs and fiber volley amplitude before and after ZX1 application (initial slope of fEPSPs: 20–25 min after ZX1 application: 148.24 ± 3.47% of baseline, n = 5, P < 0.01, Student paired t test; fiber volley amplitude: 20–25 min after ZX1 application: -4.09 ± 1.52% of baseline, Student paired t test, n = 5, P = 0.10). (G, Left) Representative traces showing granule cell response to current injections before and after ZX1 application. (Right) Summary graph of granule cell action potential threshold before and after ZX1 application (control: 48.46 ± 4.41 vs. ZX1: 45.71 ± 4.94, n = 4, P = 0.68, Wilcoxon rank sum test). (H) Summary graph of granule cell firing frequency as a function of injected current amplitude (F-I curve) before and after ZX1 application (5 pA: control: 0.83 ± 0.83, ZX1: 0.83 ± 0.83, n = 4, P = 0.84; 15 pA: control: 10.04 ± 1.60, ZX1: 10.78 ± 1.58, n = 4, P = 0.88; 25 pA: control: 34.23 ± 4.17, ZX1: 35.94 ± 5.31, n = 4, P = 0.68, Wilcoxon rank sum test). Values represent mean ± SEM.
Zinc Inhibition of AMPAR EPSCs in Hippocampal Synapses.
To determine whether synaptic zinc-mediated inhibition of AMPAR EPSCs is a general modulatory mechanism of AMPAR neurotransmission across different zinc-containing synapses, we explored the effect of zinc on AMPAR EPSCs in the hippocampus. We stimulated zinc-rich Schaffer collateral fibers (SCs) (14) (Fig. 3I), and we recorded from hippocampal CA1 neurons, which express AMPARs containing GluA1-3 subunits (35). ZX1 potentiated CA1 SC EPSCs in ZnT3WT mice, but left CA1 SC EPSCs unaffected in ZnT3KO mice (Fig. 3 J and K). Similar to the DCN, ZX1 did not affect PPR and CV of SC EPSCs in ZnT3WT mice (Fig. S3 B and C), indicating that synaptic zinc modulates CA1 SC EPSCs via postsynaptic mechanisms. Finally, PPR, CV, and kinetic properties of CA1 SC EPSCs were not different between ZnT3WT and ZnT3KO mice, suggesting that the lack of effect of ZX1 in SC EPSCs in ZnT3KO mice was due to the lack of synaptic zinc and not to differences in baseline synaptic transmission between ZnT3WT and ZnT3KO mice (Fig. S3 B–D and Table S1). Moreover, we investigated the effects of ZX1 on the presynaptic fiber volley and the size of the accompanied field EPSP (fEPSP) recorded in the stratum radiatum and evoked by afferent stimulation also in the stratum radiatum. ZX1 increased synaptic strength, measured as increases in the slope of the fEPSP, without affecting the amplitude of presynaptic fiber volley (Fig. S3 E and F). Because the amplitude of the fiber volley is proportional to the number of presynaptic fibers activated by the stimulus and thus serves as an estimate of the strength of an afferent input, we conclude that ZX1 increases synaptic strength but does not affect afferent input (Fig. S3 E and F). Next, we measured the effect of ZX1 on the spiking output of DCN granule cells, the cells from which zinc-rich parallel fibers originate. ZX1 did not affect either action potential threshold or the current-firing frequency (f–I) function in these neurons (Fig. S3 G and H), further suggesting that ZX1 does not affect the spiking output of presynaptic neurons. Taken together, our results suggest that zinc inhibition of AMPAR EPSCs is a general postsynaptic modulatory mechanism in zinc-containing synapses that express GluA1-3.
Plasticity of AMPAR EPSCs by Sound-Evoked Reduction of Presynaptic Zinc Levels in DCN Synapses.
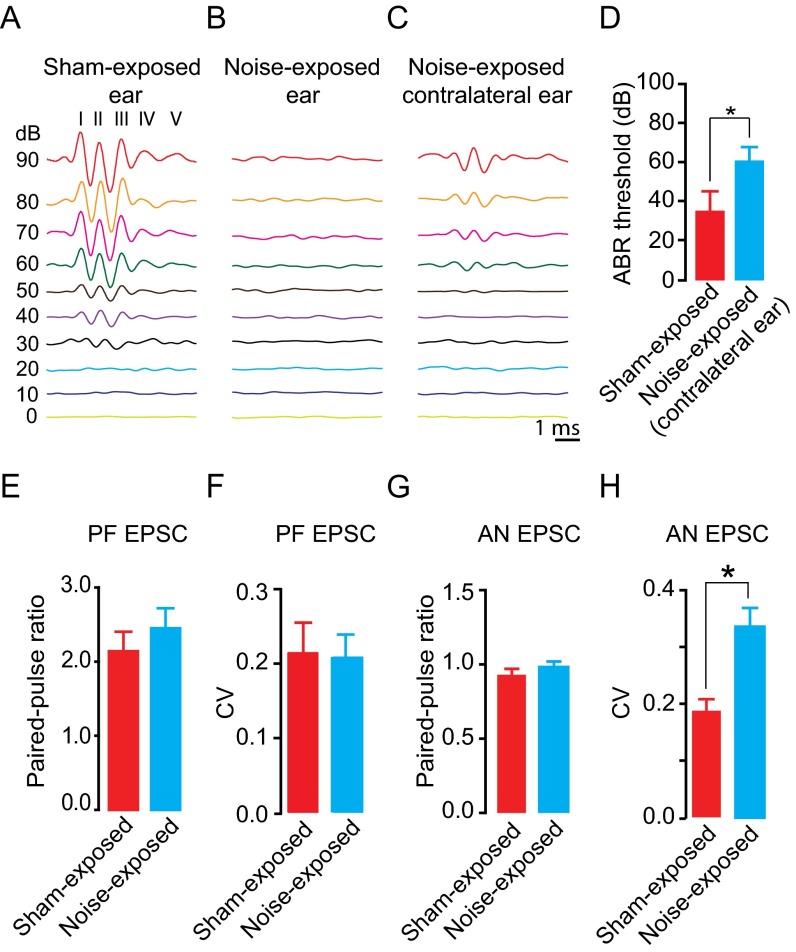
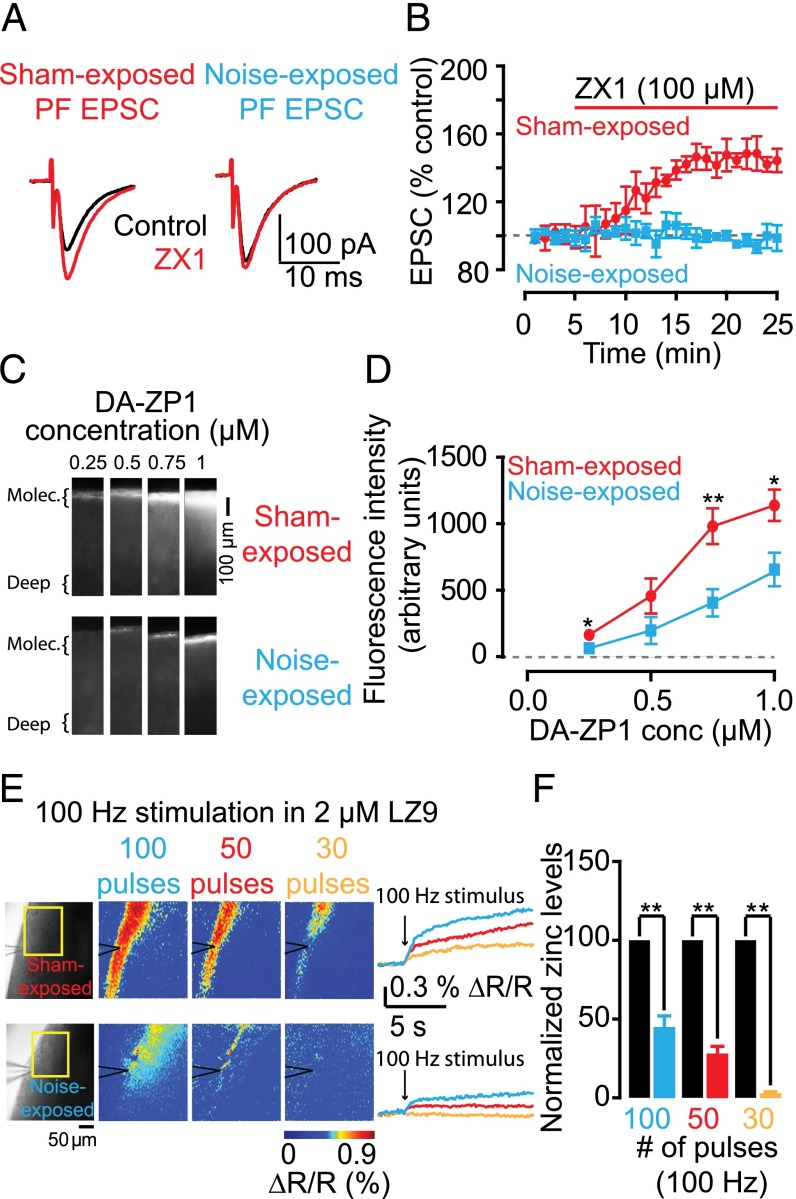
The results depicted in Figs. 1–3 show that synaptic zinc is an endogenous neuromodulator that controls the strength of baseline synaptic responses in zinc-containing synapses. Potential plasticity of zinc levels would give zinc a dynamic role in shaping excitatory synaptic transmission in an activity-dependent manner and would add to the complexity of synaptic plasticity in mammalian synapses. In the neocortex, levels of synaptic zinc are rapidly and dynamically regulated. In particular, in the barrel cortex, increased sensory stimulation leads to decreased levels of synaptic zinc, whereas decreased sensory stimulation leads to increased synaptic zinc (36). These anatomical studies have established the experience-dependent modulation of synaptic zinc levels; however, the effect of this modulation on synaptic strength remains unknown. We examined the effect of auditory experience on zinc-mediated effects on PF EPSCs in fusiform cells, which receive direct AN input in their basal dendrites (Fig. 3E). We examined mice that were exposed to sustained loud sound (see SI Materials and Methods for details), which caused hearing loss, as evidenced by increased threshold of auditory brainstem responses (ABRs) in noise-exposed mice (Fig. S4 A–D). We also studied sham-exposed mice, which underwent the same procedure but were not exposed to sound. As expected, ZX1 enhanced PF EPSCs in fusiform neurons from sham-exposed mice, but, strikingly, did not affect PF EPSCs in fusiform neurons from noise-exposed mice (Fig. 4 A and B). Moreover, PPR, CV, and kinetic properties of PF EPSCs were not different between sham- and noise-exposed mice, suggesting that the lack of an effect of ZX1 on PF EPSCs in noise-exposed mice was not a result of changes in glutamatergic synaptic transmission (Fig. S4 E and F and Table S1). Quantal analysis on stimulus-evoked AN EPSCs from sham- and noise-exposed mice showed that CV was increased in noise-exposed mice but PPR was unaltered (Fig. S4 G and H). These results indicate decreased quantal content (n Pr) without changes in Pr in sound-exposed mice; such changes are consistent with a reduced number of release sites. Moreover, these results are consistent with reduced ABR thresholds (Fig. S4 A–D) and with previous studies showing damage of AN terminals even after milder acoustic trauma (37, 38).
Fig. S4.
(A–C) Representative traces of ABR traces in response to sound clicks presented at different intensities (dB) levels from a sham-exposed, exposed ear (A), noise-exposed, exposed ear (B), and noise-exposed animal, unexposed, contralateral ear (C). I-V represent the different waves of the ABR. (D) Summary graph of ABR thresholds from sham-exposed, ipsilateral ears and noise-exposed, contralateral ears (sham-exposed: 34 ± 5.47 dB, n = 5; noise-exposed contralateral ear: 61.8 ± 8.72 dB, n = 5, P < 0.01, Student unpaired t test). ABR thresholds for noise-exposed animals, exposed ears were above 90 dB were not detected with our system. (E and F) Summary of PPR (E) and CV (F) of fusiform cell PF EPSCs from sham- and noise-exposed mice. (PPR: sham-exposed: 2.14 ± 0.17, noise-exposed: 2.45 ± 0.27; P = 0.35, n = 5, Student unpaired t test; CV: sham-exposed: 0.26 ± 0.04, noise-exposed: 0.20 ± 0.03; P = 0.26, Student unpaired t test.) (G and H) Summary graph of PPR (G) and CV (H) of AN EPSCs from sham- and noise-exposed mice (PPR: sham-exposed: 0.93 ± 0.07, noise-exposed: 0.98 ± 0.04, P = 0.42, n = 5, Student unpaired t test; CV: sham-exposed: 0.18 ± 0.02; noise-exposed: 0.33 ± 0.03, P < 0.01, Student unpaired t test). Values represent mean ± SEM.
Fig. 4.
Plasticity of AMPAR EPSCs by sound-evoked reduction of presynaptic zinc levels in DCN parallel fiber synapses. (A) Representative PF EPSCs from sham- and noise-exposed mice before and after ZX1 application. (B) Time course of PF EPSC amplitude from sham- and noise-exposed mice before and after ZX1 application (PF EPSC amplitude: sham-exposed: 145.55 ± 6.87% of baseline, n = 5, P < 0.01; noise-exposed: 96.98 ± 4.85% of baseline, n = 5, P = 0.83; sham- vs. noise-exposed: P < 0.01). (C) Representative zinc-mediated fluorescent signals in sham- and noise-exposed mice at increasing concentrations of DA-ZP1. (D) Summary graph of fluorescence intensity at different concentrations of DA-ZP1 (fluorescence intensity in arbitrary units: sham- vs. noise-exposed, n = 5, P = 0.02 for 0.25 µM; P = 0.15 for 0.5 µM; P < 0.01 for 0.75 µM; P = 0.03 for 1 µM). (E, Left) Representative evoked, zinc-mediated fluorescent signals in sham- and noise-exposed mice in response to increasing number of pulses at 100 Hz. (Right) Time course of representative ratiometric fluorescent signals. (F) Summary graph of normalized extracellular zinc concentrations. Concentrations from noise-exposed mice are normalized to sham-exposed average concentrations.
We hypothesized that the lack of a ZX1 effect on PF EPSCs may be due to a decrease in zinc inhibition in PF EPSCs. Consistent with this hypothesis, we found that DCN slices from noise-exposed mice showed a significant decrease in synaptic zinc levels, as evidenced by reduced DA-ZP1 fluorescence in these mice (Fig. 4 C and D). Next, we compared evoked zinc release between sham- and noise-exposed mice. To quantify evoked zinc levels in DCN slices, we incubated the slices in ACSF containing the ratiometric zinc sensor LZ9 (2 μM), measured zinc-mediated fluorescence in response to PF electrical stimulation (Fig. 4E), and used the equation shown in SI Materials and Methods to convert fluorescent ratios to extracellular zinc levels, which were subsequently normalized to sham-exposed levels (15). We found that evoked zinc release was significantly reduced in noise-exposed mice (Fig. 4 E and F). This result suggests that sound-dependent reduction in vesicular zinc levels and vesicular zinc release abolished the inhibitory effect of zinc on PF AMPAR EPSCs. Because noise exposure caused reduction of AN inputs, we suggest that the sound-dependent removal of the inhibitory effect of zinc enhances PF EPSCs and is consistent with a compensatory, presynaptic homeostatic response that restores the overall excitatory strength in fusiform cells. This finding indicates that experience-dependent changes of presynaptic zinc levels caused AMPAR plasticity even in the absence of changes in glutamatergic transmission.
SI Materials and Methods
Animal Handling.
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh, Pittsburgh, PA.
Electrophysiology.
Brain slice preparation, electrophysiology, and imaging experiments were carried out using artificial cerebrospinal fluid (ACSF) with the following composition (in mM): 130 NaCl, 3 KCl, 1.2 CaCl2·2H2O, 1.3 MgCl2⋅6H2O, 20 NaHCO3, 3 Hepes, and 10 d-glucose, saturated with 95% O2/5% CO2 (vol/vol), pH = 7.25–7.35, ∼300 mOsm. For experiments described in Fig. 1C, similar results were obtained using ACSF containing 1.2 mM KH2PO4. Contaminating zinc was removed from the ACSF by stirring the ACSF with Chelex 100 resin (Biorad) for 1 h. High purity CaCl2⋅2H2O and MgCl2⋅6H2O salts (99.995% purity; Sigma Aldrich) were added to the ACSF after the Chelex resin was filtered using Nalgene rapid flow filters lined with polyethersulfone (0.2-µm pore size). All plastic and glassware were washed with 5% high purity nitric acid.
DCN brain slice preparation and electrophysiology.
Male or female ICR mice (Harlan laboratory) and ZnT3 WT and KO littermate mice (Jackson Laboratory) aged between postnatal day 18 (P18) and P28 were used in this study. Mice were first anesthetized with isoflurane and then immediately decapitated. Brains were rapidly removed and coronal slices (210 µm) of the left DCN were prepared in ACSF at 35 °C using a Vibratome (VT1200 S; Leica). Slices were then transferred into a holding chamber and incubated for ∼60 min at 35 °C before electrophysiology or imaging experiments were initiated. For electrophysiological experiments, slices were transferred into the recording chamber and perfused with ACSF at a rate of 1–2 mL/min. For noise exposure experiments, brain slices were prepared 10–15 min after sham or noise exposure, when the mice had recovered from anesthesia. CWCs and FCs were recorded in the molecular layer and fusiform cell layer of the DCN, respectively, and were identified by their characteristic firing pattern in cell-attached configuration. CWCs fire a mixture of simple and complex spikes and FCs fire simple regular spikes (48). Granule cells were identified based on their small soma size (<10 μm), location, morphology, and characteristic intrinsic properties (membrane capacitance: 2.45 ± 1.23 pF, input resistance: 1.95 ± 0.93 GΩ, n = 4) (49). Electrophysiological recordings were made using a MultiClamp-700B amplifier equipped with either a Digidata-1440A A/D converter and Clampex (Molecular Devices) or a National Instruments NI-USB-6229 DAQ board (National Instruments) and ephus. Voltage-clamp recordings were conducted using recording pipettes with 3–5 MΩ resistance filled with a cesium-based internal solution with the following composition (in mM): 126 CsCH3O3S, 4 KCl, 10 Hepes, 4 Na2ATP, 0.3 Tris-GTP, 10 Tris-phosphocreatine, 1 Cs2EGTA, 1 QX-314, and 3 sodium ascorbate (pH = 7.25, 295 mOsm). Current-clamp recordings in granule cells were conducted using a K-based internal solution containing (in mM) 113 K-gluconate, 4.5 MgCl2·6H2O, 14 Tris-phosphocreatine, 9 Hepes, 0.1 EGTA, 4 Na2ATP, 0.3 Tris-GTP, and 10 sucrose (pH = 7.3, 300 mOsm). Recordings were conducted at 34–37 °C using an inline heating system (Warner instruments). Data were sampled at 10 or 20 kHz and filtered at 4 kHz. AMPAR EPSCs in CWCs were evoked by stimulating zinc-rich parallel fibers with an Isoflex stimulator (AMPI), through a glass theta electrode containing ACSF. For paired-pulse experiments in CWCs, we used an interstimulus interval of 20 ms. In FC recordings, AMPAR EPSCs were elicited by stimulation of zinc-rich parallel fibers and zinc-lacking auditory fibers, two anatomically and functionally distinct excitatory inputs onto the FCs. Previous studies have shown that single stimuli in the molecular layer of the DCN evoke PF EPSCs in CWCs and FCs that are mediated by AMPARs, when recorded at hyperpolarized potentials and with 1.3 mM Mg2+ in the extracellular solution (50). For paired-pulse experiments in FCs, we used an interstimulus interval of 50 ms. For rise times, we report the 10–90% rise times of the EPSC. EPSC decay time constants were calculated from single exponential fits. To investigate the effect of ZX1 on spontaneous EPSCs (sEPSCs), sEPSCs were recorded from CWCs under control conditions for at least 15 min and for 20 min following ZX1 administration. Event detection and analysis of sEPSC amplitude, 10–90% rise time, and decay time constant were carried out using MiniAnalysis software (Synaptosoft). The threshold for detecting sEPSCs was set at 3 times the RMS of noise level. Action potential threshold was measured in phase plane as the membrane potential at which the depolarization slope exhibited the first abrupt change (Δslope > 10 V/s). For all experiments, series resistance was monitored throughout the experiment using the size and shape of the capacitive transients in response to a 5-mV depolarization step. Experiments were not included if the series resistance changed more than 15% during the recorded session. Experiments were conducted in the presence of glycine and GABAA receptor blockers, 1 µM strychnine, and 20 µM SR95531, respectively. ZX1 (100 µM) and tricine (10 mM) were always bath applied. Electrophysiological experiments involving ZnT3WT and ZnT3KO mice were blinded.
Hippocampal slice preparation and electrophysiology.
Male or female ZnT3KO or ZnT3WT mice aged between P18 and P32 were used in this study. Electrophysiological experiments, similar to those conducted with DCN slices, were performed in a blind fashion. Animals were first anesthetized with isoflurane and then immediately decapitated. Brains were rapidly removed and coronal or transverse hippocampal slices (260–300 µm) were prepared using a Vibratome (VT1200 S; Leica) in an ice-cold sucrose-rich solution with the following composition (in mM): sucrose 250, KCl 3, NaH2PO4 1.23, MgCl2·6H2O 5, CaCl2·2H2O 0.5, NaHCO3 26, and glucose 10, saturated with 95% O2/5% CO2 (pH = 7.25–7.35). Brain slices were placed in a temporary storage chamber filled with ACSF. Slices were maintained at 35 °C for 20 min and then at room temperature for at least 1 h before electrophysiological recordings. For electrophysiological experiments, slices were transferred into the recording chamber and perfused with ACSF at a rate of 1–2 mL/min. Electrophysiological recordings of CA1 hippocampal neurons were made using a MultiClamp-700B amplifier equipped with Digidata-1440A A/D converter and Clampex (Molecular Devices). Voltage-clamp recordings were conducted using recording pipettes with 3–5 MΩ resistance filled with cesium-based internal solution, similar to the one used for DCN slice electrophysiology. Recordings were conducted at 34–37 °C using an inline heating system (Warner instruments). Data were sampled at 20 kHz and filtered at 4 kHz. AMPAR EPSCs in CA1 neurons were evoked by stimulating the zinc-rich Schaffer collaterals with an Isoflex stimulator (AMPI), through a glass theta electrode containing ACSF. In paired-pulse experiments in CA1 neurons, we used an interstimulus interval of 50 ms. Series resistance was monitored throughout the experiment using the size and shape of the capacitive transients in response to a 5-mV depolarization step. Experiments were not included if the series resistance changed more than 15% during the recorded session. Extracellular field recordings were conducted by positioning the recording ACSF-filled electrode (resistance < 1 MΩ) in the stratum radiatum of CA1 region of the hippocampus. Field EPSPs (fEPSPs) were evoked by stimulating Schaffer collaterals once every 15 s by positioning a concentric bipolar tungsten electrode (FHC) in the stratum radiatum. Recordings were conducted at 34–37 °C using an inline heating system (Warner instruments). fEPSPs were recorded and analyzed using MultiClamp-700B amplifier equipped with Digidata-1440A A/D converter and Clampex (Molecular Devices).
Glutamate Uncaging.
DCN slices were prepared as stated above. Voltage-clamp recordings were made in the presence of tetrodotoxin (TTX, 500 nM) in ACSF to prevent action potentials. AMPAR currents were elicited by photolytic uncaging of MNI-caged glutamate (100 µM) onto the dendritic arbor in the molecular layer using 1- or 2-ms pulses of UV laser light (355 nm; DPSS Lasers). Laser power, duration, and uncaging location were adjusted so that the evoked AMPAR current reached a peak amplitude of ∼500 pA. Once a stable uncaging response was established, ZX1 (100 µM) was added to the ACSF to measure the effect of zinc chelation on the AMPAR current.
Noise Exposure.
Male and female ICR (CD-1) mice (P17–P30) were used for noise and sham exposure experiments. Noise- or sham-exposed mice were anesthetized using 3% isoflurane during induction and 1–1.5% during maintenance. Noise-exposed mice were exposed unilaterally (left ear) using a narrow bandpass noise with a 1-kHz bandwidth centered at 16 kHz at 116 dB sound pressure level (SPL) for 4 h. Noise was presented through a pipette tip, one end of which was attached to the speaker (CF-1; Tucker Davis Technologies), whereas the other end was inserted to the left ear canal of the mouse. Sham-exposed animals were subjected to an identical procedure but without any noise exposure.
ABRs.
ABRs reflect the synchronous activity of auditory brainstem nuclei in response to sound, arising from the auditory nerve (wave I) to the inferior colliculus (wave V); waves II–IV represent the activity of various auditory brainstem nuclei. ABRs were measured immediately after sham or noise exposure. ABRs were detected by measuring the amplitude of the ABR in response to 1-ms click sound stimuli. The sound intensity that generated ABRs that were 4 SDs above the baseline noise level was classified as ABR threshold. Baseline noise levels were measured using the ABRs obtained at 0-dB sound intensity. For ABR measurements, mice were anesthetized using 3% isoflurane during induction and 1–1.5% during maintenance. Anesthetized animals were placed in a sound attenuating chamber with a subdermal electrode placed at the vertex, the ground electrode was placed ventral to the right pinna, and the reference electrode ventral to the left pinna (sham- and/or noise-exposed ear). To measure ABRs from unexposed, contralateral ears of noise-exposed mice, the ground electrode was placed ventral to the left pinna and the reference electrode was placed ventral to the right pinna (contralateral ear). Temperature was maintained at ∼37 °C using a heating pad. Sound stimuli were presented using a pipette tip, one end of which was attached to the speaker (CF-1; Tucker Davis Technologies), whereas the other end was inserted to the left ear (sham- and/or noise-exposed animal) or right ear (unexposed ear of the noise-exposed animal) canal of the mouse. ABR thresholds were measured for 1-ms click presented at a rate of 18.56/s using System 3 software package from Tucker Davis Technologies. Evoked potentials were averaged 1,024 times and filtered using a 300- to 3,000-Hz band-pass filter.
Fluorescence Imaging.
Synaptic vesicular zinc imaging in DCN slices with DA-ZP1.
Vesicular zinc imaging experiments were carried out using DA-ZP1, a high affinity, zinc-sensitive, membrane permeable, fluorescent sensor (34). In our experiments, DA-ZP1 was used to detect vesicular zinc in the synaptic terminals of the zinc-rich parallel fibers of the DCN. Zinc imaging was carried out in sham- and noise-exposed ICR (CD-1) mice (P17–P30) and ZnT3KO (P17–P30) mice. Sham and noise exposure experiments were performed in an interleaved manner. Immediately after sham or noise exposure (only ICR mice), animals were euthanized and DCN slices were prepared as described above. To compare synaptic vesicular zinc levels, DCN slices from all three groups of animals (sham-exposed, noise-exposed, and ZnT3KO mice) were placed in a recording chamber perfused with recirculating ACSF maintained at 32–35 °C, containing blockers of ionotropic receptors (NMDA receptor antagonist AP5, 50 µM; AMPA/kainate receptor antagonist DNQX, 20 µM; SR95531, 20 µM and strychnine, 1 µM). Fluorescence was measured at different concentrations of DA-ZP1 (0.25, 0.5, 0.75, and 1 µM), which were added sequentially to the recirculating ACSF. Fluorescence responses were acquired as time-lapse movies over 30 min using an upright microscope (Olympus BX5) with a 20× water immersion objective and epifluorescence optics. The excitation source was an ephus-driven blue LED (M470L2; Thorlabs). The zinc-sensitive green fluorescent signals were isolated using a Pinkel filter set (Semrock LF488/543/635–3X-A-000) and acquired using a CCD camera (Rolera XR; QImaging). Fluorescence intensity was measured using ImageJ software. Fluorescence intensity was measured at five different regions of interest (ROIs) in the molecular layer of the DCN. The area of each ROI was 1,962 µm2 (x axis: 44.3 µm; y axis: 44.3 µm). To account for the background fluorescence, ROIs were measured from five different locations in the deep layer of the DCN. The mean fluorescence intensity was then calculated by subtracting the background fluorescence values obtained from the zinc-free deep layer of the DCN from the fluorescence values obtained from the zinc-rich molecular layer of the DCN.
Imaging of synaptic zinc release in the DCN using ZP1-6COOH.
ZP1-6COOH is a membrane impermeable, high affinity fluorescent zinc sensor (51). For imaging synaptic zinc release, slices were incubated in recirculating ASCF at 31–33 °C containing the slow zinc chelator Ca-EDTA (200 μM) to reduce background fluorescence, the fluorescent zinc sensor ZP1-6COOH (2 μM), and blockers of ionotropic receptors: AP5 (50 μM), SR95331 (20 μM), DNQX (20 μM), and strychnine (1 μM). Synaptic zinc release from the molecular layer of the DCN was evoked with a theta stimulating electrode. Fluorescent movies were acquired with a confocal microscope (Leica TCS SP5 MP) using the Leica LAS AF software package. Imaging and stimulus synchronization was controlled by ephus. A 488-nm laser source was used to excite ZP1-6COOH, and green fluorescent emission signals were isolated with a band-pass filter (495–600 nm) and acquired with photomultiplier tubes. Confocal images were acquired at 10 Hz for 20 s. Electrical stimuli were 0.3-ms, 50-V pulses delivered at 100 Hz for 1 s, starting at 2 s into the movie. Stimulus trials were interleaved with nonstimulus trials to correct for the gradual, linear “run-up” observed during imaging. A linear fit of the nonstimulus trace was subtracted from the stimulus and nonstimulus traces, and the raw fluorescence values were then converted into ΔF/Fo, with Fo taken as the average baseline fluorescence from 1 s before the onset of the stimulus.
Synthesis of extracellular zinc probe LZ9 (ZP1-6COOH linked to lissamine rhodamine B).
The sensor LZ9 was prepared by using a modified literature procedure (15). The peptide Fmoc-P9K(Mtt)E(OtBu) was synthesized using an Aapptec Focus XI automated synthesizer on a 100-µmol scale. Fmoc-Glu(OtBu)-Wang resin (substitution 0.48 mmol/g, 208 mg) was loaded into the reaction vessel and stirred in dimethylformamide (DMF; 10 mL) for 30 min with gentle rocking. Fmoc groups were removed by subsequent 3- and 15-min treatments with 20% piperidine in DMF (5 mL). The resin beads were washed thoroughly (5 × 5 mL) with DMF before and after each coupling reaction. For coupling reactions, Fmoc-Pro-OH (500 µmol, 169 mg) or Fmoc-Lys(Mtt)-OH (500 µmol, 312 mg) were activated with HBTU (500 µmol, 190 mg) and 0.5 M diisopropylethylamine (DIPEA) in DMF (3 mL) for 1.5 min before addition to the reaction vessel. Coupling reactions were performed for 30 min with gentle agitation. Subsequent amino acids were added iteratively using the same deprotection and coupling protocols. The resin was washed with DMF and CH2Cl2 and dried under nitrogen flow. A portion of the resin (∼25 µmol peptide) was then transferred to a glass peptide synthesis vessel. The resin was stirred for 30 min in DMF (5 mL) with magnetic stirring. The solvent was removed and the resin beads were treated twice with 20% piperidine in DMF (5 mL) with stirring for successive 20-min periods to remove the N-terminal Fmoc group. The resin was washed sequentially with DMF (4 × 5 mL), 10% DIPEA in DMF (5 mL), and DMF (2 × 5 mL). A solution of Lissamine rhodamine B (LRB) sulfonyl chloride (60 µmol, 35 mg; Anaspec) in DMF (2 mL) was added to the reaction vessel and allowed to react for 1.5 h with stirring. This solution was drained from the vessel and the resin beads were washed with DMF (5 × 5 mL) and CH2Cl2 (5 × 5 mL). The Lys side chain was selectively deprotected by incubating the resin with three 3.3 mL portions of a mixture of trifluoroacetic acid (TFA; 0.3 mL) and triisopropylsilane (TIPS; 0.5 mL) in CH2Cl2 (9.2 mL) in 2 min intervals. The resin beads were washed thoroughly with CH2Cl2 and DMF. A solution of ZP1 (70 µmol, 61 mg), HBTU (70 µmol, 27 mg), and DIPEA (500 µmol, 88 µL) in DMF (2.5 mL) was added to the reaction vessel and the mixture was allowed to react for 6 h with stirring. The solution was removed from the reaction vessel and the resin was washed with DMF, treated with 20% piperidine in DMF (5 mL) for 10 min, washed again with DMF and CH2Cl2, and dried under vacuum. The peptide was cleaved from the resin by treatment with a 38:1:1 mixture of H2O/TIPS/TFA (5 mL; vol/vol/vol) for 30 min. The solution was collected and concentrated under reduced pressure. The crude residue was brought up in H2O/CH3CN (1:1, vol/vol), diluted 100-fold with H2O, and purified by preparative HPLC (15 mL/min; solvent A: 0.1% AcOH in H2O; solvent B: 0.1% AcOH in CH3CN) with a Zorbax SB-C18 column (7 µm, 21.2 × 250 mm) using a linear solvent gradient that ramped from 0% solvent B to 10% solvent B over 2.5 min, then to 25% solvent B over 2.5 min, then to 50% solvent B over 20 min, and then to 100% solvent B over 5 min, followed by a 7-min washout period. Analytically pure material was obtained through semipreparative HPLC (3 mL/min; solvent A: 0.1% TFA in H2O; solvent B: 0.1% TFA in CH3CN) with a Zorbax SB-C18 column (5 µm 9.4 × 250 mm) using a linear solvent gradient that was isocratic at 0% solvent B for 4 min, then ramped to 39% solvent B over 1 min, and then to 40.5% solvent B over 7 min, followed by a 1-min washout period. Purity was assessed by analytical HPLC (1 mL/min; solvent A: 0.1% TFA in H2O; solvent B: 0.1% TFA in CH3CN) with a Zorbax SB-C18 column (5 µm, 4.6 × 250 mm) using a linear solvent gradient that ramped from 0% solvent B to 100% solvent B over 100 min. Fractions containing the product were pooled and lyophilized. LRMS (ESI+) m/z calcd for C130H148Cl2N20O26S22+ [M + 2H]2+ 1269.5, found 1269.4; calcd for C130H149Cl2N20O26S23+ [M + 3H]3+ 846.7, found 847.3. Aliquots of 5 mM LZ9 in DMSO were stored in the dark at −20 °C.
Imaging of synaptic zinc release in the DCN using LZ9.
For imaging synaptic zinc release with the LZ9 zinc probe, slices were incubated in recirculating ASCF at 31–33 °C containing the slow zinc chelator Ca-EDTA (200 μM) to reduce background fluorescence, the fluorescent zinc sensor LZ9 (2 μM), and blockers of ionotropic receptors: AP5 (50 μM), SR95331 (20 μM), DNQX (20 μM), and strychnine (1 μM). Because of the large FRET component of LZ9 ratiometric zinc sensor (red emission from LRB via FRET) excitation by ZP1, we used an interleaved, multiplexed approach for ratiometric imaging (15). Blue excitation of LZ9 excites the ZP1 moiety, so all emission wavelengths, green or red, are due to the zinc-sensitive component of the sensor. Green excitation of LZ9 excites LRB, resulting in zinc-insensitive red emission. Using ephus, blue and green light-emitting diodes (M470L2 and M530L2; Thorlabs) were synchronized with the exposure times of a CCD camera (Rolera XR; QImaging) so that every other frame used either blue or green excitation. A Pinkel filter set was used to separate the two emission and two excitation colors (LF488/ 543/635–3X-A-000; Semrock). Two-channel, multiplexed movies were acquired at frame rates of 20 Hz, each channel at a frame rate of 10 Hz using an upright microscope (Olympus BX5) with a 20× or 40× water immersion objective and epifluorescence optics. Each frame had an exposure time of 38 ms and interframe interval of 50 ms. Synaptic zinc release from the molecular layer of the DCN was evoked with a theta stimulating electrode using 30, 50, and 100 pulses delivered at 100 Hz. The fluorescent response was visualized using a fourth-order, low-pass, 2D Butterworth filter, applied to each frame. This revealed a “hotspot” of fluorescence in the molecular layer of the DCN and this ROI was quantified in the unfiltered images. The ratio of ZP1-based fluorescence to LRB-based fluorescence (ratiometric zinc signal, R) was created by dividing the frame-averaged ROI during blue excitation by the same ROI in the subsequent frame acquired with green excitation for each pair of frames. In each experiment, LZ9 was calibrated by adding 4.5 mM of the high-affinity zinc chelator EDTA to the ACSF to measure Rmin followed by 5 mM ZnCl2 to saturate the probe and measure Rmax. Based on Rmin and Rmax, each ratiometric frame was converted into zinc concentration using the following equation: [Zn2+] = Kd × (R – Rmin)/(Rmax – R), where, Kd is the dissociation constant of the ratiometric probe and R is the ratiometric fluorescence acquired with interleaved multiplexed imaging (15). Rmin is the minimum ratiometric fluorescence of the probe in the presence of 4.5 mM EDTA, and Rmax is the maximum fluorescence obtained after application of 5 mM ZnCl2.
Statistics.
For statistical comparisons, Student paired t test, Student unpaired t test, and one-way ANOVA were used if the group data passed Lilliefor’s test for normality. Tukey’s least significant test was used for post hoc analysis of one-way ANOVA. If the group data were not normally distributed, then the Wilcoxon rank sum test was used. Significance levels are defined as P < 0.05: *P < 0.05; **P < 0.01. Error bars represent ± SEM.
Values and Statistical Tests for Main Figures.
Values.
Fig. 2 (F–I): Cumulative probability plot of sEPSC amplitude (F), frequency (G), rise time (H), and decay time (I) before and after ZX1 application (mean sEPSC amplitude: control: 18.32 ± 1.46 pA; ZX1: 18.78 ± 1.56 pA; n = 5, P = 0.24; mean sEPSC frequency: control: 6.78 ± 0.92 Hz; ZX1: 7.56 ± 1.41 Hz; n = 5, P = 0.72; mean sEPSC rise time: control: 1.03 ± 0.05 ms; ZX1: 1.07 ± 0.04 ms, n = 5, P = 0.25; mean sEPSC decay time: control: 1.72 ± 0.04 ms; ZX1: 1.78 ± 0.06 ms, n = 5, P = 0.10). Fig. 4D: Fluorescence intensity (arbitrary units) in DA-ZP1: sham-exposed: 163.32 ± 21.78 in 0.25 µM, 457.30 ± 131.56 in 0.5 µM, 981.37 ± 134.04 in 0.75 µM, 1138.83 ± 116.92 in 1 µM; noise-exposed: 64.18 ± 29.78 in 0.25 µM, 196.76 ± 101.70 in 0.5 µM, 405.80 ± 103.04 in 0.75 µM, 704.38 ± 125.32 in 1 µM; Fig. 4F: Normalized synaptic zinc release at 100-Hz stimulation measured using LZ9 zinc probe: noise-exposed animals: 100 pulses: 45.23 ± 6.64% of sham-exposed animals; 50 pulses: 28.44 ± 4.20% of sham-exposed animals; 30 pulses: 2.64 ± 2.05% of sham-exposed animals.
Statistical tests.
Fig. 1C: Student paired t test. Fig. 1E: Student paired t test. Fig. 1F: One-way ANOVA, post hoc Tukey. Fig. 1H: One-way ANOVA, post hoc Tukey. Fig. 1J: ZnT3WT PF EPSC amplitude before and after ZX1: Student paired t test; ZnT3KO PF EPSC amplitude before and after ZX1: Student paired t test; PF EPSC amplitude after ZX1, ZnT3WT vs. ZnT3KO: Student unpaired t test.
Fig. 2C: Student paired t test. Fig. 2E: Student paired t test. Fig. 2 F–I: Student paired t test.
Fig. 3H: PF EPSC amplitude before and after ZX1: Student paired t test; AN EPSC amplitude before and after ZX1: Student paired t test; EPSC amplitude after ZX1, PF vs. AN: Student unpaired t test. Fig. 3K: ZnT3WT Schaffer collateral EPSC amplitude before and after ZX1: Student paired t test; ZnT3KO Schaffer collateral EPSC amplitude before and after ZX1: Student paired t test; Schaffer collateral EPSC amplitude after ZX1, ZnT3WT vs. ZnT3KO: Student unpaired t test.
Fig. 4B: Sham-exposed PF EPSC amplitude before and after ZX1: Student paired t test; Noise-exposed PF EPSC amplitude before and after ZX1: Student paired t test; PF EPSC amplitude after ZX1, sham exposed vs. noise exposed: Student unpaired t test. Fig. 4D: Student unpaired t test. Fig. 4F: Student unpaired t test.
Discussion
Our results show that endogenous, synaptically released zinc modulates AMPAR EPSCs in two different brain areas. Although earlier studies had suggested that exogenous zinc modulates AMPARs, this modulation has been considered physiologically irrelevant, because recent work failed to reveal any effect of endogenous zinc on AMPAR EPSCs in hippocampal and in DCN synapses (14, 22). However, these studies either used tricine or compared AMPAR EPSCs between WT and ZnT3KO mice. The use of tricine is problematic, because, unlike ZX1, tricine cannot efficiently prevent zinc from binding high-affinity zinc-binding sites and therefore is not an appropriate chelator for studying the role of zinc in synapses (15). Consistent with these results, ZX1, but not tricine, revealed the effect of endogenous zinc on AMPAR EPSCs (Fig. 1 G and H).
Finally, the lack of difference in the size of AMPAR EPSCs, evoked by trains of synaptic stimuli, between WT and ZnT3KO in hippocampal synapses has been used as evidence for the lack of effect of endogenous zinc on MF AMPA EPSCs (14). However, this result does not exclude compensatory, non–zinc-mediated mechanisms that maintain AMPAR EPSCs unchanged in ZnT3KO mice. Together, our results establish vesicular zinc as an endogenous AMPAR modulator that adjusts fast excitatory synaptic transmission in the brain.
Single shocks of PFs revealed a robust ZX1 effect on PF EPSCs (Figs. 1, 3, and 4), but ZX1 application did not enhance sEPSCs (Fig. 2 D and E). These results suggest that zinc is “pooling” between release sites so as to require multisite activity to exert its inhibitory effect on AMPARs (i.e., evoked, multisynapse release). Moreover, our experiments in PF EPSCs showed no changes in the quantal content of PF glutamatergic neurotransmission after noise exposure (Fig. S4 E and F). However, our results revealed decreases in zinc content and zinc release in the same PF terminals from noise-exposed mice (Fig. 4 C–F), suggesting that activity-dependent changes in zinc-containing vesicles mediate the observed sound-dependent plasticity in presynaptic zinc levels and release (Fig. 4). These results suggest that zinc-containing vesicles may form a functionally and/or anatomically distinct population. This hypothesis is consistent with previous studies showing that ZnT3 interacts with the adaptor protein AP3 and is preferentially targeted to a distinct vesicle subpopulation (39, 40). The anatomical and functional properties of different zinc-containing synapses and the relative distribution and release dynamics of zinc-containing vesicles may determine the requirement for differential activity patterns capable of eliciting zinc modulation of AMPAR EPSCs in zinc-containing glutamatergic synapses. These differential requirements may explain the lack of effect of ZX1 in mossy fiber field potentials (13).
Whereas previous results have shown that AMPARs lacking GluA2 subunits are permeable to zinc (41), our results establish that endogenous zinc inhibits AMPAR EPSCS in zinc-containing synapses that express GluA1-3. Our results are consistent with direct binding and modulation of AMPARs by synaptic zinc. This conclusion is also consistent with structural data showing that the LBD of GluA2 contains a number of zinc binding sites formed mainly by histidine residues (42). Because the ATD and LBD are tightly packed in NMDARs but more separated in AMPARs, with the consequence that the ATD is not a regulatory site for AMPARs (8, 9), we propose that the LBD is a more likely site for allosteric AMPAR modulation by zinc. More studies are needed for determining the subunit sensitivity, the binding site, and the underlying biophysical mechanism of zinc-mediated AMPAR inhibition.
How could stimulation by noise exposure, which targets AN inputs, lead to changes in vesicular zinc in PF inputs? Recent results have revised the DCN circuitry and support the notion that, through electrical coupling with fusiform cells, stellate cells, a class of interneurons in the molecular layer, sense ongoing auditory activity, thus providing a link between AN and PF activity (43, 44). Based on these findings, auditory signals are able to rapidly recruit or suppress stellate cells and control the efficacy of PF activity. Auditory-evoked changes in PF activity through this pathway may provide the trigger for plasticity in presynaptic zinc levels. Moreover, other studies indicate that coincident synaptic activation of PF and AN inputs lead to induction of spike-timing dependent synaptic plasticity (STDP) of parallel fiber inputs (45), by analogy with the climbing fiber and parallel fiber inputs in the cerebellum. According to this scheme, auditory experience-dependent increases in fusiform cell spiking could also provide the trigger to induce plastic changes in PF inputs. Alternatively, because granule cells receive auditory nerve input from higher auditory centers such as auditory cortex, the changes in auditory stimulation might cause changes in vesicular zinc via this pathway (46).
Previous studies have established activity-dependent AMPAR synaptic plasticity via changes in pre- and postsynaptic glutamatergic neurotransmission (47). Such AMPAR plasticity is involved in memory, learning, and development of the CNS and is crucial for the proper functioning and adaptability of the mammalian brain. The sound-dependent plasticity of presynaptic zinc levels and zinc-mediated inhibition of AMPARs (Fig. 4) adds zinc as a key player in the complexity of AMPAR synaptic plasticity in the mammalian brain.
Materials and Methods
All animal procedures were approved by Institutional Animal Care and Use Committees of the University of Pittsburgh. Methods for preparing brain slices, electrophysiological recordings, noise exposure, recording of acoustic brainstem responses, and fluorescence imaging are provided in SI Materials and Methods. Data analysis, statistical tests, and detailed values presented in main figures are also provided in SI Materials and Methods.
Acknowledgments
We thank Dr. Elias Aizenman for helpful discussions and critical comments on the manuscript. This work was supported by funding from National Institutes of Health Grants R01-GM065519 (to S.J.L.), F32- DC013734 (to C.T.A.), F32-GM109516 (to J.M.G.), and R01-DC007905 (to T.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512296112/-/DCSupplemental.
References
- 1.Traynelis SF, et al. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80(2):292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S, Paoletti P. Allosteric modulators of NMDA receptors: Multiple sites and mechanisms. Curr Opin Pharmacol. 2015;20:14–23. doi: 10.1016/j.coph.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Hansen KB, Furukawa H, Traynelis SF. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol Pharmacol. 2010;78(4):535–549. doi: 10.1124/mol.110.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 6.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15(2):453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 7.Aizenman CD, Muñoz-Elías G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34(4):623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- 8.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344(6187):992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CH, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511(7508):191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462(7274):745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3(3):181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- 12.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26(1):187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 13.Pan E, et al. Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3 synapse. Neuron. 2011;71(6):1116–1126. doi: 10.1016/j.neuron.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergnano AM, et al. Zinc dynamics and action at excitatory synapses. Neuron. 2014;82(5):1101–1114. doi: 10.1016/j.neuron.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Anderson CT, et al. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc Natl Acad Sci USA. 2015;112(20):E2705–E2714. doi: 10.1073/pnas.1503348112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 17.Sindreu CB, Varoqui H, Erickson JD, Pérez-Clausell J. Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb Cortex. 2003;13(8):823–829. doi: 10.1093/cercor/13.8.823. [DOI] [PubMed] [Google Scholar]
- 18.Mayer ML, Vyklicky L, Jr, Westbrook GL. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rassendren FA, Lory P, Pin JP, Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990;4(5):733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- 20.Dreixler JC, Leonard JP. Subunit-specific enhancement of glutamate receptor responses by zinc. Brain Res Mol Brain Res. 1994;22(1-4):144–150. doi: 10.1016/0169-328x(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang DQ, Ribelayga C, Mangel SC, McMahon DG. Suppression by zinc of AMPA receptor-mediated synaptic transmission in the retina. J Neurophysiol. 2002;88(3):1245–1251. doi: 10.1152/jn.2002.88.3.1245. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Rosello T, et al. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J Neurosci. 2013;33(22):9259–9272. doi: 10.1523/JNEUROSCI.0237-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oertel D, Young ED. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27(2):104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Frederickson CJ, Howell GA, Haigh MD, Danscher G. Zinc-containing fiber systems in the cochlear nuclei of the rat and mouse. Hear Res. 1988;36(2-3):203–211. doi: 10.1016/0378-5955(88)90062-7. [DOI] [PubMed] [Google Scholar]
- 25.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsien RW, Malinow R. Changes in presynaptic function during long-term potentiation. Ann N Y Acad Sci. 1991;635:208–220. doi: 10.1111/j.1749-6632.1991.tb36493.x. [DOI] [PubMed] [Google Scholar]
- 27.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158(1):126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 28.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci USA. 1999;96(4):1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Rosello T, Anderson CT, Ling C, Lippard SJ, Tzounopoulos T. Tonic zinc inhibits spontaneous firing in dorsal cochlear nucleus principal neurons by enhancing glycinergic neurotransmission. Neurobiol Dis. 2015;81:14–19. doi: 10.1016/j.nbd.2015.03.012. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen KB, Ogden KK, Yuan H, Traynelis SF. Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron. 2014;81(5):1084–1096. doi: 10.1016/j.neuron.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005;280(45):37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- 32.Rubio ME, Juiz JM. Chemical anatomy of excitatory endings in the dorsal cochlear nucleus of the rat: Differential synaptic distribution of aspartate aminotransferase, glutamate, and vesicular zinc. J Comp Neurol. 1998;399(3):341–358. doi: 10.1002/(sici)1096-9861(19980928)399:3<341::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Petralia RS, Rubio ME, Wang YX, Wenthold RJ. Differential distribution of glutamate receptors in the cochlear nuclei. Hear Res. 2000;147(1-2):59–69. doi: 10.1016/s0378-5955(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 34.Zastrow ML, et al. Reaction-based probes for imaging mobile zinc in live cells and tissues [published online ahead of print September 23, 2015] ACS Sensors. 2015 doi: 10.1021/acssensors.5b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakashima AS, Dyck RH. Zinc and cortical plasticity. Brain Res Brain Res Rev. 2009;59(2):347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Kalappa BI, Tzounopoulos T. Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. eLife. 2015;4 doi: 10.7554/eLife.07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salazar G, et al. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell. 2004;15(2):575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavoie N, et al. Vesicular zinc regulates the Ca2+ sensitivity of a subpopulation of presynaptic vesicles at hippocampal mossy fiber terminals. J Neurosci. 2011;31(50):18251–18265. doi: 10.1523/JNEUROSCI.4164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Y, Jeng JM, Sensi SL, Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol. 2002;543(Pt 1):35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: Crystal structures of the GluR2 ligand binding core. Neuron. 2000;28(1):165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 43.Apostolides PF, Trussell LO. Regulation of interneuron excitability by gap junction coupling with principal cells. Nat Neurosci. 2013;16(12):1764–1772. doi: 10.1038/nn.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apostolides PF, Trussell LO. Superficial stellate cells of the dorsal cochlear nucleus. Front Neural Circuits. 2014;8:63. doi: 10.3389/fncir.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Rubio M, Tzounopoulos T. Mechanisms underlying input-specific expression of endocannabinoid-mediated synaptic plasticity in the dorsal cochlear nucleus. Hear Res. 2011;279(1-2):67–73. doi: 10.1016/j.heares.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryugo DK, Haenggeli CA, Doucet JR. Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res. 2003;153(4):477–485. doi: 10.1007/s00221-003-1605-3. [DOI] [PubMed] [Google Scholar]
- 47.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7(7):719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- 49.Balakrishnan V, Trussell LO. Synaptic inputs to granule cells of the dorsal cochlear nucleus. J Neurophysiol. 2008;99(1):208–219. doi: 10.1152/jn.00971.2007. [DOI] [PubMed] [Google Scholar]
- 50.Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci USA. 2003;100(1):265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodroofe CC, Masalha R, Barnes KR, Frederickson CJ, Lippard SJ. Membrane-permeable and -impermeable sensors of the Zinpyr family and their application to imaging of hippocampal zinc in vivo. Chem Biol. 2004;11(12):1659–1666. doi: 10.1016/j.chembiol.2004.09.013. [DOI] [PubMed] [Google Scholar]