Abstract
After the discovery of retroviral reverse transcriptase in 1970, there was a flurry of activity, sparked by the “War on Cancer,” to identify human cancer retroviruses. After many false claims resulting from various artifacts, most scientists abandoned the search, but the Gallo laboratory carried on, developing both specific assays and new cell culture methods that enabled them to report, in the accompanying 1980 PNAS paper, identification and partial characterization of human T-cell leukemia virus (HTLV; now known as HTLV-1) produced by a T-cell line from a lymphoma patient. Follow-up studies, including collaboration with the group that first identified a cluster of adult T-cell leukemia (ATL) cases in Japan, provided conclusive evidence that HTLV was the cause of this disease. HTLV-1 is now known to infect at least 4–10 million people worldwide, about 5% of whom will develop ATL. Despite intensive research, knowledge of the viral etiology has not led to improvement in treatment or outcome of ATL. However, the technology for discovery of HTLV and acknowledgment of the existence of pathogenic human retroviruses laid the technical and intellectual foundation for the discovery of the cause of AIDS soon afterward. Without this advance, our ability to diagnose and treat HIV infection most likely would have been long delayed.
Keywords: human retrovirus, adult T-cell leukemia
The late 1970s and early 1980s were banner years for the study of cancer. Until 1980, research on molecular, genetic, and viral mechanisms of oncogenesis was largely confined to experimental animal models—particularly mice and chickens, and cells derived from them—that dated back to near the beginning of the century. The applicability of the findings of these studies, and of the tools used in them, to human disease remained to be established. Indeed, the 1979 Cold Spring Harbor Symposium—entitled “Viral Oncogenes”—focused entirely on animal viruses and cancers, and not one of the 125 talks from most of the leading tumor virology laboratories discussed human cancer (1). At that time, only Burkitt’s lymphoma, a rare disease limited to malaria-rich regions of Africa, was known to be associated with virus infection (2), and there was only one “human” retrovirus, an uncommon nonpathogenic spuma (or foamy) virus causing occasional zoonotic infections after transmission from infected chimpanzees (3). This situation was soon to change dramatically.
This time period was noteworthy for many discoveries, based on earlier virus studies, that provided the foundation for modern cancer research, as well as therapy and prevention. Among these discoveries were the following: (i) the identification and clarification of numerous retroviral oncogenes (4–11), (ii) the discovery that these genes are derived from normal cellular genes (protooncogenes) of the host and that close homologs are present in human DNA (12), (iii) the identification of a novel enzymatic activity (protein tyrosine kinase) of a number of oncogene products (13), (iv) the discovery that retroviruses that lack oncogenes (often called leukemia viruses) can cause cancer by rare integration of their DNA in the vicinity of protooncogenes, thereby altering the regulation of their expression (14), (v) the recognition that many human cancers (including Burkitt’s lymphoma) contained protooncogenes altered by mutation or chromosomal rearrangement in ways similar to what happens in retroviruses, but in the absence of viral involvement (15–17), and (v) the discovery that a relatively common and widespread cancer (cervical carcinoma) was caused by infection with a DNA virus, human papilloma virus (18). Rounding out this list is the discovery of human T-cell leukemia virus (HTLV; now known as HTLV-1), the first pathogenic human retrovirus, reported in the paper reprinted in this issue of PNAS as part of the 100th anniversary series (19).
The discovery of retroviral reverse transcriptase (RT) in 1970 (20, 21) had a number of consequences beyond providing dramatic proof for the provirus theory of retrovirus replication put forward by Temin years earlier and discussed by Vogt (22, 23). For one, it provided an important tool for molecular biologists, enabling them to make DNA copies of RNA at will for studies using molecular hybridization, and, eventually, cloning, PCR, and deep sequencing. Second, because of its presence in virus particles (virions), RT provided a simple means of detecting and quantitating viruses—particularly ones that lacked oncogenes, which often had no visible effect on cell cultures—far superior to the cumbersome and insensitive electron microscope techniques then in use. As we shall see, this tool turned out to be a two-edged sword. Third, by validating a plausible molecular mechanism by which certain RNA viruses—now called retroviruses—could modify the genomes of normal cells to transform them into cancer cells, it provided a significant impetus to the Special Virus Cancer Program (SVCP), originated about 10 y earlier, which was to become a major part of the “War on Cancer” proclaimed by then US President Richard Nixon and made real by the passage of the National Cancer Act in 1971 (24).
The availability of new tools, including reverse transcriptase assays, as well as substantial funding available through SVCP contracts, led many well-established investigators to join in renewed efforts to identify retroviruses associated with human cancer, and, as incisively reviewed by Weiss and coworkers (25, 26), the early 1970s saw numerous publications reporting the detection of new retroviruses in human cancers, all of which proved to be due to artifacts of one sort or another, such as contamination of cell lines with infectious viruses of other species, often during transmission of human tumors as xenotransplants, or simply during growth in the same laboratory. In other cases, investigators were misled by the production in some cancer cell lines of noninfectious particles arising from expression of certain endogenous proviruses common to all humans. A number of investigators also reported the presence in some cancers of particles containing both RNA and associated reverse transcriptase, which could have been retrovirus virions, but turned out to be complexes of cellular RNA and DNA polymerase (particularly γ), which, under certain conditions, can use RNA as a template. By the end of the decade, most investigators had abandoned the search.
Prelude to the 1980 PNAS Paper
According to his autobiography (27), Robert Gallo was drawn to medicine, particularly to cancer research, by the death of his sister from leukemia at a very young age, and, after receiving a Doctor of Medicine degree and subsequent training, he gravitated to the National Cancer Institute (NCI), becoming head of its Laboratory of Tumor Cell Biology (LTCB) in the early 1970s. Like many other groups, the Gallo laboratory was apparently motivated to search for retrovirus–cancer associations by the discovery of reverse transcriptase and both its mechanistic implications and its practical utility in virus discovery. Their early papers on distinguishing viral from cellular DNA polymerases (28–31) were important in clearing up some of the confusion as to what constituted a true retroviral RT signal. The group also made important advances in the technology for growing blood cells—particularly T cells—in culture, culminating in the discovery and partial purification of T-cell growth factor (now called IL-2) from phytohemagglutinin-stimulated T cells (32), and its use to establish, for the first time, continuous cultures of normal and malignant T cells. These tools—the culture techniques and the availability of the key growth factor—not only enabled the discovery of HTLV described in the accompanying PNAS Anniversary paper, but also subsequently made possible the rapid discovery and characterization of HIV by both the Gallo and Montagnier groups several years later (33, 34).
The lesson that many researchers apparently took from the plethora of false discoveries of supposedly human retroviruses, dubbed “human rumor viruses” (25), was that the search was a futile one, and most turned their attention elsewhere. The Gallo laboratory, although not immune to the same problem (35), nonetheless carried on, only setting the bar much higher. According to a later memoir (36), they now had a list of requirements (a sort of updated Koch’s postulates) that any candidate human retrovirus must pass before it could be reported (here slightly modified from the original): (i) It must be present in freshly isolated uncultured (or briefly cultured) tissue from a patient, as well as cultured cells or cell lines; (ii) it must be novel, not closely related to any known animal retrovirus; (iii) it must infect human target cells in vitro; (iv) there must be specific antibodies against it in the patient from which it was isolated; (v) integrated (proviral) DNA must be present in the cells from which it was isolated; and (vi) there must be evidence of infection (e.g., serological) with the same virus in other patients with the same disease.
At about the same time, Japanese researchers identified a previously unknown form of T-cell malignancy, referred to as adult T-cell leukemia (ATL), an often rapidly fatal disease characterized by both cutaneous nodules and internal (lymph nodes, spleen, liver) involvement and cell morphology distinct from known similar T-cell tumors such as Sezary syndrome or mycosis fungoides (37). The most striking characteristic of ATL noted in this report was its distribution. Of 16 patients studied, 13 came originally from a small region on the southern Japanese island of Kyushu, far removed from the major population centers of Japan, leading the authors to speculate, rather presciently, “Genetic background may play an important role, but other features such as an oncogenic virus must be explored.”
The Highlighted Paper (19)
In 1978, patient C.R., a 28-y-old black male, was diagnosed (most likely incorrectly, in retrospect) with cutaneous T-cell lymphoma, or mycosis fungoides. He was treated at the NCI–Department of Veterans Affairs (VA) Medical Center and studied by oncologists (and coauthors of ref. 19) Adi Gazdar, Paul Bunn, and John Minna. Using IL-2 provided by the Gallo laboratory, they cultured T cells from a lymph node biopsy and blood, enabling them to isolate a continuously growing line, designated HUT-102, which was maintained in culture for over 200 passages, during which time its growth became independent of IL-2.
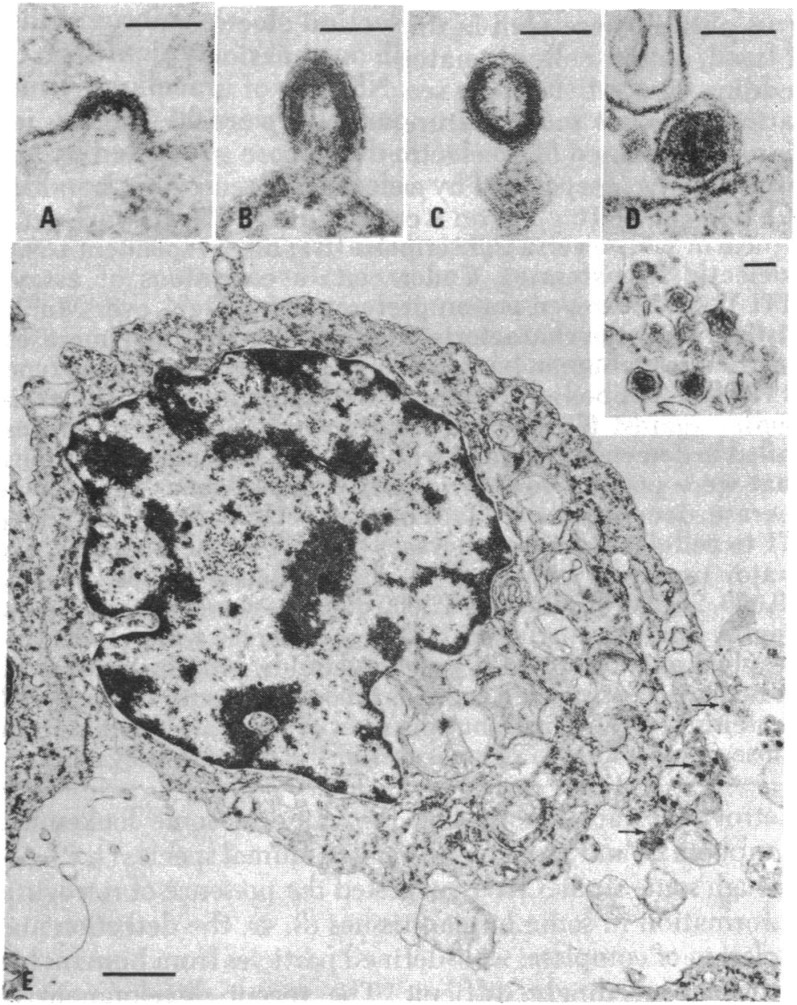
HUT-102 was subjected to a series of studies, standard for the time, that revealed the presence of a previously undescribed retrovirus. First, karyotypic analysis showed a near normal (pseudodiploid) human male chromosome pattern, excluding contamination with cells from other animals, or many other human cell lines (including the highly aggressive aneuploid and female HeLa cells). Electron microscopy of thin sections of pelleted cells showed occasional cells producing what seemed to be typical type C retrovirus particles. “Type C” is an old ultrastructural designation (38) that includes taxonomically unrelated retroviruses (particularly alpha- and gammaretroviruses) that assemble during budding from the cell membrane and subsequently mature from a hollow shell inside the virion envelope to a condensed central core, as in figure 2 of ref. 19, reprinted here as Fig. 1.
Fig. 1.
Thin section electron micrographs of HUT-102 cells. (A–D) “C-type” particles in various stages of budding and maturation. (E) IUdR-induced cell showing large numbers of budding and released particles, some of which are enlarged in the Inset. (Scale bars, A–D and Inset, 100 nm; E, 1,000 nm.) Reproduced with permission from ref. 19.
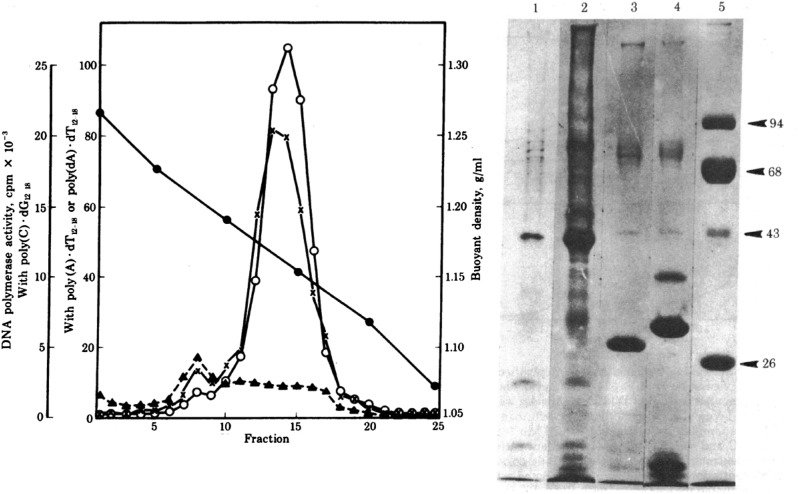
In the initial passages, virion production by HUT-102 was weak and sporadic, requiring treatment with 5-iodo, 2′-deoxyuridine (IUdR) (which reverses silencing of proviruses by causing demethylation of methyl cytosine residues in DNA) to obtain usable amounts of virus for characterization. Even so, for the biochemical studies shown in figure 3 in ref. 19 (Fig. 2), the authors started with 20 L of cell culture supernatant, a remarkable amount, from which virus was concentrated by ultracentrifugation and purified by equilibrium density centrifugation, revealing the presence of particles with a density of about 1.16 g/mL, typical for retroviruses, containing a DNA polymerase (i.e., reverse transcriptase) capable of incorporating 3H-labeled TTP into poly (dT) when provided with a template-primer combination of poly(A):dT12–18. This combination of density gradient centrifugation and assay for RT activity was a standard procedure, used by many laboratories to identify and characterize retroviruses. Indeed, I had used it extensively for my PhD thesis work about 8 y earlier.
Fig. 2.
Properties of HTLV particles released by HUT-102 cells. (Left) RT activity of purified virions after equilibrium density gradient ultracentrifugation in a sucrose gradient. Incorporation of radiolabeled deoxynucleotide was monitored for each fraction with a variety of template primers: poly(C):oligo (dG) (x), poly(A):oligo (dT) (open circles), poly (dA):oligo (dT) (with Mn++ instead of Mg++; filled triangles). Filled circles show the density, in gm/mL. Banding of RT-containing particles at 1.16 g/mL is typical of retroviruses. (Right) Polyacrylamide gel electrophoresis of proteins from purified virions. Lanes 1 and 2, HTLV from HUT-102 cells; lane 3, simian sarcoma virus; lane 4, murine leukemia virus; lane 5, markers, whose molecular weight is shown to the right. Reproduced with permission from ref. 19.
The partially purified virions obtained by sucrose density gradient centrifugation allowed their further biochemical characterization. Polyacrylamide gel electrophoresis revealed a pattern of virion proteins, consistent with those of known retroviruses, but sufficiently distinct to rule out the more likely sources of contamination (Fig. 2, Right). Further characterization of the virion-associated RT, presented in table 1 in ref. 19 again with assays that had been in general use for a long time, showed that, unlike cellular DNA polymerases, the enzyme preferred RNA (poly(A)) over DNA (poly(dA)) templates and had a slight preference for Mg++ over Mn++ for divalent cation. The latter point was an important one because gammaretroviruses, the most likely sources of laboratory contamination, all exhibit a preference for Mn++. To further exclude such contamination, the authors found that antibodies against the RTs of likely contaminating viruses failed to neutralize the RT activity of the virions of the newly discovered HTLV.
Finally, to satisfy the first of the postulates listed above, the authors reported (without showing any data) that cells freshly isolated from the same patient about a year after HUT-102 released a similar virus 3 d after stimulation with IUdR and, like the first cell line, eventually grew into an immortal line, called CTCL-3, that was also IL-2–independent and constitutively produced HTLV.
Follow-Up Studies
At this point, the reader will have noticed that the PNAS paper fulfilled only the first two of the six postulates listed above. Particularly lacking were evidence for infectivity of the virus particles and evidence that they were the result of infection of the individual, and not inheritance of an endogenous retrovirus. Although endogenous retroviruses of humans (HERVs) were not to be definitively identified for a few more years, they were well known in some animals, including chickens, mice, and cats, as sometimes infectious type C viruses that could be produced from uninfected cultured cells spontaneously or after induction with halogenated pyrimidines (like IUdR) (39). This possibility was ruled out in a subsequent study that used nucleic acid hybridization with labeled HTLV cDNA to demonstrate the presence of HTLV DNA and RNA sequences in HUT-102 cells, but not in cells from other human tissues (40). These studies (41) and others (42) also reinforced the unique nature of HTLV relative to known retroviruses. Also in 1981, the Gallo group reported isolation of a closely related virus from another T-cell lymphoma patient (43) and the detection of antibodies against HTLV in serum from C.R., the original patient (44).
In the meantime, Hinuma et al., in Japan, reported the presence of distinct antigens in a T-cell line from an ATL patient called MT-1 (which, like HUT-102, also produced IUdR-inducible type C particles) and the presence of antibodies against them in all ATL patients tested, as well as 26% of clinically normal individuals from the affected area, and hypothesized correctly that this result might be due to widespread asymptomatic infection (45). They were also able to demonstrate infectivity of the virus they called ATLV by transfer of the virus produced by a female ATL patient after cocultivation with primary cord blood lymphocytes from a male baby, leading to a highly ATLV productive transformed cell line, called MT-2 (46). Further evidence of infectivity and transformation was also subsequently reported by the Gallo group (47).
Finally, the Japanese and American groups collaborated to show that HTLV and ATLV were different isolates of the same virus (48), eventually shown to have nearly identical nucleotide sequences (49), and to be related to the previously discovered bovine leukemia virus (50). Given the priority of the 1980 PNAS paper (19), the name HTLV was agreed to for the virus by all researchers, and the associated disease is still known as ATL. It was a while, however, before the meaning of the initials in HTLV was finally agreed on, with “lymphotropic” rather than “leukemia” or “lymphoma” being determined to be the “L” word.
Impact of the Discovery
HTLV was the first infectious, oncogenic human retrovirus discovered. It was also the last. With the possible exception of the related, but distinct, HTLV-2, discovered by the Gallo group in a different type of T-cell leukemia a few years later (51), which forced the renaming of HTLV to HTLV-1, and whose role in oncogenesis remains to be established (52), no other directly oncogenic human retrovirus has been found. More recently, infections with two other related viruses, HTLV-3 and HTLV-4, have been reported in a few individuals in sub-Saharan Africa, apparently the result of zoonotic infection from local primates (53). Worldwide, HTLV-1 is a significant, but not major, cause of morbidity and mortality: From a survey encompassing about 1.5 billion of the world’s 7 billion inhabitants, a prevalence of between 4 and 10 million HTLV-1–infected individuals has been estimated (54), concentrated in pockets of infection in Japan, the West Indies, and parts of South America and sub-Saharan Africa. Of these, about 5% will succumb to ATL, and another 5% will acquire another disease caused by the same virus, HTLV-associated myelopathy/tropical spastic paraparesis, a serious neurological condition. The large majority of infected individuals remain healthy carriers for life (52). Although the molecular mechanisms of HTLV oncogenesis are a very active area of research (55), knowledge gained has not yet led to any new treatment or prevention measures, beyond screening of the donated blood supply.
The most significant impact of the discovery of HTLV may well have been on another retroviral disease altogether. The first reports of US cases of the disease that came to be known as AIDS appeared in June 1981, within 7 mo of the publication of the highlighted PNAS paper (56, 57). The first identification, by the group headed by Luc Montagnier of the Pasteur Institute, using many of the same protocols as in the PNAS paper, of the virus now called HIV appeared about 2 y later (33), with confirmation by the Gallo laboratory and others within another year (43, 58). Although unrelated to one another, HTLV and HIV have in common that they both replicate in CD4+ T calls and that similar techniques are used to grow them in cell culture. There is little doubt that, without the trail blazed by the highlighted 1980 PNAS paper and its predecessors, the discovery of HIV, which led directly both to effective screening technology to eliminate blood-borne transmission, and eventually to effective suppressive antiviral therapy now prolonging life for millions of infected individuals worldwide, would have been greatly delayed. Contributing to delays would also have been the pre-1980 mindset among virologists of the unlikelihood of human retroviruses. Thus, both the technology for growing T cells in culture and isolating virus from them and the paradigm shift represented by the discovery of a pathogenic human retrovirus laid the critical foundation for some of the most important medical advances of the late 20th century, without which many millions more would have died of AIDS.
Acknowledgments
I thank Peter Vogt and Robin Weiss for helpful guidance and encouragement. I was a Research Professor of the American Cancer Society, and work in my laboratory is supported by NIH Grant R37 CA089441.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century. See the companion article, “Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma,” on page 7415 in issue 12 of volume 77.
References
- 1. Watson, JD, ed (1980) Viral Oncogenes, Cold Spring Harbor Symposia on Quantitative Biology (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY), Vol XLIV.
- 2.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 3.Heneine W, Schweizer M, Sandstrom P, Folks T. Human infection with foamy viruses. Curr Top Microbiol Immunol. 2003;277:181–196. doi: 10.1007/978-3-642-55701-9_8. [DOI] [PubMed] [Google Scholar]
- 4.Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- 5.Coffin JM, et al. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duesberg PH, Bister K, Vogt PK. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci USA. 1977;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duesberg PH, Vogt PK. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci USA. 1970;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin GS. Rous sarcoma virus: A function required for the maintenance of the transformed state. Nature. 1970;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- 9.Parks WP, Scolnick EM. In vitro translation of Harvey murine sarcoma virus RNA. J Virol. 1977;22(3):711–719. doi: 10.1128/jvi.22.3.711-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih TY, Weeks MO, Young HA, Scholnick EM. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- 11.Stehelin D, Varmus HE, Bishop JM. Detection of nucleotide sequences associated with transformation by avian sarcoma viruses. Bibl Haematol. 1975;(43):539–541. doi: 10.1159/000399214. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg N, Jolicoeur P. Retroviral pathogenesis. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 475–585. [PubMed] [Google Scholar]
- 13.Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward WS, Neel BG, Astrin SM. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- 15.Dalla-Favera R, et al. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1982;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Der CJ, Krontiris TG, Cooper GM. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci USA. 1982;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parada LF, Tabin CJ, Shih C, Weinberg RA. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297(5866):474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- 18.Dürst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poiesz BJ, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temin HM, Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- 21.Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- 22.Temin HM. The participation of DNA in Rous sarcoma virus production. Virology. 1964;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- 23.Vogt PK. Historical introduction to the general properties of retroviruses. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. [PubMed] [Google Scholar]
- 24.Wade N. Special virus cancer program: Travails of a biological moonshot. Science. 1971;174(4016):1306–1311. doi: 10.1126/science.174.4016.1306. [DOI] [PubMed] [Google Scholar]
- 25.Voisset C, Weiss RA, Griffiths DJ. Human RNA “rumor” viruses: The search for novel human retroviruses in chronic disease. Microbiol Mol Biol Rev. 2008;72(1):157–196. doi: 10.1128/MMBR.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss RA. The search for human RNA tumor viruses. In: Weiss RA, Teich N, Varmus HE, Coffin JM, editors. RNA Tumor Viruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1982. [Google Scholar]
- 27.Gallo RC. Virus Hunting: AIDS, Cancer, and the Human Retrivirus. Basic Books; New York: 1991. [Google Scholar]
- 28.Bhattacharyya J, Xuma M, Reitz M, Sarin PS, Gallo RC. Utilization of mammalian 70S RNA by a purified reverse transcriptase from human myelocytic leukemic cells. Biochem Biophys Res Commun. 1973;54(1):324–334. doi: 10.1016/0006-291x(73)90926-1. [DOI] [PubMed] [Google Scholar]
- 29.Bobrow SN, Smith RG, Reitz MS, Gallo RC. Stimulated normal human lymphocytes contain a ribonuclease-sensitive DNA polymerase distinct from viral RNA-directed DNA polymerase. Proc Natl Acad Sci USA. 1972;69(11):3228–3232. doi: 10.1073/pnas.69.11.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert MS, Smith RG, Gallo RC, Sarin PS, Abrell JW. Viral and cellular DNA polymerase: Comparison of activities with synthetic and natural RNA templates. Science. 1972;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- 31.Sarin PS, Abrell JW, Gallo RC. Comparison of biochemical characteristics of reverse transcriptase from human acute leukemic cells and several RNA tumor viruses. Basic Life Sci. 1974;3:345–354. doi: 10.1007/978-1-4613-4529-9_27. [DOI] [PubMed] [Google Scholar]
- 32.Poiesz BJ, Ruscetti FW, Mier JW, Woods AM, Gallo RC. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci USA. 1980;77(11):6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barré-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 34.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 35.Chan E, et al. Characterisation of a virus (HL23V) isolated from cultured acute myelogenous leukaemic cells. Nature. 1976;260(5548):266–268. doi: 10.1038/260266a0. [DOI] [PubMed] [Google Scholar]
- 36.Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24(39):5926–5930. doi: 10.1038/sj.onc.1208980. [DOI] [PubMed] [Google Scholar]
- 37.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–492. [PubMed] [Google Scholar]
- 38.Teich N. Taxonomy of retroviruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA Tumor Viruses. Vol 1. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1982. pp. 25–208. [Google Scholar]
- 39.Boeke JD, Stoye JS. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 343–435. [PubMed] [Google Scholar]
- 40.Reitz MS, Jr, Poiesz BJ, Ruscetti FW, Gallo RC. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci USA. 1981;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalyanaraman VS, Sarngadharan MG, Poiesz B, Ruscetti FW, Gallo RC. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J Virol. 1981;38(3):906–915. doi: 10.1128/jvi.38.3.906-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oroszlan S, et al. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci USA. 1982;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Gallo RC. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- 44.Posner LE, et al. Natural antibodies to the human T cell lymphoma virus in patients with cutaneous T cell lymphomas. J Exp Med. 1981;154(2):333–346. doi: 10.1084/jem.154.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinuma Y, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyoshi I, et al. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 47.Popovic M, Lange-Wantzin G, Sarin PS, Mann D, Gallo RC. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci USA. 1983;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popovic M, et al. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982;300(5887):63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- 49.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: Complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagata N, et al. Comparison of the entire genomes of bovine leukemia virus and human T-cell leukemia virus and characterization of their unidentified open reading frames. EMBO J. 1984;3(13):3231–3237. doi: 10.1002/j.1460-2075.1984.tb02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalyanaraman VS, et al. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- 52.Ciminale V, Rende F, Bertazzoni U, Romanelli MG. HTLV-1 and HTLV-2: Highly similar viruses with distinct oncogenic properties. Front Microbiol. 2014;5:398. doi: 10.3389/fmicb.2014.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfe ND, et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc Natl Acad Sci USA. 2005;102(22):7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuoka M, Jeang KT. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: Viral infectivity, Tax, HBZ and therapy. Oncogene. 2011;30(12):1379–1389. doi: 10.1038/onc.2010.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control (CDC) Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30(25):305–308. [PubMed] [Google Scholar]
- 57.Centers for Disease Control (CDC) Pneumocystis pneumonia--Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30(21):250–252. [PubMed] [Google Scholar]
- 58.Levy JA, et al. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]