Abstract
Background
The genetic engineering of T cells through the introduction of a chimeric antigen receptor (CAR) allows for generation of tumor targeted T cells. Once expressed by T cells, CARs combine antigen-specificity with T cell activation in a single fusion molecule. Most CARs are comprised of an antigen binding domain, an extracellular spacer/hinge region, a trans-membrane domain, and an intracellular signaling domain resulting in T cell activation following antigen binding.
Methods
The authors performed a search of the literature regarding tumor immunotherapy using CAR modified T cells to provide a concise review of this topic.
Results
This review will focus on the elements of CAR design required for successful application of this technology in cancer immunotherapy. Most notably, proper target antigen selection, co-stimulatory signaling, and the ability of CAR modified T cells to traffic, persist, and retained function following adoptive transfer are required for optimal tumor eradication. Furthermore, recent clinical trials have demonstrated tumor burden and chemotherapy conditioning prior to adoptive transfer as critically important for this therapy. Future research into counteracting the suppressive tumor microenvironment and the ability to activate an endogenous anti-tumor response by CAR modified T cells may enhance the therapeutic potential of this treatment.
Conclusion
In conclusion CAR modified T cell therapy is a highly promising treatment for cancer having already demonstrated both promising pre-clinical and clinical results. However, further modification and additional clinical trials will need to be conducted to ultimately optimize the anti-tumor efficacy of this approach.
Keywords: chimeric antigen receptor, immunotherapy, adoptive cell therapy, T cell
Introduction
The ability of the immune system to recognize and eradicate cancer is well established. This principle is most clearly demonstrated in the context of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for hematologic malignancies [1, 2]. However, in the allogeneic setting the benefit of “graft vs leukemia” is offset by the complications of graft vs host disease (GVHD). In the context of autologous “adoptive cellular therapy” (ACT) the risk of GVHD is minimal and the eradication of cancer using autologous tumor-infiltrating lymphocytes (TILs) has been demonstrated in patients with melanoma [3]. However, the process by which tumor reactive TILs are isolated and expanded is technically difficult, labor intensive, and time consuming. To overcome these obstacles and broaden the application of ACT many groups have developed methods of genetically engineering T lymphocytes to target tumor cells. These strategies include engineering T cells with a chimeric antigen receptor (CAR) which redirects T cell specificity and function [4]. Pioneering studies by Eshhar and colleagues demonstrated the proof of principle that expression of an antibody derived single chain variable fragment (scFv) coupled to a T cell signaling domain will redirect T cell specificity and function [5]. With the advent of efficient methods of human T cell modification and the ability to rapidly expand these tumor targeted T cells, this strategy has become a feasible treatment option [6-9]. In addition to CAR modification an alternative method of generating tumor targeted T cells is by the introduction of a T cell receptor (TCR) alpha and beta chains with known anti-tumor specificity [3]. However, this method of T cell modification is outside the scope of this review. This review will focus on CAR design and the elements required for successful eradication of malignancies by CAR modified T cells. In addition we will discuss findings from early clinical trials testing CAR modified T cells as well as future challenges to this evolving field.
Advantages of CAR Modified T cells
There are several advantages to utilizing CAR-modified T cells for cancer immunotherapy. CARs recognize tumor antigens in a HLA-independent manner [4]. This allows CAR-modified T cells to overcome the tumor's ability to escape immunodetection by down regulation of HLA molecules on the cell surface [10, 11]. Furthermore, since tumor targeting is HLA independent, use of CARs is applicable to a broad range of patients irrespective of HLA-type. Targeting of tumor antigens by CAR modified T cells is applicable to any cell surface antigen including proteins, carbohydrates and glycolipids for which a monoclonal antibody can be generated. This enables CAR modified T cells to respond to a broader range of targets compared to the native T cell receptor (TCR). CAR modification can redirect the specificity of most T cell subsets including CD4, CD8, naïve, memory, or effector T cells. This is critically important since naïve and antigen experienced T cells have different functional capacities that may make them more or less favorable for use in adoptive cell therapy [12]. Introduction of one or more T cell activating signals is possible with CAR modification, which may enhance the ability of T cells to proliferate, persist, and lyse targeted cells. Furthermore, the genetic modification of T cells is not limited to activating signals. T cells can be engineered to deliver potent anti-tumor immunomodulators (e.g. cytokines) to the hostile tumor microenvironment, which may enhance their anti-tumor effect [13]. Finally and most significantly, the ability to generate a large quantity of tumor-specific T cells in a relatively short period of time makes this strategy feasible for use in the clinical setting [9, 14].
CAR Modified T cells: Targeting
The basic design of a CAR includes a tumor-associated antigen (TAA) binding domain (most commonly a scFv (single chain variable fragment) derived from the antigen binding region of a monoclonal antibody), an extracellular spacer/hinge region, a trans-membrane domain, and an intracellular signaling domain [4, 15]. The target antigen chosen for CAR specificity is a critical determinant for the effectiveness and safety of the genetically engineered T cell. The ideal target antigen is expressed only on tumor cells and will produce the greatest effect if it is required for tumor cell survival. This type of TAA will have little chance for immune editing and tumor escape since it is critical for survival of the tumor [16]. Unfortunately, expression of most TAAs is not restricted to tumor cells, leading to undesirable effects of “on target/off tumor” toxicity. Historically, CAR modified T cell have only targeted antigens expressed on the target cell surface. However, CARs that target intracellular antigens (via HLA-restricted presentation) have also been developed [17, 18].
One widely utilized target of CAR modified T cells is the CD19 antigen expressed on nearly all normal B cells but also most B cell malignancies [19]. Since CD19 is not expressed on hematopoietic stem cells the toxicity of targeting this antigen is limited to B cell aplasia following treatment with anti-CD19 CAR modified T cells [20-22]. This “on target/off tumor” toxicity, in the form of B cell aplasia, is considered a tolerable side effect of this therapy. Some other examples of targets for CAR modified T cells include ERRB2 (HER-2/neu) for the treatment of breast and prostate cancer [23, 24] prostate specific membrane antigen (PSMA) for the treatment of prostate cancer [25, 26], Carboxy anhydrase-IX, (CAIX) for the treatment of renal cell carcinoma [27, 28] Lewis Y (LeY) for the treatment of lung and ovarian tumors [29] [30], carcinoembryonic antigen (CEA), for the treatment of colon cancer [31, 32], folate binding protein (FBP), folate receptor (FR), or MUC-CD for the treatment of ovarian cancer [33, 34] and the diasialoganglioside GD2 for the treatment of neuroblastoma [35]. While this is not an exhaustive list it clearly illustrates the wide range of tumors which can be targeted by CAR modified T cells.
While most CARs utilize a scFv for tumor targeting, some groups have developed CARs consisting of receptor ligands fused to intracellular signaling domains. Examples include, a polypeptide against vascular endothelial growth factor receptor (anti-VEGFR2), an integrin binding peptide (anti-αvβ6), heregulin (anti-Her3/4 receptor), or Interleukin (IL)-13 mutein (anti-IL13 receptor-α) [36-39]. In yet another approach Sentman and colleagues have demonstrated the anti-tumor efficacy of a CAR containing the NKG2D receptor coupled to the CD3 zeta (CD3ζ) chain [40]. In this manner genetically modified T cells will target the ligands of NKG2D (e.g. MIC A/B) that are overexpressed on a number of tumors. In an attempt to develop a “universal” CAR, Cooper and colleagues have developed an anti-fluorescein isothiocyanate (anti-FITC) CAR [41]. In this manner FITC-conjugated therapeutic molecules (e.g. monoclonal antibodies, ligands, or nucleic acid based aptamers) that are specific to one or more TAAs can be employed to target the tumor of interest. T cells genetically modified to express the anti-FITC CAR will then target the FITC-labeled tumors. This method allows targeting of multiple TAAs which will limit the ability of tumors to evade targeting by downregulating of a single target antigen [41]. Furthermore, this approach allows treatment of several tumor types with one CAR.
Attempts at improving CAR design and T cell activation have involved increasing the affinity of the antibody-recognition domain. It has been postulated that increased CAR antigen affinity will improve targeting of tumors with low antigen expression and amplify T cell activation. However, Chmielewski et. al., illustrated the maximum activation of T cells via CAR-antigen binding was independent of the antigen binding affinity [42]. It was also noted that low affinity receptors could discriminate between tumors with low and high expression of the target antigen [42]. This characteristic could be used to reduce the severity of “on target/off tumor” toxicity, in that CAR-modified T cell activation would be limited to cells with overexpression of the target antigen. In addition, careful consideration must be used in selecting CARs with high antigen affinity. When antigen binding is too avid the effector T cell might be unable to engage multiple targets thereby limiting its effectiveness [43].
Since most currently designed CARs are based on scFv's derived from murine antibodies these foreign molecules may elicit an undesired anti-CAR response by the host. This anti-CAR effect was demonstrated by Lamers et. al., who found both antibody and cell mediated responses to cells expressing CARs [44]. A strategy to prevent this outcome includes humanization of scFv fractions or utilizing human antibodies to generate CARs [45]. The optimal format for developing the antigen binding domain of a CAR remains to be established and warrants further pre-clinical and clinical investigation.
CAR Modified T cells: Signaling
The optimal T cell activation signaling domains incorporated into a CAR remains a topic of debate. First generation CARs mediate T cell activation through immunoreceptor tyrosine-based activating motif (ITAM) of the CD3ζ chain or the FcεRIγ [46]. The CD3ζ signal was found to provide the requisite “signal 1” resulting in T cell activation, target cell lysis, modest IL-2 secretion, and in vivo anti-tumor function [46, 48, 49]. Since these initial reports, we and others have demonstrated the anti-tumor potential of T cells modified with first generation CARs in pre-clinical studies [48-52]. Despite these in vivo results, the anti-tumor effect of first generation CARs is limited. Suboptimal stimulation of the T cell with only “signal 1” results in T cell anergy leading to poor cytokine secretion, poor proliferation, and eventual apoptosis of the genetically modified T cell [53, 54]. Furthermore, tumor eradication was predicated on the expression of co-stimulatory molecules (e.g. B7.1/CD80) on the tumor cell surface [50].
To enhance CAR activation signals and overcome the limitation of first generation CARs, second generation CARs were developed which incorporated co-stimulatory domains. The most well studied T cell costimulatory receptor is CD28 which interacts with the B7 family molecules, B7.1 and B7.2, located on the surface of target cells [55, 56]. According to the current model of T cell activation CD28 provides a second activation signal (co-stimulation; “signal 2”) which augments “signal 1” from the TCR/CD3 complex [57, 58]. Costimulation by CD28 enhances T cell proliferation, IL-2 synthesis, and expression of the anti-apoptotic protein Bcl-xL [56]. To replicate this endogenous T cell activation second generation CARs were designed to deliver both “signal 1” from the CD3ζ domain and “signal 2” from a CD28 signaling domain. Maher et. al., tested a CD28 containing second generation CAR targeted to prostate specific membrane antigen (PSMA) [26]. When compared to a first generation anti-PSMA CAR (signal 1 only), the second generation CAR led to enhanced IL-2 production, increased proliferation in response to antigen, and sustained lysis of PSMA+ targets by genetically modified T cells. In a preclinical model of B cell malignancy Brentjens et. al., demonstrated enhanced eradication of established tumors using a CD28 containing second generation CAR when compared to a first generation CAR specific for the same antigen (CD19) [59]. Several groups have similarly demonstrated increased proliferation, increased cytokine production, upregulation of anti-apoptotic proteins (e.g. Bcl-xL), and delayed activation induced cell death (AICD) with CD28 containing second generation CARs [32, 60-64]. In addition, CD28 containing second generation CARs may enable genetically modified T cells to counteract the inhibitory effects of the “hostile tumor microenvironment.” Studies have demonstrated T cell genetically modified with a CD28 containing CAR proliferate and express nuclear factor kappa-B (NF-κB) despite the repressive effects of both transforming growth factor (TGF)-β and T regulatory cells (Tregs) [47, 65]. Persistence of genetically modified T cells is also enhanced when utilizing CD28 containing second generation CARs [66].
Second generation CARs have also been developed using alternative co-stimulatory molecules. Coupling CD3ζ signaling with other B7 family members (e.g. inducible costimulation (ICOS)) or tumor necrosis factor receptor (TNFR) family (e.g. CD137/4-1BB or CD134/OX-40) has been described [67]. Finney et. al., demonstrated enhanced antigen-induced cytokine (e.g. IL-2, interferon (INF)-γ, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony stimulating factor (GM-CSF)) production by T cells modified to express B7 family and TNFR family containing second generation CARs [67]. Co-stimulation with CD28 produced the highest level of IL-2 and B7 family co-stimulation resulted in higher levels of INF-γ, TNF-α, and GM-CSF compared to TNFR containing CARs. All second generation CARs enabled resting T cells to express the anti-apoptotic protein Bcl-2 and enhanced proliferation in response to antigen when compared to first generation CARs. Target cell lysis was enhanced by inclusion of CD28, ICOS, and CD134 with ICOS co-stimulation exhibiting the highest in vitro target cell lysis [67]. However, the incorporation of ICOS signaling may result in suboptimal IL-2 production, which may detract from using this co-stimulatory domain in CAR design [68]. Our group also compared a similar panel of second generation CARs that included CD28, DAP10, OX-40, and 4-1BB co-stimulatory domains [59]. In these studies CD28 containing CARs demonstrated superior in vitro proliferation and cytokine secretion when compared to the alternative constructs [59]. In addition CD28 containing second generation CARs had enhanced in vivo antitumor activity compared to first generation CARs in the setting of tumors that fail to express costimulatory ligands [59].
In contrast, June and colleagues compared the in vivo anti-tumor efficacy of CD28 vs CD137 (4-1BB) second generation CARs in an acute lymphoblastic leukemia (ALL) xenograft model of disease [69]. In this report a CD19 targeted CD137 containing second generation CAR had enhanced anti-leukemic effect, improved persistence, and an antigen independent activation of T cells resulting in improved efficacy following adoptive transfer [69]. However, in a mesothelioma tumor model, June and colleagues also demonstrated equivalent anti-tumor efficacy for both CD28 and 4-1BB containing second generation CARs [70]. Other groups have also shown similar results with CD137 containing second generation CARs, noting that CD137 co-stimulation results in improved T cell survival, AICD resistance, increased expression of anti-apoptotic proteins (e.g. Bcl-xL), sustained proliferation and persistence, enhanced cytokine production, and increased antigen specific tumor cell lysis [71, 72].
In addition to the work on CD28 and 4-1BB other groups have developed alternative methods to provide co-stimulation. The incorporation of CD244, a NK cell receptor, into CAR design resulted in the acquisition of a cytolytic effector memory phenotype and augmented CAR mediated responses when compared to first generation CAR (CD3ζ only) modified T cells [73, 74]. However, comparisons between CD28, CD137, and CD244 containing second generation CARs have yet to be performed. In an alternative method Stephan et. al., demonstrated potent auto- and trans-costimulation of T cells modified to express a first generation CAR and co-transduced to express co-stimulatory ligands (e.g. CD80 and 4-1BBL) [75]. T cells modified in this manner have increased proliferation, cytokine secretion, in vitro survival, in vivo expansion, in vivo persistence and eradication of systemic malignancy in mouse model of disease [75].
In an attempt to further optimize CAR design several groups have developed “third generation” CARs that provide signal 1, signal 2, and an additional costimulatory signal (e.g. CD28/4-1BB/CD3ζ signaling). Comparisons between second generation CARs and third generation CARs have demonstrated conflicting results. Several studies have reported enhanced cytokine production, T cell survival and anti-tumor efficacy for T cells that express a third generation CAR [70-72, 76, 77]. Zhong et. al., have demonstrated enhanced cytokine production, improved in vivo T-cell survival, enhanced tumor elimination, improved PI3K/AKT pathway activation, enhanced Bcl-xL expression, and reduced T cell apoptosis for a third generation CAR (CD28/4-1BB/CD3ζ signaling) against prostate specific membrane antigen (anti-PSMA) [76]. Pule et. al., demonstrated sustained in vitro proliferation, enhanced IL-2 production, and the ability to maintain cytolytic function following repeated antigenic stimulation for a CD28-OX40 containing third generation CAR [78]. Wilkie et. al., compared third generation CARs (CD28/4-1BB/CD3ζ vs CD28/OX-40/CD3ζ) targeting MUC1 (expressed on breast and ovarian tumors) [79]. In this report T cells modified with either third generation CAR had equivalent in vitro cytotoxicity as T cells modified with a CD28 containing second generation CAR directed against the same target. However, CD28/OX-40 containing third generation CAR modified T cells had improved in vitro IFN-γ secretion [79]. Unfortunately, a direct comparison between the in vivo anti-tumor efficacies of these third generation CARs was not performed.
It must be noted that differences between second and/or third generation CARs may not be solely attributable to the signaling domains incorporated in their design. Rather, the difference in antigen binding domains (i.e. scFv), method of transduction (lentivirus vs retrovirus), tumor model (systemic vs subcutaneous), route of T cell administration (intravenous vs intraperitoneal vs intra-tumoral), culture conditions, and a number of other variables could account for the differences found in these studies. To this end, the optimal combination of T cell activation signaling is still debated. However, several groups are currently testing second and third generation CARs in clinical trials and a more thorough understanding should be forthcoming.
CAR Modified T cells: Trafficking
Genetically modified tumor targeted T cells must traffic to the site(s) of disease to effectively eradicate disease. The ability of CAR modified T cells to localize to the site(s) of tumor has been demonstrated using a dual bioluminescent imaging of genetically modified T cells and tumor cells in a mouse model of cancer [80]. In this report CAR modified T cells accumulated at most site(s) of systemic tumor and persisted over time [80]. More significant are the findings of recent clinical trials that show T cells genetically modified to express a second generation CAR localize to sites of disease [20, 22, 66]. Our group has demonstrated CD19 targeted T cells expressing a CD28 second generation CAR trafficked to several sites of disease (e.g. bone marrow, lymph nodes, liver) following adoptive transfer in patients with chronic lymphocytic leukemia (CLL) [20]. Savoldo et. al., have also demonstrated the trafficking of CAR modified T cells to a skin lesion two weeks after ACT in a patient with non-Hodgkin lymphoma (NHL) [66]. In this study, T cells targeted to the same antigen (CD19) but genetically modified with a first generation CAR (CD3ζ only) or a CD28 containing second generation CAR were simultaneously infused into patients. Strikingly, only T cells modified with the CD28 containing second generation CAR were found to traffic to the tumor site [66]. In another report using a 4-1BB containing second generation CAR, genetically modified T cells were found in the bone marrow of CLL patients following adoptive transfer [22]. Taken together, these results are consistent with the ability of CAR modified T cells to localize to site(s) of disease
While the above reports are promising it remains possible that genetic engineering and ex vivo expansion of T cells may alter the expression of one or more chemokine/cytokine receptors necessary for trafficking. One strategy to enhance the trafficking of T cells to site(s) of disease is through the genetic expression of chemokine receptors. Several groups have demonstrated this principle through the expression of CXCR2 (CXCL1 receptor) and CCR4 (CCL17 receptor) in CAR modified T cells [81, 82]. As this therapeutic strategy evolves finding the optimal methods that enable T cells to traffic to site(s) of tumor will be critical for successful tumor eradication.
CAR Modified T cells: Persistence
Tumor targeted T cells must persist for a sufficient period of time to result in successful tumor elimination. In ACT utilizing TILs, the persistence of adoptively transferred T cells correlated with improved clinical response [83]. Conditioning chemotherapy can enhance the persistence of adoptively transferred T cells through a number of mechanisms which has been demonstrated in pre-clinical models and in the setting of ACT using TILs in patients with melanoma [84, 85]. Our group recently verified the principle of CAR modified T cells persistence following conditioning chemotherapy [20]. In this report, an initial cohort of CLL patients treated with CD19 targeted T cells and without conditioning chemotherapy had limited to undetectable persistence. In contrast, a subsequent cohort of patients who received prior conditioning chemotherapy (cyclophosphamide) demonstrated evidence of genetically modified T cells in the bone marrow for up to 6 weeks following adoptive transfer [20]. Conditioning chemotherapy may also reduce the patient's disease burden which will enhance the persistence of the adoptively transferred T cells. In one study, lower disease burden was correlated with T cell persistence in patients with metastatic neuroblastoma treated with CD171-targeted T cells [86]. This finding was also demonstrated in our study, with an inverse relationship between persistence of genetically modified cells and the peripheral blood tumor burden [20].
The link between T cell phenotype and persistence has also been investigated in the context of ACT. Following antigen exposure, naïve T cells can develop into one of two memory subsets termed effector memory (TEM) or central memory (TCM) [87]. Naïve and antigen experienced T cells have different functional capacities that may make them more or less favorable for use in adoptive cell therapy [12]. In non-human primates adoptive transfer of a CMV specific CD8+ T cell clones derived from a TCM (CD62L+) but not from a TEM (CD62L−) can establish persistent T cell memory [88]. In a recent report of CAR modified viral specific and non-viral specific T cells, persistence of adoptively transferred T cells was predicated on increased frequency of helper (CD4+) and central memory (CD45RO+CD62L+) cells within the infused product [89].
Persistence of modified T cells may also be contingent on the signaling domain incorporated into the CAR. Savoldo et. al., demonstrated enhanced persistence (up to 6 months) of T cells modified with a CD28 containing second generation CAR compared to first generation modified T cells targeting the same antigen (CD19) [66]. Other reports have supported the ability of T cells modified with a CD28 containing second generation CAR to persist in the blood and bone marrow following adoptive transfer into patients [20, 21]. In comparison June and colleagues demonstrated persistence of CD19 targeted T cells containing a 4-1BB second generation CAR for >9 months following adoptive transfer [22]. In this report, patients exhibited a significant benefit from CAR modified T cells and this may be the clearest indication that persistence of CAR+ T cells may be required for optimal clinical responses.
The administration of IL-2 may also enhance the persistence of genetically modified T cells. Till et. al., treated seven patients with indolent NHL with T cells modified to express a first generation CAR targeted to CD20 [90]. Persistence following infusion of modified T cells was enhanced in patients who received low-dose subcutaneous IL-2 [90]. However, while patients in the trial had minimal toxicity following the infusion of low-dose IL-2, the use of exogenous IL-2 is tempered by toxicity profile seen in other studies [91].
One strategy to enhance the persistence of CAR+ T cells is through the use of virus-specific T cells. In this manner, co-stimulation of CAR modified T cells occurs through the engagement of the native TCR against viral antigens. Pule et. al., reported on the safety of this approach and demonstrated enhanced survival of CAR modified autologous Epstein Barr-virus specific cytotoxic T lymphocytes (CAR-CTLs) compared to activated T cells (CAR-ATC) when modified with a distinguishable first generation CAR against the same tumor antigen (GD2) [92]. While initially CAR-CTLs survived in the circulation at higher level than CAR-ATCs this difference was lost by 6 weeks following infusion [92]. In a recent update of this trial persistence of either CAR-CTLs or CAR-ACTs beyond the 6 week time point was not reported to be different [89]. Persistence of either modified T cell population was contingent on the frequency of helper (CD4+) and central memory (CD45RO+CD62L+) cells within the infused product [89]. However, despite these findings it must be noted that the use of genetically modified viral specific T cells is still an attractive method to broaden ACT using allogeneic donor derived virus specific T cells which reduced risk of GVHD following adoptive transfer [93].
Factors affecting the persistence of adoptively transferred T cells include the use of conditioning chemotherapy, patient tumor burden, cytokine supplementation, co-stimulatory signaling, T cell phenotype, and possibly the use of viral specific T cells. The optimal method for persistence of CAR modified T cells is yet to be fully defined, however it is clear that efficacy of ACT is contingent on the persistence of the T cells following adoptive transfer.
CAR Modified T cells: Function
Optimal tumor eradication by CAR modified T cells requires the ability to maintain cytolytic function following adoptive transfer. The hostile tumor microenvironment includes T regulatory cells (Tregs), myeloid derived suppressor cells (MDSCs), and several immunosuppressive molecules/cytokines (e.g. TGF-β) that inhibit the anti-tumor function of adoptively transferred or endogenous T cells [10, 94]. Early clinical trials testing CAR+ T cell therapy had disappointing results, which could have been due to the suppressive effects of this hostile tumor microenvironment [86, 90, 91]. It has been shown that T cells modified to express a CD28 containing second generation CAR were resistant to in vitro inhibition by Tregs [47]. However, this study used inducible Tregs (iTregs), which have unstable Foxp3 expression [95]. Natural Tregs (nTregs) have been demonstrated to inhibit the in vitro and in vivo anti-tumor function of T cells modified to express a second generation CAR [96]. Conditioning with irradiation or chemotherapy can reduce the number of Tregs allowing gene modified T cells to eradicate tumor cells. This principal was demonstrated by our group using cyclophosphamide to restore the antitumor activity of CAR modified T cells despite targeted nTregs inhibition in a mouse model of cancer [96].
Tumor eradication by CAR modified T cells remains the best measure of retained anti-tumor function (clinical responses reported in clinical trials will be discussed below). A surrogate indication of retained function following adoptive transfer is the development of “on target/off tumor” toxicity (e.g. B cell aplasia and/or hypogammaglobinemia when targeting the CD19 antigen). Recent trials utilizing anti-CD19 CARs have demonstrated B cells aplasia in the peripheral blood following adoptive transfer [20-22]. Elevation of pro-inflammatory cytokine profiles is another surrogate marker for T cell function following adoptive transfer. June and colleagues demonstrated this in CLL patients with an increase in several pro-inflammatory cytokines in the peripheral blood and bone marrow which was correlated with peak expansion of CAR modified T cells [22]. In this report, adoptively transferred T cells also maintained their ex vivo capacity to degranulate in response to target antigen as assessed by CD107a surface expression [22].
CAR Modified T cells: Toxicity
The implications of “on target/off tumor” toxicity can be far more serious than B cell aplasia. One patient with metastatic colon cancer died five days after lymphocyte depleting chemotherapy (cyclophosphamide and fludarabine) followed by infusion of modified T cells targeted to ERBB2 [97]. The authors speculated that large number of infused modified T cells localized to the lung resulting in release of pro-inflammatory cytokines following recognition of low levels of ERBB2 on lung epithelia [97]. The resulting pro-inflammatory cytokine release triggered pulmonary toxicity, multiorgan failure and eventual death of the patient [97]. In another trial, liver toxicity was reported in five out of eleven patients with renal cell carcinoma treated with T cells modified to express a first generation CAIX specific CAR [28, 98]. The authors concluded the toxicity was the result of modified T cells targeting bile duct epithelial cells which had low levels of CAIX expressions [28]. These studies highlight the need for target antigens that are uniquely expressed on cancer cells while sparing normal tissue.
At our center, we safely infused CD19 targeted T cells into three CLL patients without conditioning chemotherapy but our first patient who received conditioning chemotherapy (cyclophosphamide) followed by CAR modified T cells died two days after T cell infusion [99]. After a thorough review of all clinical data, including an autopsy, the likely cause of death in this patient was infection and not the infused modified T cells [99]. Despite these findings, our clinical trial was modified to divide the infusion of T cells over two days to enhance safety. Since this modification, we have infused an additional five patients with CAR modified T cells following chemotherapy without any further adverse events [20].
First Generation CAR Modified T cell Trials
Several centers have conducted clinical trials testing the anti-tumor efficacy of first generation CAR modified T cells. Overall, early clinical trials using first generation CAR modified T cells have failed to demonstrate significant clinical benefit [28, 86, 89-92, 98, 100]. Furthermore, significant variation exists between these trials including targeted antigen, gene transfer method, method of ex vivo expansion, cell dose, use of exogenous IL-2, and use of conditioning therapy. Nevertheless, we have gained invaluable insight into the factors that constitute effective ACT using CAR modified T cells.
Lamers et. al., reported on their experience in patients with metastatic clear cell renal cell carcinoma (RCC) treated with T cells expressing an anti-carbonic anhydrase IX (CAIX) CAR with and without subcutaneous IL-2 [28, 98]. As mentioned, infusions of the CAR modified T cells resulted in liver toxicity in five out of the eight patients infused. The authors concluded the hepatic toxicity seen in this trial was most likely related to targeting of CAIX on bile duct epithelial cells [28]. Three additional patients were treated without liver toxicity on a modified protocol which included infusion of an anti-CAIX monoclonal antibody (to block CAIX on normal liver tissue) prior to T cell infusion [98]. Persistence of CAR modified T cells was limited for all patients on this trial and no objective clinic responses were observed. Significantly, patients developed both a humoral and cellular anti-CAR response which could help explain the limited persistence of modified T cells [98].
Kershaw et. al., described the treatment of 14 ovarian cancer patients with CAR modified T cells directed against the α-folate receptor (FR) [91]. Subcutaneous IL-2 was given to a majority of patients (n=8) and adverse events were consistent with the toxicity profile of IL-2. Again, modified T cells had limited persistence and limited tumor localization as determined using 111Indium-labeled T cells. No objective responses were observed and an inhibitory factor developed in three patients (out of six tested) which, when tested, reduced the in vitro anti-FR tumor response of modified T cells [91].
Park et. al., reported the adoptive transfer of CAR modified CD8+ clones directed against the L1-cell adhesion molecule (L1-CAM; CD171) in six patients with neuroblastoma [86]. No clinical responses were observed, however increased T cell persistence (as measured by quantitative polymerase chain reaction (Q-PCR) of peripheral blood) was noted in a patient with limited disease burden when compared to patients with larger tumor burdens [86].
Till et. al., reported on the adoptive transfer of CAR modified T cells targeting CD20 in seven patients with refractory or relapsed indolent NHL [90]. In the first cohort no clinical responses were seen and persistence of T cells was limited. In the second cohort, who received subcutaneous IL-2, persistence of modified T cells was enhanced. One patient in second cohort exhibited a partial remission and one patient had decreased metabolic activity of their tumor as measured by positron emission tomography (PET) [90].
Brenner and colleagues have treated 19 patients with neuroblastoma using two populations (viral and non-viral specific) of T cells modified with a CAR directed against GD2 [89, 92]. Three patients with active disease (bone or bone marrow disease) achieved complete remission (CR) following adoptive transfer, of which two remain in CR (1 and 4 years following T cell infusion). As mentioned previously, persistence of modified T cell was contingent on the increased frequency of helper (CD4+) and central memory (CD45RO+CD62L+) cells within the infused product. However, over time viral specificity had no impact on the persistence of genetically modified T cells [89]. Furthermore, persistence of modified T cells correlated with superior clinical outcome [89]. Modified T cells persisted at low levels up to 192 weeks and 96 weeks for non-viral and viral specific CAR modified T cells respectively as determined by Q-PCR of peripheral blood [89].
Jensen et. al., treated four patients with recurrent NHL with CAR modified T cells [100]. Two patients with diffuse large B cell lymphoma were treated with cloned CD8+ CTLs expressing a CD20 specific CAR after autologous hematopoietic stem cell transplant. Two patients with follicular lymphoma were treated with polyclonal CAR modified T cells expressing a CD19-specific CAR and low dose subcutaneous IL-2. Modified T cell persistence was again limited, cellular anti-transgene immune responses was noted for two patients, and no objective clinical responses were found [100].
Second Generation CAR Modified T cell Trials
Based on promising pre-clinical data, several centers initiated clinical trials using T cells modified with second generation CARs. Kochenderfer et. al., described the treatment of one NHL patient with a CD28 containing second generation CAR directed against the CD19 antigen [21]. This patient received conditioning therapy which included cyclophosphamide and fludarabine followed by modified T cells and IL-2. This therapy resulted in partial remission of the patient's lymphoma (for up to 32 weeks), B-cell aplasia (for up to 39 weeks) and persistence of CAR modified T cells shown by Q-PCR of the blood (for up to 27 weeks) [21].
Savoldo et. al., treated six patients with relapsed or refractory NHL with CAR modified T cells [66]. In this trial non-preconditioned patients received simultaneous infusion of two autologous T cell products (first generation and CD28 containing second generation CARs) both specific for CD19. Superior persistence, expansion and trafficking to a site of disease (cutaneous skin lesion) for CD28 containing second generation CAR modified T cells were demonstrated [66]. However, patients did not show evidence of sustained tumor regression in this cohort.
Our group described the treatment of nine patients (8 with CLL and 1 with ALL) with CD28 containing second generation CAR modified T cells directed against the CD19 antigen [20]. As mentioned, T cell infusion was well tolerated in all but one patient who died two days after modified T cell infusion [99]. Six patients received conditioning chemotherapy with cyclophosphamide prior to T cell infusion. Persistence of modified T cells was enhanced with prior chemotherapy and inversely proportional to the peripheral blood tumor burden [20]. Three out of four evaluable patients with bulky CLL exhibited a response to treatment with conditioning chemotherapy followed by modified T cell infusion, including one patient who had a marked clinical and radiographic reduction in lymphadenopathy. The one patient treated with ALL exhibited a B cell aplasia prior to allo-HSCT. Modified T cells were also found to traffic to sites of disease (bone marrow, lymph nodes, liver) and retained ex vivo cytotoxicity after adoptive transfer as previously discussed [20].
June and colleagues described the treatment of three CLL patients with a 4-1BB containing second generation CAR targeting CD19 [22, 101]. In these reports patients received conditioning chemotherapy prior to T cell infusion. Strikingly, modified T cells were demonstrated to expand (up to 10,000 fold) following adoptive transfer, traffic to site of disease (bone marrow) and were detected at high levels (by flow cytometry and Q-PCR of peripheral blood and bone marrow) for up to 6 months post infusion [22]. All three patients exhibited a response following treatment (2 CR and 1 PR), and development of a tumor lysis syndrome correlated with elevation of peripheral blood CAR modified T cells number and pro-inflammatory cytokine levels [22]. In addition, B-cell aplasia, decreased plasma cell number, and hypogammaglobulinemia were evident in these patients [22].
Third Generation CAR Modified T cell Trial
As mentioned above one patient with colon cancer was treated with a third generation (CD28/4-1BBCD3ζ) targeting ERBB2 [97]. This report details the death of the patient five days following lymphocyte depleting chemotherapy (cyclophosphamide and fludarabine) followed by infusion of modified T cells. As mentioned, the “on target/off tumor” toxicity from the modified T cells resulted in a clinically significant release of pro-inflammatory cytokines resulting in pulmonary toxicity, multiorgan failure and eventual death of the patient [97].
Conclusions/Future Challenges
The ultimate promise of tumor immunotherapy is the cure of cancer without the toxicity of conventional treatments. The pre-clinical and early clinical results of CAR modified T cells have given hope that this promise is within reach. The treatment of cancer with CAR modified T cells has several advantages: HLA independent recognition of target antigens, broad applicability to most patients, ability to circumvent “tumor escape,” and the ability to rapidly deliver a population of tumor specific T cells. The successful application of this technology will require the identification of target antigens that are uniquely expressed on tumor cells thereby minimizing the risk for toxicity. In addition, as demonstrated in recent clinical reports, a prerequisite for success of this therapy is the in vivo persistence of CAR modified T cells following adoptive transfer [20-22]. We have shown multiple injections of modified T cells can artificially increase T cell persistence and enhance anti-tumor efficacy in a mouse model of cancer [59]. However, this technology should allow for a single injection of T cells which engraft, proliferate, persist, and retain targeted cytotoxic function for a lifetime. Otherwise this technology is ultimately an expensive and ineffective intervention. Continued investigation into the elements which govern persistence of tumor targeted T cells is essential. Thus far, signaling, tumor burden, conditioning chemotherapy, T cell phenotype, and use of supplementary cytokines have all been implicated. Furthermore, as noted in this review, several of the early trials demonstrated the development of immune response against the adoptively transferred cells [91, 98, 100]. Understandably, this phenomenon has limited the persistence and efficacy of adoptively transferred cells. Moving forward the development of less immunogenic CARs (e.g. humanized scFvs), promotion of tolerance, and/or optimizing immunosuppression will need to be investigated for successful application of this therapy.
While recent reports have demonstrated success for this technology in hematologic malignancies the application of this therapy to solid malignancies may need further development. While early research has focused on the ability to reliably generate tumor targeted T cells it is possible CAR modified T cells will be rendered ineffective upon entering the suppressive tumor microenvironment. Thus the future of this therapy is in the generation of CAR modified T cells that will resist the anergy and apoptosis that occurs for all immune effectors within the tumor microenvironment. Furthermore, more than overcoming the hostile tumor microenvironment this therapy should have the capacity to recruit an endogenous anti-tumor response. Targeting a single antigen on a tumor cell may initially result in a decrease in tumor burden, but may also select for the expansion of tumor cells lacking this target antigen. Therefore, while genetic modifications of T cells may allow us to specifically target the tumor and potentially overcome the hostile tumor microenvironment without recruitment and activation of the endogenous immune system it is likely this therapy will be insufficient to cure the majority of patients. What is further required is for the activation of an endogenous anti-tumor response (e.g. TILs, NK cells, innate immune system) by the CAR modified T cells. If effective it is plausible this activation will induce epitope spreading against several antigens expressed by the tumor thereby reducing the ability of tumors to escape eradication.
While scientifically this field has significantly progressed in a relatively short period of time, the success of all cancer therapies is only measured by the impact they have on patients in the clinic. To fully define the efficacy of this therapy multi-institutional clinical trials will need to be conducted which require a significant expenditure of labor and funding. Unfortunately, these expenditures can be prohibitive and currently available funding mechanisms inadequately cover their costs. However, will the recent landmark responses seen in smaller single institutional studies there is a new hope resources will be made available for the development of this promising therapy.
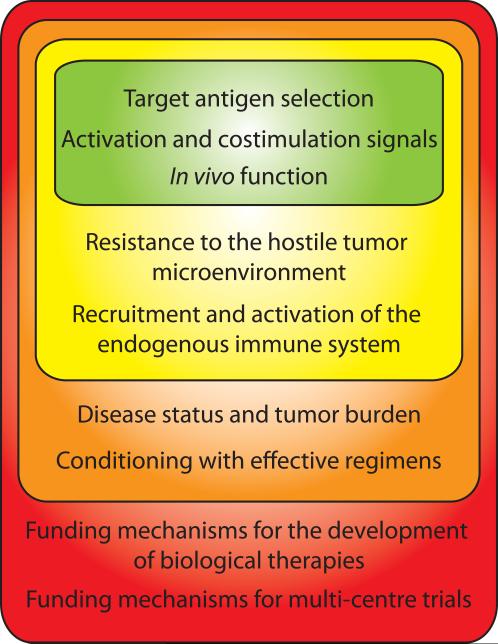
With this review we have attempted to outline the qualities that translate into successful tumor immunotherapy using CAR modified T cells (Figure 1). Thus far the target antigen, signaling domain, ability to traffic, persist, retained function, patient disease status and conditioning regime have all been critically important. We conclude that the future of this field will be in the development of genetically engineered tumor target T cells that can overcome the hostile tumor microenvironment and recruit an endogenous anti-tumor response. The final hurdle for researchers in this field is for the implementation of clinical trials and securing the funding needed to complete them. As this technology progresses and more reports of successful eradication of malignancy occur these hurdles should be easily overcome.
Figure 1. Considerations for effective CAR modified T cell Immunotherapy.
Successful application of CAR modified T cell immunotherapy requires the consideration of several qualities, including the CAR design, tumor microenvironment, patient clinical status, and funding mechanisms required for CAR development and clinical trials.
Acknowledgments
Supported in part by grants from the National Institutes of Health (CA-138738, CA-59350), Alliance for Cancer Gene Therapy, Damon Runyon Clinical Investigator Award (R.J.B.), The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York, Kate's Team, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of MSKCC, Geoffrey Beene Cancer Foundation, William Lawrence and Blanche Hughes Foundation, and the St. Baldrick's Foundation Post Doctoral Fellowship in Childhood Cancer (K.J.C.)
The authors would like to thank Joe Olechnowicz for his skilled work in reviewing this manuscript.
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose
References
- 1.Weiden PL, Sullivan KM, Flournoy N, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. The New England journal of medicine. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nature reviews Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 5.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunnell BA, Muul LM, Donahue RE, et al. High-efficiency retroviral-mediated gene transfer into human and nonhuman primate peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1995;92:7739–7743. doi: 10.1073/pnas.92.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam JS, Reeves ME, Cowherd R, et al. Improved gene transfer into human lymphocytes using retroviruses with the gibbon ape leukemia virus envelope. Hum Gene Ther. 1996;7:1415–1422. doi: 10.1089/hum.1996.7.12-1415. [DOI] [PubMed] [Google Scholar]
- 8.Gallardo HF, Tan C, Ory D, et al. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952957. [PubMed] [Google Scholar]
- 9.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. 00002371-200902000-00009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Seliger B. Different regulation of MHC class I antigen processing components in human tumors. Journal of immunotoxicology. 2008;5:361–367. doi: 10.1080/15476910802482870. [DOI] [PubMed] [Google Scholar]
- 12.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. S1074-7613(09)00507-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridgeman JS, Hawkins RE, Hombach AA, et al. Building better chimeric antigen receptors for adoptive T cell therapy. Current gene therapy. 2010;10:77–90. doi: 10.2174/156652310791111001. [DOI] [PubMed] [Google Scholar]
- 16.Goldberger O, Volovitz I, Machlenkin A, et al. Exuberated numbers of tumor-specific T cells result in tumor escape. Cancer Res. 2008;68:3450–3457. doi: 10.1158/0008-5472.CAN-07-5006. 68/9/3450 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Tassev DV, Cheng M, Cheung NK. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2011 doi: 10.1038/cgt.2011.66. [DOI] [PubMed] [Google Scholar]
- 18.Stewart-Jones G, Wadle A, Hombach A, et al. Rational development of high-affinity T- cell receptor-like antibodies. Proc Natl Acad Sci U S A. 2009;106:5784–5788. doi: 10.1073/pnas.0901425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leukemia & lymphoma. 1995;18:385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 20.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011 doi: 10.1182/blood-2011-04-348540. blood-2011-04-348540 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. blood-2010-04-281931 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. 3/95/95ra73 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinthus JH, Waks T, Kaufman-Francis K, et al. Immuno-gene therapy of established prostate tumors using chimeric receptor-redirected human lymphocytes. Cancer Res. 2003;63:2470–2476. [PubMed] [Google Scholar]
- 24.Teng MW, Kershaw MH, Moeller M, et al. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther. 2004;15:699–708. doi: 10.1089/1043034041361235. [DOI] [PubMed] [Google Scholar]
- 25.Gong MC, Latouche JB, Krause A, et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. nbt0102-70 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Weijtens ME, Willemsen RA, van Krimpen BA, et al. Chimeric scFv/gamma receptor-mediated T-cell lysis of tumor cells is coregulated by adhesion and accessory molecules. Int J Cancer. 1998;77:181–187. doi: 10.1002/(SICI)1097-0215(19980717)77:2<181::AID-IJC2>3.0.CO;2-M. [pii] [DOI] [PubMed] [Google Scholar]
- 28.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–22. doi: 10.1200/JCO.2006.05.9964. 24/13/e20 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Mezzanzanica D, Canevari S, Mazzoni A, et al. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 1998;5:401–407. [PubMed] [Google Scholar]
- 30.Westwood JA, Smyth MJ, Teng MW, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A. 2005;102:19051–19056. doi: 10.1073/pnas.0504312102. 0504312102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darcy PK, Kershaw MH, Trapani JA, et al. Expression in cytotoxic T lymphocytes of a single-chain anti-carcinoembryonic antigen antibody. Redirected Fas ligand-mediated lysis of colon carcinoma. Eur J Immunol. 1998;28:1663–1672. doi: 10.1002/(SICI)1521-4141(199805)28:05<1663::AID-IMMU1663>3.0.CO;2-L. doi: 10.1002/(SICI)1521-4141(199805)28:05<1663::AID-IMMU1663>3.0.CO;2-L. [pii] [DOI] [PubMed] [Google Scholar]
- 32.Haynes NM, Trapani JA, Teng MW, et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol. 2002;169:5780–5786. doi: 10.4049/jimmunol.169.10.5780. [DOI] [PubMed] [Google Scholar]
- 33.Hwu P, Shafer GE, Treisman J, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chekmasova AA, Rao TD, Nikhamin Y, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16:3594–3606. doi: 10.1158/1078-0432.CCR-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossig C, Bollard CM, Nuchtern JG, et al. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 36.Niederman TM, Ghogawala Z, Carter BS, et al. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci U S A. 2002;99:7009–7014. doi: 10.1073/pnas.092562399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pameijer CR, Navanjo A, Meechoovet B, et al. Conversion of a tumor-binding peptide identified by phage display to a functional chimeric T cell antigen receptor. Cancer Gene Ther. 2007;14:91–97. doi: 10.1038/sj.cgt.7700993. [DOI] [PubMed] [Google Scholar]
- 38.Muniappan A, Banapour B, Lebkowski J, et al. Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther. 2000;7:128–134. doi: 10.1038/sj.cgt.7700100. [DOI] [PubMed] [Google Scholar]
- 39.Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ang SO, Hartline C, Champlin R, et al. Generating a Chimeric Antigen Receptor to Redirect T-Cell Specificity after Infusion. Molecular Therapy. 2011;19:S–137. [Google Scholar]
- 42.Chmielewski M, Hombach A, Heuser C, et al. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. DOI: 173/12/7647 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Eshhar Z. Tumor-specific T-bodies: towards clinical application. Cancer Immunol Immunother. 1997;45:131–136. doi: 10.1007/s002620050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamers CH, Gratama JW, Warnaar SO, et al. Inhibition of bispecific monoclonal antibody (bsAb)-targeted cytolysis by human anti-mouse antibodies in ovarian carcinoma patients treated with bsAb-targeted activated T-lymphocytes. Int J Cancer. 1995;60:450–457. doi: 10.1002/ijc.2910600405. [DOI] [PubMed] [Google Scholar]
- 45.Hombach A, Schneider C, Sent D, et al. An entirely humanized CD3 zeta-chain signaling receptor that directs peripheral blood t cells to specific lysis of carcinoembryonic antigen-positive tumor cells. Int J Cancer. 2000;88:115–120. doi: 10.1002/1097-0215(20001001)88:1<115::AID-IJC18>3.0.CO;2-E. [pii] [DOI] [PubMed] [Google Scholar]
- 46.Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loskog A, Giandomenico V, Rossig C, et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. 2404366 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Heuser C, Hombach A, Losch C, et al. T-cell activation by recombinant immunoreceptors: impact of the intracellular signalling domain on the stability of receptor expression and antigen-specific activation of grafted T cells. Gene Ther. 2003;10:1408–1419. doi: 10.1038/sj.gt.3302023. 3302023 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Haynes NM, Snook MB, Trapani JA, et al. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-zeta vs Fc epsilon RI-gamma. J Immunol. 2001;166:182–187. doi: 10.4049/jimmunol.166.1.182. [DOI] [PubMed] [Google Scholar]
- 50.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. nm827 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Gilham DE, O'Neil A, Hughes C, et al. Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother. 2002;25:139–151. doi: 10.1097/00002371-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Stancovski I, Schindler DG, Waks T, et al. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–6582. [PubMed] [Google Scholar]
- 53.Harding FA, McArthur JG, Gross JA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 54.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 55.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. 10.1146/annurev.immunol.021908.132706 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chambers CA, Allison JP. Co-stimulation in T cell responses. Current opinion in immunology. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 57.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 58.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–190. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:54265435. doi: 10.1158/1078-0432.CCR-07-0674. 1078-0432.CCR-07-0674 [pii] [DOI] [PubMed] [Google Scholar]
- 60.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. 66/22/10995 [pii] [DOI] [PubMed] [Google Scholar]
- 61.Beecham EJ, Ma Q, Ripley R, et al. Coupling CD28 co-stimulation to immunoglobulin T-cell receptor molecules: the dynamics of T-cell proliferation and death. J Immunother. 2000;23:631–642. doi: 10.1097/00002371-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Hombach A, Sent D, Schneider C, et al. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res. 2001;61:1976–1982. [PubMed] [Google Scholar]
- 63.Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 64.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koehler H, Kofler D, Hombach A, et al. CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res. 2007;67:2265–2273. doi: 10.1158/0008-5472.CAN-06-2098. [DOI] [PubMed] [Google Scholar]
- 66.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. 46110 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 68.Harada Y, Ohgai D, Watanabe R, et al. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. mt200983 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. 0813101106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, Wang QJ, Yang S, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. 183/9/5563 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 73.Speiser DE, Colonna M, Ayyoub M, et al. The activatory receptor 2B4 is expressed in vivo by human CD8+ effector alpha beta T cells. J Immunol. 2001;167:6165–6170. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- 74.Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T cells. Cancer Immunol Immunother. 2009;58:1991–2001. doi: 10.1007/s00262-009-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 76.Zhong XS, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. mt2009210 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang L, Chang WC, McNamara G, et al. Transgene-enforced co-stimulation of CD4+ T cells leads to enhanced and sustained anti-tumor effector functioning. Cytotherapy. 2007;9:771–784. doi: 10.1080/14653240701656079. 782862895 [pii] [DOI] [PubMed] [Google Scholar]
- 78.Pule MA, Straathof KC, Dotti G, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 79.Wilkie S, Picco G, Foster J, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:49014909. doi: 10.4049/jimmunol.180.7.4901. DOI: 180/7/4901 [pii] [DOI] [PubMed] [Google Scholar]
- 80.Santos EB, Yeh R, Lee J, et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 2009;15:338–344. doi: 10.1038/nm.1930. nm.1930 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kershaw MH, Wang G, Westwood JA, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 82.Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. blood-2009-03-209650 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. DOI: 173/12/7125 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nature clinical practice Oncology. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Current opinion in immunology. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. 6300104 [pii] [DOI] [PubMed] [Google Scholar]
- 87.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 88.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Louis CU, Savoldo B, Dotti G, et al. Anti-tumor activity and long-term fate of chimeric antigen receptor positive T-cells in patients with neuroblastoma. Blood. 2011 doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. blood-2007-12-128843 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. 12/20/6106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.l882. nm.1882 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curran K, Taylor C, Doubrovina E, et al. Virus Specific T-Lymphocytes Genetically Modified to Target the CD19 Antigen Eradicates Systemic Lymphoma in Mice. Blood. 2010;116 [Google Scholar]
- 94.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koenecke C, Czeloth N, Bubke A, et al. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 96.Lee JC, Hayman E, Pegram HJ, et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011;71:2871–2881. doi: 10.1158/0008-5472.CAN-10-0552. 0008-5472.CAN-10-0552 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. mt201024 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 99.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. S1083-8791(10)00119-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]