Abstract
Stroke is a leading cause of adult disability. Stem/progenitor cell transplantation improves recovery after stroke in rodent models. These studies have 2 main limitations to clinical translation. First, most of the cells in stem/progenitor transplants die after brain transplantation. Second, intraparenchymal approaches target transplants to normal brain adjacent to the stroke, which is the site of the most extensive natural recovery in humans. Transplantation may damage this tissue. The stroke cavity provides an ideal target for transplantation because it is a compartmentalized region of necrosis, can accept a high volume transplant without tissue damage, and lies directly adjacent to the most plastic brain area in stroke. However, direct transplantation into the stroke cavity has caused massive death in the transplant. To overcome these limitations, the authors tested stem/progenitor transplants within a specific biopolymer hydrogel matrix to create a favorable environment for transplantation into the infarct cavity after stroke, and they tested this in comparison to stem cell injection without hydrogel support. A biopolymer hydrogel composed of cross-linked hyaluronan and heparin sulfate significantly promoted the survival of 2 different neural progenitor cell lines in vitro in conditions of stress and in vivo into the infarct cavity. Quantitative analysis of the transplant and surrounding tissue indicates diminished inflammatory infiltration of the graft with the hydrogel transplant. This result indicates that altering the local environment in stem cell transplantation enhances survival and diminishes cell stress. Stem cell transplantation into the infarct cavity within a pro-survival hydrogel matrix may provide a translational therapy for stroke recovery.
Keywords: stem cell, neural repair, inflammation, gliosis, neovascularization, infarct cavity, bioengineering
Introduction
Stroke is the leading cause of adult disability because of the brain’s limited capacity for repair.1 Once damage from a stroke is established, little can be done to recover lost function. With the increased incidence of and declining mortality from stroke, large increases in the number of disabled stroke survivors are expected.2 Treatments that improve repair and recovery in stroke may reduce this clinical burden.
Stem/progenitor cell transplantation after stroke produces an improvement in behavioral recovery in most preclinical studies.3 Multiple therapeutic pathways are proposed for the behavioral recovery. Transplanted stem/progenitor cells may differentiate into neurons or glia and integrate into the host brain.4 Cell transplantation after stroke may also enhance endogenous repair processes after stroke, such as axonal sprouting,5,6 neurogenesis, and angiogenesis.7,8 Acute stem cell transplantation also may protect neurons and attenuate inflammation after stroke.9 Despite these promising studies, cell transplantation in stroke has not seen clinical translation.
There are at least 2 limitations in present cell transplantation approaches in stroke. The delivery methods for stem/progenitor cells are limited. Most transplanted cells die when directly injected into the brain3 or distribute to peripheral organs when given systemically.10,11 Furthermore, scaling up vascular delivery of stem/progenitor cells can actually block blood flow in the brain and worsen cerebral ischemia.10,12 In terms of direct brain transplantation, an ideal site for stem cell delivery is the infarct cavity. This location is a compartmentalized area of loose tissue that has undergone necrosis and can accept a large volume injection, and it is directly adjacent to peri-infarct tissue, the site of greatest neuroplasticity after stroke.13 However, transplants within the infarct cavity die, and the closer transplants are made to the stroke cavity, the greater the cell death in the transplant.14 Transplantation into the brain thus targets peri-infarct tissue and involves multiple injections into this site. Because this is the site of the most active functional remapping after stroke in humans,15 multiple stem cell injections into peri-infarct tissue in humans may damage the very tissue that undergoes repair and recovery. Strategies that overcome these limitations may promote a clinical translation for intraparenchymal transplantation of stem/progenitor cells.
Recent advances in tissue engineering have produced applications that may provide solutions to the problem of stem cell transplant death and damage associated with the transplant. Biopolymer hydrogels have been designed that promote stem cell survival, minimize wound scar formation, and enhance stem cell engraftment.16,17 Hydrogels for CNS applications have the same viscoelastic properties as the brain16,18,19 and are easily transplanted into the adult brain without damage.20 Hydrogels alter the survival and differentiation of stem/progenitor cells in vitro and in vivo.17,18 Several hydrogel approaches use the normal brain extracellular matrix component hyaluronan.21 Hyaluronan gels have mechanical properties similar to brain tissue and do not promote local scarring or tissue reaction.21,22 These gels influence neural differentiation and allow neuronal sprouting and ingrowth into the gel.21-23
Hyaluronan and other bioengineered scaffolds have been shown to promote survival and engraftment of cells in the brain and spinal cord.16 A limitation to this scaffold approach is that their semirigid nature prevents injection into most locations of human stroke. However, hydrogels that “gel” within the brain allow minimally invasive injection of a liquid and use the same neurosurgical approaches now commonly used in human stroke therapy.24,25 Here, we used a hyaluronan/heparin/collagen hydrogel that can be injected in liquid form and gels within the brain to examine whether it can modify the normally hostile environment of the infarct core into a pro-survival and progrowth niche and promote the survival of stem cells.
Materials and Methods
Neural Progenitor Cells (NPCs) Derived From Embryonic Stem (ES) Cell Cultures
Undifferentiated mouse ES cells were propagated using standard techniques.26 Mouse ES cell lines were then transfected with flap-Ub promoter-GFP-WRE (FUGW) lentivirus carrying a green fluorescent protein (GFP) construct. Colonies expressing GFP were replated onto a feeder layer. Neuronal stem cells were derived from GFP-transfected ES cells using the monolayer differentiation protocol as described in Ying et al,27 with several modifications. Briefly, undifferentiated ES cells were dissociated and plated into 0.1% gelatin-coated tissue culture plastic in N2B27 medium. Cells were then trypsinized into single cells and transferred into a petri dish in N2B27 medium supplemented with beta fibroblast growth factor (bFGF) and epidermal growth factor (EGF), which were added daily. Neurospheres formed in 3 days and were then transferred to a gelatin-coated plate and allowed to attach. A population of bipolar neural stem cells grew from the neurosphere and were selected and passaged every 2 to 3 days. This procedure produces ES cell–derived neural precursors that can differentiate into astrocytes, oligodendrocytes, and neurons and remain stable for multiple passages.27
NPCs Derived From Embryonic Cortex Cultures
Mouse neural precursors were derived from GFP reporter transgenic E12.5 embryo mouse cortex as described in Currle et al,28 with modifications. In brief, telencephalic vesicles were dissected from E12.5 embryos and cut into small pieces of tissue. Tissues were then dissociated using several rounds of titration with fire-polished Pasteur pipettes. Cells were washed once with 0.2% bovine serum albumin (BSA) in Hanks balanced salt solution (HBSS) and plated at 50 000 cells/mL on uncoated culture flask in media with 10 ng/mL FGF2 (R&D Systems or Peprotech, Invitrogen, Carlsbad, CA), and 2 μg/mL heparin (Sigma, Invitrogen, Carlsbad, CA), which were added every 3 to 4 days. Neurospheres were formed in 3 to 4 days, and cells were passaged every 7 days. This procedure produces multipotent neural progenitors capable of in vitro differentiation into astrocyte, oligodendrocyte, and neuronal lineages.28
Hyaluronan–Heparin–Collagen Hydrogel
A hyaluronan–heparin–collagen hydrogel (HyStem-HP, Glycosan, Salt Lake City, UT) was made from thiol-modified sodium hyaluronate, heparin sulfate, and gelatin and was cross-linked with polyethylene glycol diacrylate. Solutions of this hydrogel form a transparent gel when mixed with a cross-linker over a period of 30 minutes. Then, 100 000 neuronal stem cells in 1 μL were mixed with 4 μL hyaluronan/heparin sulfate, 1 μL gelatin, and 1 μL cross-linker to form a stem-cell–hydrogel complex.
NPC Survival in Culture
NPC survival was quantified in culture with or without hydrogel in 96-well plates 24 hours after treatment by measuring lactate dehydrogenase (LDH) released into the culture media (Promega, Madison, WI). Cell survival was further confirmed by a tetrazolium reduction assay to measure viable mitochondrial function (Dojindo, Gaithersburg, MD). In brief, for LDH measurement, 50 μL culture medium was mixed with 50 μL substrate and incubated at 37°C for 30 minutes; 50 μL stop solution was then added, and absorbance at 490 nm was recorded. For the tetrazolium reduction assay, 10 μL CCK-8 solution was added to the cells, and they were incubated at 37°C for 2.5 hours. Absorbance at 450 nm was then recorded.
Induction of Focal Ischemia and Stem Cell Transplantation
All procedures were performed in accordance with National Institutes of Health Animal Protection Guidelines and approved by the UCLA Chancellor’s Animal Research Committee. A cortical photothrombotic stroke was produced in 2-month-old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA).29 Mice were anesthetized with 5% isoflurane and maintained with 2.5% isoflurane in N2O:O2 (2:1). A midline skin incision was made after the mice were placed in the stereotactic apparatus. Rose Bengal (10 mg/mL) was injected intraperitoneally at 10 μL/g of mouse body weight, and 5 minutes allowed for dye absorption. Blood vessels were then irradiated through the intact skull for 15 minutes with a 2-mm diameter cold fiberoptic light source at 0 mm anterior and 1.5 mm lateral left of the bregma.
NPCs with or without hydrogel matrix were transplanted into the stroke cavity 7 days after the stroke surgery. Partial liquefaction of the necrotic tissues occurs at 7 days poststroke,30 providing an area devoid of normal brain structure for transplantation into the infarct core at this time without damaging peri-infarct tissue. A burr hole was drilled on the skull overlying the infarct cavity at the same coordinates as the focal point of the light source. Then, 100 000 NPCs in 1 μL saline were mixed with 4 μL hyaluronan/heparin, 1 μL gelatin, and 1 μL cross-linker. The 7 μL complex was then injected while in liquid form stereotaxically with a 30-gauge needle and 25 μL Hamilton syringe into the infarct cavity at a depth of 1 mm from the surface of the brain at a rate of 0.7 μL/min. The needle was left in place for an additional 5 minutes after the injection before withdrawal. No immunosuppression agents were used for the transplantation because no significant difference in graft volume is observed in allogeneic NPC transplantation into the mouse brain with or without cyclosporine A.31
Immunohistochemistry and Microscopy
After a 2-week survival, mice were deeply anesthetized and perfused with buffered saline and then 4% paraformaldehyde, cryoprotected, and frozen-sectioned. Brains were cut in 4 parallel series of 50 μm thickness. Immunohistochemistry was performed as described in Ohab et al.32 Briefly, brain sections were rinsed with phosphate-buffered saline blocked in normal donkey serum plus Triton-X100. Brain sections were then incubated overnight at 4°C with primary antibodies. Secondary antibodies from the appropriate hosts conjugated to cyanine 2, cyanine 3, and cyanine 5 (Jackson Immuno Research, West Grove, PA) were used. Brain sections were then counterstained with the nuclear marker DAPI (4′,6-diamidino-2-phenylindole). Primary antibodies were used as follows: chicken anti-GFP (1:200, Abcam Cambridge, MA); goat anti-doublecortin (DCX; C18, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA); rat anti-platelet-endothelial cell adhesion molecule-1 (PECAM-1; 1:300; BD PharMingen, San Diego, CA); rabbit anti-GFAP (glial fibrillary acidic protein) (1:1000; Zymed, San Francisco, CA); rabbit anti-NG2 (1:400; Chemicon); rabbit anti-Iba1(1:400, Wako Chemicals USA, Richmond, VA). Quantitative estimates of the cell numbers were stereologically determined using the optical fractionator procedure.33 Six serial sections, spaced 200 μm apart through the subventricular zone (SVZ) and hippocampus, were quantified for transplanted GFP positive cells. For Iba1-positive cells, cells were counted in an area around the edge of the GFP-positive cell transplant site. For GFAP quantification, 3 areas around the peri-infarct cortex (2 medial and 1 lateral to the infarct core) per section were randomly chosen and photographed. GFAP fluorescent signals were then analyzed with Image J software.34 PECAM-positive vessels were traced, and the length of the vessels was measured (Stereoinvestigator, Microbrightfield, Colchester, VT).35 Double labeling was determined using a confocal laser scanning microscope (Leica TCS SP MP, Leica, Deerfield, IL) with z-plane reconstruction of image stacks. All quantification was performed with the investigator blinded to the treatment condition.
Statistics
Differences between stem cells with vehicle and stem cells with hydrogel were tested with 2-sample t tests assuming unequal variance (Excel; Microsoft, Seattle, WA).
Results
Hyaluronan–Heparin–Collagen Hydrogel Promotes Survival of NPCs Transplanted Into the Infarct Cavity After Stroke
Hyaluronan–heparin–collagen interact with stem/progenitor cells to support survival and/or differentiation.17,36-38 These constituents are naturally occurring brain macromolecules and when gelled have a viscoelastic profile resembling the normal brain.38 For these reasons a hyaluronan–heparin–collagen hydrogel was tested in these studies. The particular formulation was chosen because it remains in liquid form long enough to allow injection through a small needle directly into the brain cavity and then gels within the cavity. In comparing stroke with hydrogel and stroke without hydrogel, the hydrogel caused no apparent deformation of the brain or change in infarct size (1.25 ± 0.18 mm3 without hydrogel vs 1.18 ± 0.35 mm3 with hydrogel; P = .77).
A total of 100 000 GFP transgenic NPCs derived from the embryonic cortex were transplanted into the infarct cavity the number of 7 days after stroke with or without hydrogel (n = 6 each group). Then, 14 days after transplantation, transplanted cells were identified by the immunostaining of GFP (Figure 1). In the cells-only group, an average of 4000 cells survived transplantation at 2 weeks. Cells transplanted with hydrogel had an average of 8000 cells—a statistically significant 2-fold expansion in cell survival (P = .035). Transplantation with hydrogel altered the cellular morphology of the transplanted cells. With hydrogel transplantation, stem cells that remained in the transplant formed a flattened or spheroid shape without local processes (Figures 1 and 2). In both cells-only and cells + hydrogel groups, the transplanted cells that did migrate beyond the infarct adopted a more elaborate morphology, with local processes extending into the surrounding tissue (Figures 2, 3, and 4). There was no difference in the degree of migration of transplanted cells out of the stroke cavity in the cells-only and cells + hydrogel groups. In both cases, cells migrated 200 μm or less into the peri-infarct cortex or underlying white matter.
Figure 1.
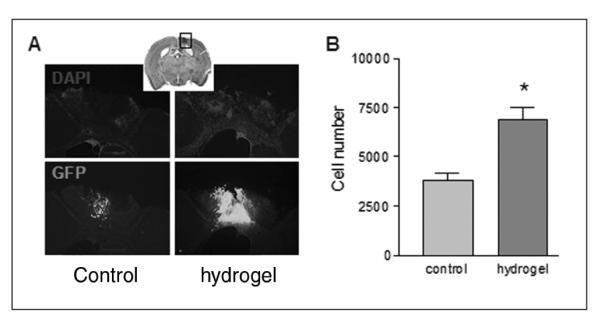
Hyaluronan–heparin–collagen hydrogel promotes the survival of neural progenitor cells (NPCs) transplanted into the infarct cavity after stroke: (A) 100 000 NPCs derived from the embryonic cortex were transplanted into the infarct cavity 7 days after stroke, with or without hydrogel (n = 6 each group); 14 days after transplantation, transplanted cells were identified by the immunostaining of green fluorescent protein (GFP); the infarct cavity was identified by counterstaining with DAPI (4′,6-diamidino-2-phenylindole). (B) In the cells-only group, an average of 4000 cells survived transplantation at 2 weeks. Cells transplanted with hydrogel had an average of 8000 cells, a statistically significant 2-fold expansion in cell survival (P = .035)
Figure 2.
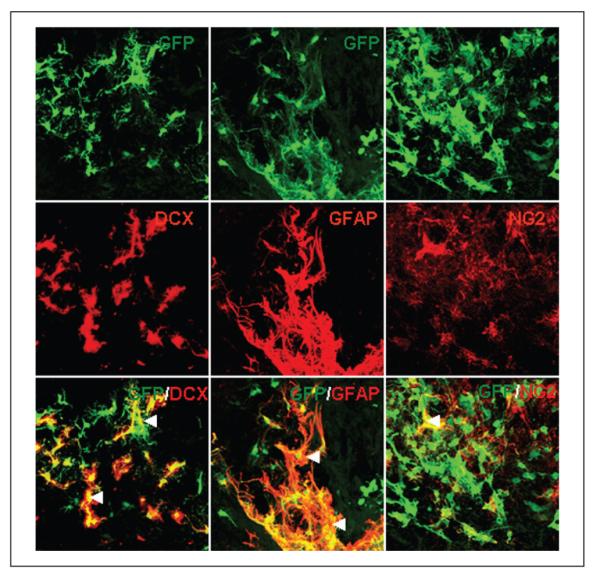
Differentiation of neural progenitor cells (NPCs) transplanted into the infarct cavity after stroke. Transplanted NPCs were identified by green fluorescent protein (GFP) immunostaining (top row); the middle row shows double staining for a marker of immature migrating neurons (anti-doublecortin [DCX]), an astrocyte marker (GFAP), and a polydendrocyte or oligodendrocyte precursor marker, NG2; the transplant condition did not have an effect on the degree of differentiation of stem cells (◀ double-labeled cell)
Figure 3.
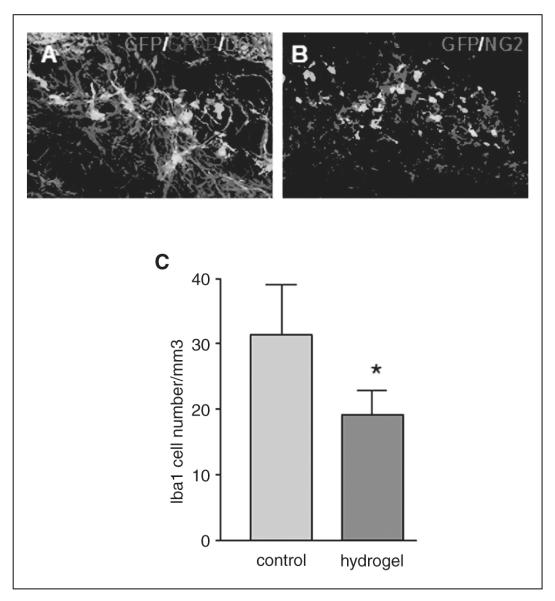
Effect of transplanted stem cells on neovascularization, peri-infarct reactive astrocytosis, and infiltration of microglia cells: (A) Vessels in the peri-infarct area were identified by anti-platelet-endothelial cell adhesion molecule-1 immunostaining; the total length of the vessels was compared between transplanted cells with or without hydrogel; there was no significant difference between the cells-only and cells + hydrogel groups. (B) Peri-infarct glial fibrillary acidic protein (GFAP) reactive astrocytosis was identified by GFAP immunostaining; the GFAP immunofluorescence signal was compared between transplanted cells with or without hydrogel, and no significant difference was found. (C) Transplanted stem cells were identified by green fluorescent protein (GFP) immunostaining, and active microglia/macrophages were identified by immunostaining with Iba1. (D) The number of activated microglia/macrophages was quantified in the transplant site; active microglia/macrophages infiltrating the stem cell graft were significantly decreased with hydrogel (P = .004)
Figure 4.
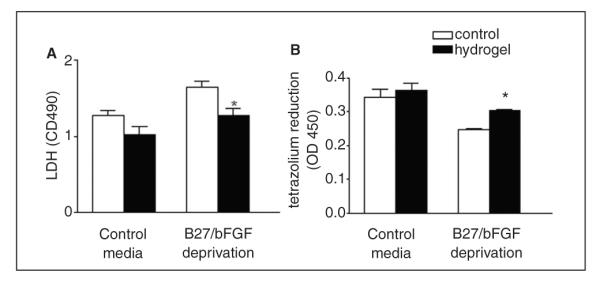
Hyaluronan–heparin–collagen hydrogel supports the survival of neural progenitor cells (NPCs) deprived of growth factors in culture; NPCs were cultured in 96-well plates with or without B27 and bFGF; survival was then compared in NPC cultures with or without hydrogel (A, B); NPC survival was measured by lactate dehydrogenase (LDH) released from dead cells and tetrazolium reduction by live cells; in stem cells cultured without B27 and beta fibroblast growth factor (bFGF), hydrogel substantially increased the survival as shown by as shown by the following: (A) decreased LDH (P = .007) and (B) increased tetrazolium reduction (P = .002; n = 3)
The transplant condition did not have an effect on the differentiation of stem cells. Most cells with or without hydrogel expressed the astrocyte marker GFAP. Few cells stained with DCX, a marker of immature or migrating neurons, or NG2, a marker for oligodendrocyte precursor cells (Figure 3).
Hyaluronan–Heparin–Collagen Hydrogel Diminishes the Infiltration of Microglia/Macrophage Cells Into the Graft
Implanted hydrogels can modify the local brain environment.21,39 To explore how the hydrogel promoted the survival of transplanted stem cells, angiogenesis, neurogenesis, reactive astrocytosis, and inflammatory cell infiltration of the transplant site and stroke cavity were compared between the cells-only and cells + hydrogel groups. There was no significant difference in angiogenesis or neovascularization as measured by peri-infarct PECAM-positive vessel length in transplanted cells with or without gel (1.20 ± 0.15 mm without hydrogel vs 1.56 ± 0.35 mm with hydrogel; P = .22; Figure 3A). Peri-infarct reactive astrocytosis, quantified by GFAP immunostaining, was similar between the transplanted cells with or without hydrogel (ratio of optical density [OD] between peri-infarct and contralateral area was 5.63 ± 1.34 without hydrogel vs 5.82 ± 0.68 with hydrogel; P = .92; Figure 3B). However, there was a significant decrease in activated microglia/macrophages that infiltrated the graft site as indicated by Iba1 staining (Figures 3C and 3D; P = .004). The gel formed a zone around the transplanted cells in which activated microglia/macrophages appeared to be excluded (Figure 3C).
Hyaluronan–Heparin–Collagen Hydrogel Supports the Survival of NPCs Deprived of Growth Factors in Culture
The above data indicate a reduced inflammatory cell infiltration into the graft site as a mechanism of increased survival after stroke. However, direct cell-contact-mediated survival effects between hydrogels and stem/progenitor cells have been described.17 To examine whether the hydrogel itself can directly support the survival of stem cells, cell survival was compared in culture with or without hydrogel. In the isolated in vitro condition, effects of immune, astrocyte, or endothelial interactions are removed, and NPC survival relates only to the presence or absence of hydrogel. Stem cell survival was tested under conditions of growth factor and nutritional support and under conditions of stress induced by growth factor and nutrition withdrawal to mimic the initial transplant state. In stem cells cultured with nutrient and growth factor support, the hydrogel modestly but significantly increased survival. In stem cells cultured without such support, the hydrogel substantially increased the survival, as shown by decreased LDH and increased tetrazolium reduction (Figures 5A and 5B; P = .007 and .002, respectively).
Figure 5.
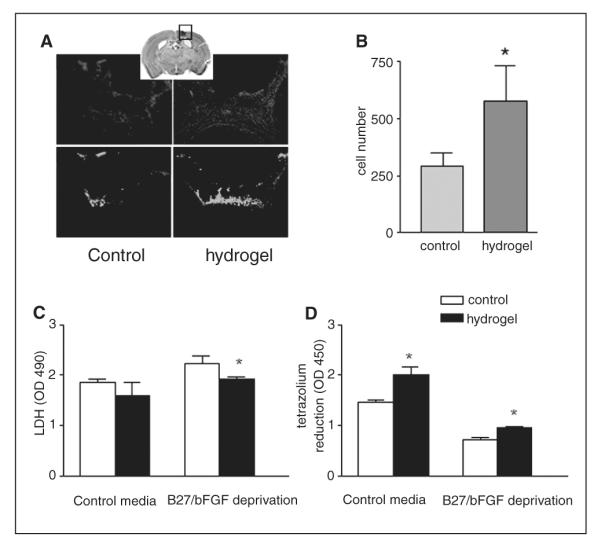
Hyaluronan–heparin–collagen hydrogel promotes survival of embryonic stem neural progenitor cells (ES-NPCs) transplanted into the infarct cavity after stroke and in culture: (A) 100 000 ES-NPCs were transplanted into the infarct cavity 7 days after stroke with or without hydrogel (n = 6 each group); 14 days after transplantation, transplanted cells were identified by the immunostaining of green fluorescent protein (GFP), and the infarct cavity was identified by counterstaining with DAPI (′,6-diamidino-2-phenylindole). (B) An average of 300 cells in the cells-only group survived, and an average of 600 cells in the hydrogel group survived, which is a statistically significant 2-fold expansion in cell survival (P = .002); ES-NPCs were cultured in 96-well plates with or without B27 and beta fibroblast growth factor (bFGF); survival was then compared in NPC cultures with or without hydrogel; ES-NPC survival was measured by LDH released from the dead cells, and tetrazolium reduction by live cells; in stem cells cultured without B27 and bFGF; hydrogel substantially increased the survival, as shown by as shown by the following: (C) decreased LDH (P = .005) and (D) increased tetrazolium reduction (P = .004; n = 3)
Hyaluronan–Heparin–Collagen Hydrogels Promote Survival of ES-NPCs Transplanted Into the Infarct Cavity After Stroke and in Culture
Neuronal progenitor cells derived from ES cells possess a nearly unlimited self-renewal capacity and constitute an attractive source of cells for regenerative medicine.40 However, distinct NPCs exhibit varying properties in their interaction with hyaluronan hydrogels.37 We next examined whether hydrogel transplantation with ES-NPCs, as opposed to the mouse cortical NPCs above, promotes survival. Similar to NPCs derived from the embryonic cortex, the transplantation of ES-NPCs with hydrogel significantly increases the cell survival 2-fold (P = .002; Figures 6A and 6B). However, much fewer ES-NPCs survive overall compared with NPCs derived from the fetal cortex: 0.3% and 0.6% of transplanted cells survive with or without hydrogel, respectively, at 2 weeks after the transplant. Similar to the results from embryonic cortical NPCs, there is also a significant decrease of Iba1-positive cell infiltration into the graft with the hydrogel (31 ± 7/mm3 without hydrogel vs 19 ± 4/ mm3 with hydrogel; P = .003; Figure 4C). In cell culture, the hyaluronan–heparin–collagen hydrogel also significantly supports the survival of ES-NPCs. The pro-survival effect of the hydrogel is seen in conditions of nutritional and growth factor support as well as during stress, when nutritional and growth factor supplementation is withdrawn (Figures 6C and 6D). In terms of cell differentiation, unlike NPCs derived from the embryonic cortex, most ES-NPCs remained in an undifferentiated state with or without hydrogel. Cells did not stain for GFAP, DCX, or NG2 after being transplanted into the infarct cavity (Figures 4A and 4B).
Figure 6.
Differentiation of embryonic stem neural progenitor cells (ES-NPCs) transplanted into the infarct cavity after stroke and infiltration of microglia cells into graft: transplanted ES-NPCs were identified by green fluorescent protein (GFP) immunostaining; photos show triple staining for the astrocyte marker glial fibrillary acidic protein (GFAP) and immature migrating neuronal marker DCX (A) and the polydendrocyte or oligodendrocyte precursor marker NG2 (B); no double staining cells were found. (C) Active microglia/macrophages infiltrating the stem cell engraftment were significantly decreased with hydrogel (P = .0003)
Discussion
Stem cell transplantation is a promising therapy for stroke, given its success in preclinical studies. However, several limitations prevent translation from basic research to clinical application. These major limitations are the potential damage to healthy and regenerating brain tissue adjacent to the infarct core during transplantation and the limited stem cell survival. Here, we report that a brain-compatible hyaluronate-based hydrogel overcame these limitations by delivering stem cells directly into the infarct cavity and promoting the survival of stem cells in a normally hostile environment.
The hydrogel in the present study contains thiol cross-linked hyaluronan, heparin sulfate, and collagen. Hyaluronan is a major constituent of the brain extracellular matrix. It is biocompatible in CNS and other wound models, biodegradable, and immunologically neutral.41 Hyaluronan inhibits scar formation and promotes angiogenesis.42 In other applications, hyaluronan hydrogels induce neurite outgrowth,21 reduce glial scar formation,43 and promote synapse formation of cultured NPCs.44 The cell attachment properties of hyaluronan alone can be sparse; collagen (gelatin) promotes cell attachment and adds an additional substrate for cell migration.45 Heparin binds and stabilizes a large number of growth factors and is a key component in several stages of neuronal differentiation for NPCs.36
Hyaluronan–heparin–collagen hydrogels promoted the survival of NPCs derived from both the fetal cortex and ES cells. There was no effect on cellular differentiation or migration of the cells from the stroke site. The hydrogel effect appears to be at least 2-fold in the present study. First, hyaluronan–heparin–collagen hydrogels provide direct support to stem cells, which is seen in isolated cell culture in which only stem cells and hydrogel are present. Second, the hydrogel diminishes the inflammatory reaction around the graft. This is seen in the significant reduction in microglia/macrophage cells in the hydrogel location within the transplant site. The inflammatory response is linked to diminished stem cell survival after transplantation in stroke.40 Inflammation in stem cell transplants stems in large part from the foreign nature of the transplanted cells. In stroke treatments, stem cell transplants are either allogeneic in human transplants or, in experimental stroke studies, allogeneic or xenotransplants. The degree of inflammation in stem/progenitor transplants is less than in solid organ or bone marrow transplantation.40,46,47 However, inflammatory processes mediate a large component of transplant death, even in the presence of immunosuppression.48 The present data indicate that the microenvironment of the transplant influences immunogenicity, and hydrogels may control this without systemic drug administration.
Hyaluronan–heparin–collagen hydrogels did not modify gliosis, neovascularization, or the response of endogenous neural precursors after stem cell transplant. Other hyaluronan hydrogels can support angiogenesis and diminish glial scar formation after stroke and in spinal cord injury.21 In these cases, the hydrogel is often modified with laminin or laminin motifs.23,49 In these studies, the particular hydrogels in use were not in liquid form and had to be mechanically implanted into the injury site as a scaffold. These differences highlight an important aspect of the hydrogel approach. Hydrogels can be modified to present several different cell adhesion motifs within the matrix of a transplant, such as with laminin; can be mixed with proteins or drugs for slow release; and can be modified in consistency to accommodate brain injection.
Recently, 2 other applications of bioengineering technology have been applied to stem cell transplants with stroke. Stem/progenitor cells have been transplanted into the infarct cavity within poly(d,l-lactic acid-co-glycolic acid) and with Matrigel scaffolds.50,51 These studies provide important proof of concept that a modification of the stem/progenitor transplant environment at the time of transplantation can promote stem cell survival and or differentiation. As this field progresses, a key element in the next steps will be the hydrogel. The properties of the present hydrogel are advantageous for brain transplantation in stroke because they can be sourced or produced in precise quantities, unlike other hydrogels, such as Matrigel, which are derived from mouse sarcoma cells and contain varying amounts of growth factors, cytokines, and extracellular matrix molecules.52,53 Hyaluronan hydrogels,54 including the one used in the present study,42 can also be modified to release specific growth factors along with stem/progenitor transplantation, which may enhance survival and engraftment of the transplanted cells and directly modify the poststroke CNS environment. A combination of stem cell transplantation and drug/protein release in an injectable hydrogel form may hold great promise for promoting stem cell survival and engraftment in the brain. Modern neurosurgical operating rooms now routinely carry intraoperative MRI and stereotaxic guidance for such stroke brain injection approaches.24,25
A variety of types of stem cells have been tested in the experimental stroke.3 Our study uses both embryonic cortex–derived NPCs and ES cell–derived NPCs. A significant difference exists between these 2 cell types regarding survival and differentiation after transplantation. Many more NPCs derived from the embryonic cortex survive and differentiate in 2 weeks and exhibit markers for immature neurons and glia. However, though survival of ES-NPCs is potentiated by the hydrogel, they remain undifferentiated. The underlying mechanism responsible for this difference is not clear. Studies with in vitro cultures of hyaluronan-collagen hydrogels and different NPC subtypes have also shown distinct survival and differentiation effects on different NPC lines.37 With the availability of multiple types of stem/progenitor cells, such as NPCs, neural cell lines, blood, bone marrow, and adipose tissue-derived progenitor cells, it is important to determine which type of stem cell has an optimal effect in terms of stroke therapy. The present data underscore the importance of testing the efficacy of different types of stem cells in the same stroke models with a similar paradigm. Old age is known to be associated with poor recovery and exacerbated microglial and astrocyte response in stroke.55 Young adult animals were used in our transplantation study and indeed have been the focus of most of the preclinical transplant studies in this field. It is important to determine whether and how the environment of the aged brain affects the survival and differentiation of transplanted stem cells and whether this hydrogel has similar anti-inflammatory and pro-survival effects on transplanted stem cells in the aged brain.
Acknowledgments
Funding
This work was supported by the Dr Miriam and Sheldon G. Adelson Medical Research Foundation and CIRM grant RC1-111.
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, William M. Feinberg lecture: stroke therapy in the year 2025: burden, breakthroughs, and barriers to progress. Stroke. 2004;35:205–211. doi: 10.1161/01.STR.0000106160.34316.19. [DOI] [PubMed] [Google Scholar]
- 3.Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38(2 suppl):817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 4.Daadi MM, Lee SH, Arac A, et al. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 2009;18:815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- 5.Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 6.Xiao J, Nan Z, Motooka Y, Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005;14:722–733. doi: 10.1089/scd.2005.14.722. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 9.Lee ST, Chu K, Jung KH, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(pt 3):616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 10.Lappalainen RS, Narkilahti S, Huhtala T, et al. The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci Lett. 2008;440:246–250. doi: 10.1016/j.neulet.2008.05.090. [DOI] [PubMed] [Google Scholar]
- 11.Schwarting S, Litwak S, Hao W, Bahr M, Weise J, Neumann H. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke. 2008;39:2867–2875. doi: 10.1161/STROKEAHA.108.513978. [DOI] [PubMed] [Google Scholar]
- 12.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 14.Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mountz JM. Nuclear medicine in the rehabilitative treatment evaluation in stroke recovery: role of diaschisis resolution and cerebral reorganization. Eura Medicophys. 2007;43:221–239. [PubMed] [Google Scholar]
- 16.Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 2008;13:806–821. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- 17.Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15:467–480. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thonhoff JR, Lou DI, Jordan PM, Zhao X, Wu P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008;1187:42–51. doi: 10.1016/j.brainres.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian WM, Hou SP, Ma J, et al. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng. 2005;11:513–525. doi: 10.1089/ten.2005.11.513. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore JL, Yi X, Quan L, Kabanov AV. Novel nanomaterials for clinical neuroscience. J Neuroimmune Pharmacol. 2008;3:83–94. doi: 10.1007/s11481-007-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou S, Xu Q, Tian W, et al. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J Neurosci Methods. 2005;148:60–70. doi: 10.1016/j.jneumeth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Lapitsky Y, Kang CE, Shoichet MS. Accelerated release of a sparingly soluble drug from an injectable hyaluronan-methylcellulose hydrogel. J Control Release. 2009;140:218–223. doi: 10.1016/j.jconrel.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Wei YT, Tian WM, Yu X, et al. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2:S142–S146. doi: 10.1088/1748-6041/2/3/S11. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael ST. Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 2008;39:1380–1388. doi: 10.1161/STROKEAHA.107.499962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller CM, Vespa P, Saver JL, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69:441–446. doi: 10.1016/j.surneu.2007.12.016. discussion 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 27.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 28.Currle DS, Hu JS, Kolski-Andreaco A, Monuki ES. Culture of mouse neural stem cell precursors. J Vis Exp. 2007;(2):152. doi: 10.3791/152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JK, Park MS, Kim YS, et al. Photochemically induced cerebral ischemia in a mouse model. Surg Neurol. 2007;67:620–625. doi: 10.1016/j.surneu.2006.08.077. discussion 625. [DOI] [PubMed] [Google Scholar]
- 30.Lin TN, Sun SW, Cheung WM, Li F, Chang C. Dynamic changes in cerebral blood flow and angiogenesis after transient focal cerebral ischemia in rats: evaluation with serial magnetic resonance imaging. Stroke. 2002;33:2985–2991. doi: 10.1161/01.str.0000037675.97888.9d. [DOI] [PubMed] [Google Scholar]
- 31.Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86:1936–1943. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- 32.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai PT, Ohab JJ, Kertesz N, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schabitz WR, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 35.Strieth S, Eichhorn ME, Werner A, et al. Paclitaxel encapsulated in cationic liposomes increases tumor microvessel leakiness and improves therapeutic efficacy in combination with Cisplatin. Clin Cancer Res. 2008;14:4603–4611. doi: 10.1158/1078-0432.CCR-07-4738. [DOI] [PubMed] [Google Scholar]
- 36.Vazin T, Chen J, Lee CT, Amable R, Freed WJ. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem Cells. 2008;26:1517–1525. doi: 10.1634/stemcells.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brannvall K, Bergman K, Wallenquist U, et al. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J Neurosci Res. 2007;85:2138–2146. doi: 10.1002/jnr.21358. [DOI] [PubMed] [Google Scholar]
- 38.Wang TW, Spector M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009;5:2371–2384. doi: 10.1016/j.actbio.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 39.Ma J, Tian WM, Hou SP, Xu QY, Spector M, Cui FZ. An experimental test of stroke recovery by implanting a hyaluronic acid hydrogel carrying a Nogo receptor antibody in a rat model. Biomed Mater. 2007;2:233–240. doi: 10.1088/1748-6041/2/4/005. [DOI] [PubMed] [Google Scholar]
- 40.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release. 2000;69:169–184. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 42.Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25:2789–2798. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 43.Hou S, Tian W, Xu Q, et al. The enhancement of cell adherence and inducement of neurite outgrowth of dorsal root ganglia co-cultured with hyaluronic acid hydrogels modified with Nogo-66 receptor antagonist in vitro. Neuroscience. 2006;137:519–529. doi: 10.1016/j.neuroscience.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Ma W, Fitzgerald W, Liu QY, et al. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol. 2004;190:276–288. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Nakamura M, Okano H, Toyama Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen type-1 gel. Res Neurol Neurosci. 2007;25:109–117. [PubMed] [Google Scholar]
- 46.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65:452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 47.Koppula PR, Chelluri LK, Polisetti N, Vemuganti GK. Histocompatibility testing of cultivated human bone marrow stromal cells: a promising step towards pre-clinical screening for allogeneic stem cell therapy. Cell Immunol. 2009;259:61–65. doi: 10.1016/j.cellimm.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Buhnemann C, Scholz A, Bernreuther C, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129(pt 12):3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- 49.Cui FZ, Tian WM, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogel immobilized with RGD peptides for brain tissue engineering. J Mater Sci Mater Med. 2006;17:1393–1401. doi: 10.1007/s10856-006-0615-7. [DOI] [PubMed] [Google Scholar]
- 50.Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30:2985–2994. [Google Scholar]
- 51.Jin K, Mao X, Xie L, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats [published online ahead of print October 14, 2009] J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2009.219. doi:10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novikova LN, Mosahebi A, Wiberg M, Terenghi G, Kellerth JO, Novikov LN. Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation. J Biomed Mater Res. 2006;77:242–252. doi: 10.1002/jbm.a.30603. [DOI] [PubMed] [Google Scholar]
- 53.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Ventura C, Cavallini C, Bianchi F, Cantoni S. Stem cells and cardiovascular repair: a role for natural and synthetic molecules harboring differentiating and paracrine logics. Cardiovasc Hematol Agents Med Chem. 2008;6:60–68. doi: 10.2174/187152508783329975. [DOI] [PubMed] [Google Scholar]
- 55.Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC. The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res. 2007;4:216–227. doi: 10.2174/156720207781387213. [DOI] [PubMed] [Google Scholar]