Abstract
A liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI+-MS/MS) method for the analysis of the tobacco-specific carcinogens N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their glucuronides (total NNN and total NNAL) in human urine was developed. The method has excellent accuracy and intra-day and inter-day precision, and limits of quantitation of 0.015 and 0.075 pmol/ml urine, respectively, for total NNN and total NNAL. A unique aspect of this method is internal assessment of possible artifactual formation of NNN by inclusion of the monitor amine [pyridine-D4]nornicotine. We found that artifactual formation of NNN comprised only 2.5% of the measured amounts of total NNN in urine of cigarette smokers, under our conditions using ammonium sulfamate as an inhibitor of nitrosation. The method was applied to urine samples from cigarette smokers and e-cigarette users. Levels of total NNN and total NNAL in the urine of cigarette smokers averaged 0.060 ± 0.035 pmol/mL and 2.41 ± 1.41 pmol/mL urine, (N = 38), respectively, which were both significantly greater than in the urine of 27 e-cigarette users.
Keywords: Combined analysis, N′-nitrosonornicotine, NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, smokers’ urine, e-cigarette users’ urine, liquid chromatography-electrospray ionization-tandem mass spectrometry
Introduction
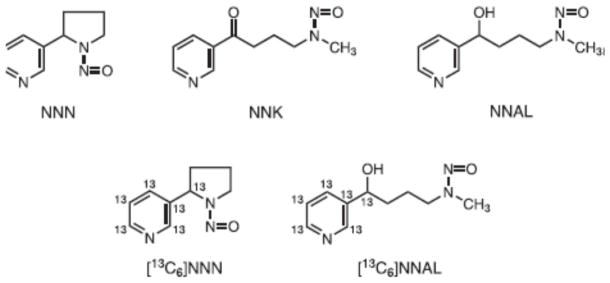
The tobacco-specific nitrosamines N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-(pyridyl)-1-butanone (NNK)(Figure 1) are present in all tobacco products and are considered “carcinogenic to humans” by the International Agency for Research on Cancer [1;2]. NNN and NNK are formed during the processing and curing of tobacco. During cigarette smoking, NNN and NNK are transferred through the smoke to both the oral tissues and lungs of smokers [3]. Each cigarette typically delivers about 100–150 ng NNN and 50–100 ng NNK to the smoker [4]. NNN causes oral cavity and esophageal cancer in rats, and tumors of the respiratory tract in mice, hamsters, and mink. NNK is a powerful organoselective lung carcinogen in rats, mice, and hamsters while also inducing tumors at other sites including the pancreas and nasal mucosa [5–7]. Because of their potent carcinogenicity and tobacco-specificity, NNN and NNK are widely acknowledged as important causes of cancer in tobacco users.
Figure 1.
Structures of NNN, NNK, NNAL, [13C6]NNN, and [13C6]NNAL.
Human exposure to NNN and NNK can be assessed by analysis of urine. Unchanged NNN as well as its pyridine-N-glucuronide are excreted in the urine [8–12]. Unchanged NNK is not generally detected in human urine. Rather, its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, Figure 1) and its O- and N-glucuronides are present in the urine of all smokers [3;13;14]. Total NNN and total NNAL (the sum of the free compounds and their glucuronide metabolites) are useful biomarkers of NNN and NNK exposure. Total NNAL in particular has been quantified in thousands of urine samples from smokers [3]. Total NNN and total NNAL are also risk biomarkers; levels of urinary total NNN have been strongly related to the risk of esophageal cancer and total NNAL to the risk of lung cancer in nested case-control studies carried out within a prospective epidemiology study in Shanghai [15–17].
Accurate assessment of total NNN and total NNAL is critical in cancer prevention strategies related to tobacco products. A reliable combined assay for quantifying these metabolites in human urine would therefore be an important addition to a panel of carcinogen exposure and cancer risk biomarkers. Our group was the first to describe an assay for free NNN and its glucuronide, as well as free and glucuronidated N′-nitrosoanabasine (NAB) and N′-nitrosoanatabine (NAT) in human urine [8]. The levels of total NNN were compared to those of total NNAL, determined separately. Two research groups have subsequently described combined assays for total NNAL, total NNN, total NAB, and total NAT in human urine [10;11;18]. A method for total NNN has also been briefly summarized [19].
The two studies by Kavvadias and co-workers mention the problem of potential artifactual formation of NNN, but this was not addressed in the study by Xia et al. We are aware from years of experience in the trace analysis of NNN that artifact formation can present problems because all tobacco products contain nornicotine, which is readily transferred to the saliva and urine of smokers and can be easily nitrosated to form NNN [20]. We have addressed and excluded this problem in our previous studies [8;12;16;21–24]. However, one must be continually aware of the potential for artifactual formation of NNN, particularly when low levels such as those expected in the urine of e-cigarette users are being analyzed. Therefore, in the study described here for analysis of total NNN and total NNAL in urine, we carefully monitored for artifactual formation of NNN by addition of [pyridine-D4]nornicotine to each urine sample. We have applied our method to the analysis of total NNN and total NNAL in the urine of cigarette smokers and users of e-cigarettes. The use of e-cigarettes has increased dramatically while they remain completely unregulated and little information is available regarding their toxicological effects [25–27]. It is possible that NNN could be formed endogenously in e-cigarette users by the reaction of nornicotine, a metabolite and common contaminant of nicotine, with salivary nitrite.
Materials and methods
Materials
We purchased [pyridine-D4]NNN and [13C6]NNN from Cambridge Isotope Laboratories (Andover, MA), while [pyridine-D4]nornicotine, NNN, NNAL, and [13C6]NNAL (Figure 1) were obtained from Toronto Research Chemicals (Ontario, Canada). Recombinant β-glucuronidase (catalog # G8295) was purchased from Sigma-Aldrich (Milwaukee, WI). Phosphate buffered saline was procured from Invitrogen (Grand Island, NY). All other chemicals were from Sigma-Aldrich, Fisher Scientific (Fairlawn, NJ), or Alfa Aesar (WardHill, MA). True Taper® 96-well plates were procured from Analytical Sales & Services (Pompton Plains, NJ) and silicone cap mats required to cover the 96-well plates were from Phenomenex (Torrance, CA). Five ml Biotage Isolute Supported Liquid Extraction+ (SLE+) diatomaceous earth-based liquid-liquid extraction cartridges were obtained from Biotage (Charlotte, NC) and Oasis MCX 60 mg, 60 μm solid-phase extraction 96-well plates were from Waters (Milford, MA). Strata SI-1 Silica (55 μm, 70 A, 100 mg) 96-well SPE plates were purchased from Phenomenex (Torrance, CA). A Cerex 96-well positive pressure processor (Chromtech, Apple Valley, MN) and an Eppendorf multi-channel pipette were used during sample processing.
Urine samples
The urine samples used in this work were obtained from ongoing studies of the University of Minnesota Tobacco Research Programs, approved by the University of Minnesota Institutional Review Board. The validated method was applied for analysis of total NNN and total NNAL in urine samples from 27 e-cigarette users and 38 cigarette smokers. The urine samples from the e-cigarette users have been previously analyzed and reported by our group [27]. One sample with a relatively high NNAL level of 0.953 pmol/ml was excluded from this secondary analysis. The urine samples from cigarette smokers were baseline samples from clinical studies on tobacco harm reduction.
Combined analysis of total NNN and total NNAL in urine
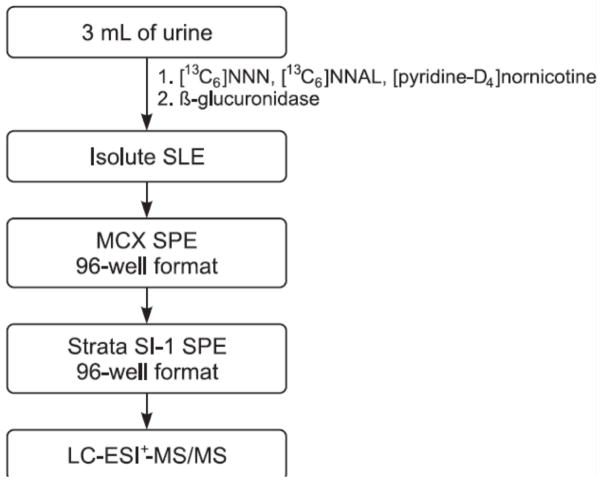
The urine samples, which had been kept frozen at −20 °C, were thawed by allowing them to stand at 4 °C overnight the day before the experiment. Three mL aliquots of urine or H2O blanks were added to 10 mL disposable glass centrifuge tubes with screw caps (Fisher Scientific). Milli-pure water (Milli-Q Advantage A10 Ultrapure water purification system, EMD Millipore, Billerica, MA) was used for preparation of all solutions. [13C6]NNN (1.09 pmol) and [13C6]NNAL (0.93 pmol) in 50 μL 1X phosphate buffered saline (1.05 mM KH2PO4, 155.2 mM NaCl, 2.96 mM Na2HPO4, pH 7.4) were added to each of the tubes containing urine along with 50 μL of 0.1 mg/μL ammonium sulfamate, 16.9 nmol [pyridine-D4]nornicotine, 400 μL of 10X phosphate buffer and 8000 units of β-glucuronidase in 80 μL of 1X phosphate buffered saline, pH 7.4. The tubes were incubated in a shaking water bath at 37 °C overnight. The amount of β-glucuronidase used was previously shown to be sufficient for complete hydrolysis [11].
The mixtures in the tubes were transferred onto 5 mL Biotage Isolute SLE+ cartridges. The aqueous solutions were applied, pushed past the hydrophobic frits with slight N2 pressure, and allowed to absorb into the diatomaceous earth for 10 min. Each cartridge was eluted 3 times with 6 mL CH2Cl2 and once with 2 mL CH2Cl2 and all the eluents were collected through gravity in 15 mL glass tubes. The combined eluents in each tube were mixed with 50 μL of 0.1 mg/μL ammonium sulfamate, then dried under vacuum in a SpeedVac® at room temperature for 1.5–2 h.
The dried samples were reconstituted in 1 mL of 1N HCl by sonication for 15 min and purified by a second solid-phase extraction using 60 mg Oasis MCX 96-well plates. The MCX plates were equilibrated by successive washings with 2 mL CH3OH and 2 mL of H2O. The reconstituted samples were then added to the MCX plates and allowed to absorb into the matrix. The MCX plates were then sequentially washed with 2 mL each of 1N HCl, CH3OH, and 1 mL of 90:5:5/H2O:CH3OH:NH4OH (v/v/v). The analytes were collected by elution with two 0.8 mL portions of 15:85:5/H2O:CH3OH:NH4OH (v/v/v) into a 2 mL square bottomed-well plate. Then 50 μL of 0.1 mg/μL ammonium sulfamate was added to each well and the plate was dried under vacuum in a SpeedVac® overnight. Samples were stored at −20 °C until the third solid-phase extraction on a Strata SI-1 Silica (55 μm, 70A, 100 mg) 96-well plate. To the dried samples were added 100 μL of tetrahydrofuran and the samples were sonicated for 15 min followed by addition of 900 μL of CHCl3. The resulting mixture was sonicated for 5 min. The Strata SI-1 96-well plate was equilibrated with 2 mL of CHCl3 after which the samples were loaded and allowed to absorb onto the silica. The plates were then serially washed with 1 mL each of CHCl3 and 50:50/hexane:EtOAc (v/v). The analytes were eluted with two 0.8 mL portions of 4:96/CH3OH:EtOAc into a True Taper® 96-well plate, with each well containing 10 μL of 0.1 mg/μL ammonium sulfamate. The plate was dried in a SpeedVac® for 1–2 h, and the samples were dissolved in 30 μL of 5 mM NH4OAc followed by sonication for 10 min. Eight μL of each sample was analyzed by LC-ESI+-MS/MS.
LC-ESI+-MSMS Analysis of NNAL and NNN
Samples were analyzed by capillary HPLC-ESI+-MS/MS with an Ultra triple quadrupole mass spectrometer (Thermo Scientific, Pittsburgh PA) interfaced with a Waters Nano Acquity HPLC system (Waters, Milford, MA). Chromatographic separation was achieved using a Luna C18 (2) 5μ, 150 × 0.5 mm column (Phenomenex) equipped with a pre-filter and eluted at a flow rate of 10 μL/min at 40 °C. The column was eluted with 25% CH3OH in H2O for 13 min for each run. The column was washed with 99% CH3OH for 5 min after every 10 samples. During sample analysis, the flow was diverted from the mass spectrometer for the first 6 min, then to the mass spectrometer from 6 – 13 min, then back to waste. NNAL and [13C6]NNAL eluted at 8.65 min while NNN and [13C6]NNN eluted at 9.22 min. The transitions monitored were m/z 178.12 → m/z 148.12 for NNN, m/z 184.12 → m/z 152.12 for [13C6]NNN, m/z 210.15 → m/z 93.17 for NNAL, m/z 216.15 → m/z 98.17 for [13C6]NNAL. The transition m/z 182.12 → m/z 154.12 was monitored for [pyridine-D4]NNN that could result from artifactual formation of [pyridine-D4]NNN due to nitrosation of [pyridine-D4]nornicotine added to each sample before processing. The mass spectrometer was tuned using [pyridine-D4]NNN. Typical tube lens offset values were 63 V for NNN and 65 V for NNAL. A capillary temperature of 270 °C and spray voltage of 2.7 kV was used. N2 was the sheath gas (35 units) with a skimmer offset of 10. Quantitative analyses were conducted in the SRM mode, with collision energy of 11 V for NNN and 20 V for NNAL. Ar was used as the collision gas with a pressure of 0.9 mTorr. The scan width was m/z 0.2 and the scan time was 0.125 sec. Quadrupole resolution was achieved with Q1 set at m/z 0.5 and Q3 set at m/z 0.7.
Calibration standards and quality control samples
For preparation of calibration standards, urine samples (100 ml each) from five non-smokers, which we had shown were free of NNAL or NNN, were mixed to form a pooled sample. NNAL was added in the range 0.075 – 3 pmol/ml urine and NNN was added in the range 0.015 – 0.3 pmol/ml, as summarized in Table 1. For quality control samples, we pooled urine samples from 8 individual smokers with no added levels of NNAL or NNN. The smokers smoked 15.4 ± 6.1 cigarettes per day.
Table 1.
Accuracy and precision of the combined assay for NNN and NNAL in urinea
| Nominal concentration (pmol/ml) | Intra-day
|
Inter-day
|
||||
|---|---|---|---|---|---|---|
| Foundb (pmol/ml) | % RSD | % Deviation from nominal | Foundc (pmol/ml) | % RSD | % Deviation from nominal | |
| NNN | ||||||
| 0.015 | 0.0161 | 10 | 7.4 | 0.0165 | 16 | 9.8 |
| 0.040 | 0.0396 | 5.4 | −1.0 | 0.0400 | 14 | −0.1 |
| 0.100 | 0.0963 | 3.3 | −3.7 | 0.0965 | 15 | −3.5 |
| 0.300 | 0.290 | 1.9 | −3.4 | 0.291 | 16 | −3.1 |
| NNAL | ||||||
| 0.075 | 0.093 | 3.4 | 24 | 0.0897 | 11 | 20 |
| 0.200 | 0.210 | 2.5 | 4.8 | 0.207 | 11 | 3.4 |
| 1.00 | 1.03 | 2.4 | 2.7 | 1.003 | 12 | 0.30 |
| 3.00 | 3.06 | 2.1 | 2.1 | 3.03 | 12 | 1.0 |
Four different amounts of NNN or NNAL were added to pooled urine samples from 5 non-smokers and the analysis was performed 6 times on one day (intra-day) or 6 times on each of 3 days (inter-day).
mean of 6 determinations at each level
mean of 18 determinations, 6 at each level on 3 separate days
Accuracy and precision
The calibration standards were analyzed 6 times on each of 3 days, and deviation from nominal concentrations (accuracy) and percent relative standard deviation (precision), both intra-day and inter-day, were calculated.
Statistical analysis
The comparison of total NNAL and total NNN levels in the urine of e-cigarette users vs. cigarette smokers was performed essentially as previously described [27].
Results
The analytical method is summarized in Scheme 1. [13C6]NNN and [13C6]NNAL were used as internal standards and [pyridine-D4]nornicotine was added to monitor for potential artifactual formation of NNN, which would be detected as [pyridine-D4]NNN. The urine samples were partially purified by supported liquid-liquid extraction on diatomaceous earth cartridges, followed by successive solid-phase extraction on mixed mode cation exchange-reverse phase and silica 96-well plates. This yielded material that was analyzed by LC-ESI+-MS/MS for m/z 178.12 → m/z 148.12 (NNN), m/z 184.12 → m/z 154.12 ([13C6]NNN), m/z 210.15 → m/z 93.17 (NNAL), m/z 216.15 → m/z 98.17 ([13C6]NNAL), and m/z 182.12 → m/z 152.12 ([pyridine-D4]NNN).
Scheme 1.
Scheme for combined analysis of NNN and NNAL in human urine.
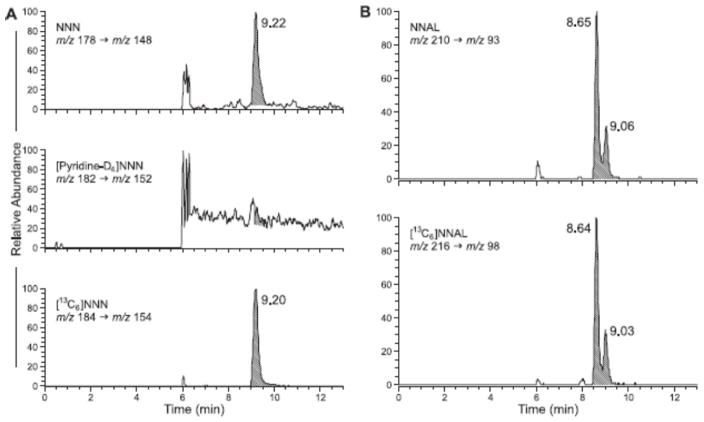
Typical LC-ESI+-MS/MS traces obtained upon analysis of a smokers’ urine sample are illustrated in Figure 2A, B. The column effluent was diverted to waste for the first 6 min, then to the mass spectrometer for 6–17 min, and subsequently back to waste. Figure 2A demonstrates a clear peak for NNN in the upper trace and [13C6]NNN in the lower trace while the middle trace shows no significant peak corresponding to [pyridine-D4]NNN, demonstrating that there was no detectable artifactual formation of NNN in this sample. Figure 2B shows the results of a typical analysis for NNAL. The upper panel is the analyte and the lower is the internal standard. The characteristic two peaks for NNAL, representing the E- and Z-rotamers, are observed in each trace. Limits of detection were 0.003 pmol/mL for NNN and 0.015 pmol/mL for NNAL while limits of quantitation were 0.015 pmol/mL for NNN and 0.075 pmol/mL for NNAL.
Figure 2.
LC-ESI+-MS/MS analysis of NNN in a smokers’ urine. The upper trace represents NNN in urine (shaded peak). The lower trace is the internal standard [13C6]NNN. The middle trace shows no significant formation of [pyridine-D4]NNN in the artifact test using added [pyridine-D4]nornicotine as the monitor amine. B. Analysis of NNAL in the same urine sample. The upper trace is NNAL, the E- and Z-rotamers eluting at 8.65 and 9.06 min, respectively, while the lower trace is the internal standard [13C6]NNAL. The column effluent was diverted to waste for the first 6 min, then to the mass spectrometer.
Accuracy and precision are summarized in Table 1. Intra-day precision ranged from 1.9 – 10% for NNN and 2.1 – 3.4% for NNAL, while intra-day deviations from the nominal added amounts ranged from −3.4 to 7.4% for NNN and 2.1 to 24% for NNAL. Inter-day precision ranged from 14 – 16% for NNN and 11 –12% for NNAL, and deviations from nominal ranged from −3.1 to 9.8% for NNN and 0.3 to 20% for NNAL. For the quality control samples (N = 21), intra-day precision was 8.6% for NNN and 2.8% for NNAL while the inter-day values were 11.8% for NNN and 2.9% for NNAL. The analytes were previously shown to be stable in urine under short term (24 h, 21 °C) and long term storage conditions (8 months, −20 °C), and under freeze/thaw cycles and autosampler conditions [10].
We examined artifactual formation of NNN by adding [pyridine-D4]nornicotine to each urine sample. The conversion of [pyridine-D4]nornicotine to [pyridine-D4]NNN in 18 urine samples averaged 0.00030 ± 0.00015%. Since we also had nornicotine measurements for these same urine samples, averaging 598 ± 301 pmol/mL, we were able to determine that the average amount of artifactual formation of NNN was 0.0018 ± 0.0011 pmol/mL. This was 2.5% of the average measured NNN (0.071 pmol/mL) in these samples.
We applied this method for a comparison of total NNN and total NNAL levels in the urine of e-cigarette users versus cigarette smokers. Demographics of the 27 e-cigarette users have been described [27]. Among the 38 cigarette smokers, 12 were female. The average age of the cigarette smokers was 42 ± 12 years and they smoked 18 ± 8.9 cigarettes per day. Total NNN was below the limit of detection in 21 of the 27 e-cigarette users. The geometric mean level of total NNN in these 27 subjects was 0.0055 pmol/mL, calculated using one half the limit of detection for the samples in which NNN was not detected. Total NNAL was below the limit of detection in 17 of the 27 e-cigarette users, and the geometric mean level of total NNAL was 0.024 pmol/mL. Levels of total NNN and total NNAL in the urine of the 38 cigarette smokers analyzed in this study were 0.060 ± 0.035 pmol/mL and 2.41 ± 1.41 pmol/mL, respectively, significantly greater than the amounts in the urine of e-cigarette users (p<0.001).
Discussion
We present a validated method for the combined determination of the important tobacco-specific nitrosamines total NNN (the sum of free NNN and its N-glucuronide) and total NNAL in human urine. The method takes advantage of 96-well technology and can potentially be applied to large numbers of urine samples being generated in ongoing clinical and epidemiologic studies. A significant advantage of this method is an integrated test for artifactual formation of NNN, which can be a vexing problem when low levels of this analyte are being quantified, as in the users of e-cigarettes reported here.
Mirvish was apparently the first to introduce the concept of a monitor amine, cis-2,6-dimethylmorpholine, to assess potential artifactual formation of nitrosamines during analysis [28]. This is particularly important when the precursor amine in question – in this case nornicotine - is readily nitrosated as we have shown in our previous studies [20;21], and when the expected amounts of nitrosamine detected - in this case NNN - may be quite low, as in e-cigarette users. Our initial unpublished analyses of the urine of e-cigarette users showed sporadic, poorly reproducible, and relatively low levels of total NNN, which we attribute to uncontrolled artifact formation resulting from nitrosation of nornicotine via an unknown source of nitrite or nitrogen oxides. This problem has been essentially eliminated by including ammonium sulfamate, a known inhibitor of nitrosation, in each step of the analysis. Thus, amounts of NNN formed by nitrosation of nornicotine during analysis by the method described here were only 2.5% of the NNN present in the samples. In contrast to the facile formation of NNN from nornicotine, we have never observed evidence for significant artifactual formation of NNAL during analysis.
This is the first study to report levels of NNN in the urine of e-cigarette users. The levels detected were significantly lower than the amounts of 0.060 pmol/mL urine which we found in cigarette smokers. This latter value is consistent with previous reports in the literature, which range from 0.023–0.12 pmol/mL urine [8;9;19;23;29]. The low amounts found in the urine of e-cigarette users could come from contamination of e-liquid nicotine with small amounts of NNN. Replacement liquids were reported to contain an average of 4 μg/L NNN while the amount in vapor per 15 puffs of an e-cigarette was reported to be 0.08 – 0.43 ng (0.45 – 2.43 pmol) [30;31]. This is in contrast to NNN amounts of 100–150 ng in the smoke of a single cigarette [32]. Another route to urinary NNN in e-cigarette users would be its endogenous formation from nornicotine. Etter et al reported the presence of nornicotine, up to 0.1% of nicotine content, in some refill liquids [33]. This nornicotine could be easily nitrosated in a user’s saliva, stomach, or urine - a scenario which is quite similar to the endogenous formation of NNN in oral nicotine replacement product users, or in the saliva of people who use tobacco products [12;21].
We previously reported levels of total NNAL in the urine of these e-cigarette users, determined using an LC-ESI-MS/MS method for NNAL only [27]. The data we obtained using the combined method reported here are fully in agreement with the previous study, which reported a geometric mean of 0.02 pmol/mL. Further, we replicated the relatively high values seen in 3 of the e-cigarette users: 0.613, 0.261, and 0.789 pmol/mL in the previous study compared to 0.732, 0.271, and 0.908 pmol/mL, respectively, in the current study. Overall, our results demonstrate that urinary levels of both total NNN and total NNAL are significantly lower in e-cigarette users than in smokers, although the relatively high levels of total NNAL in 3 e-cigarette users are a cause for concern.
In summary, the method described here for the combined analysis of total NNN and total NNAL in human urine is accurate, precise, and suitable for the analysis of low levels of NNN without appreciable artifact formation. The method has been validated and applied to urine samples from e-cigarette users and cigarette smokers. We expect this method to be applicable to large numbers of samples generated in ongoing clinical studies.
Highlights.
Urinary total NNN and total NNAL are important human carcinogen biomarkers.
An LC-ESI+-MS/MS method was developed for their combined analysis in human urine.
Use of monitor amine [pyridine-D4]nornicotine assessed artifact formation.
Smokers had significantly higher levels of total NNN and NNAL than e-cigarette users.
Acknowledgments
This study was supported by the U.S. National Institutes of Health CA-81301, U54 DA-031659, and U19 CA-157345. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, funded in part by Cancer Center Support Grant CA-77598. We thank Peter Villalta and Xun Ming for their valuable help with mass spectrometry. Nornicotine data were kindly provided by the laboratory of Professor S.E. Murphy. We thank Bob Carlson for editorial assistance.
Abbreviations
- NNN
N′-nitrosonornicotine
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- SLE
supported liquid extraction
- SPE
solid-phase extraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hecht SS, Hoffmann D. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. IARC; Lyon, FR: 2007. pp. 41–583. [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht SS. Cancer Prev Res. 2014;7:639–647. doi: 10.1158/1940-6207.CAPR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Regul Toxicol Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 6.Balbo S, James-Yi S, Johnson CS, O’Sullivan G, Stepanov I, Wang M, Bandyopadhyay D, Kassie F, Carmella S, Upadhyaya P, Hecht SS. Carcinogenesis. 2013;34:2178–2183. doi: 10.1093/carcin/bgt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balbo S, Johnson CS, Kovi RC, James-Yi SA, O’Sullivan MG, Wang M, Le CT, Khariwala SS, Upadhyaya P, Hecht SS. Carcinogenesis. 2014;35:2798–2806. doi: 10.1093/carcin/bgu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanov I, Hecht SS. Cancer Epidemiol Biomarkers Prev. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 9.Kavvadias D, Scherer G, Cheung F, Errington G, Shepperd J, McEwan M. Biomarkers. 2009;14:547–553. doi: 10.3109/13547500903242883. [DOI] [PubMed] [Google Scholar]
- 10.Kavvadias D, Scherer G, Urban M, Cheung F, Errington G, Shepperd J, McEwan M. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1185–1192. doi: 10.1016/j.jchromb.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Xia B, Xia Y, Wong J, Nicodemus KJ, Xu M, Lee J, Guillot T, Li J. Biomed Chromatogr. 2014;28:375–384. doi: 10.1002/bmc.3031. [DOI] [PubMed] [Google Scholar]
- 12.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami DK, Hecht SS. Cancer Res. 2009;69:8236–8240. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. Chem Res Toxicol. 2013;26:1209–1217. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmella SG, Akerkar S, Hecht SS. Cancer Res. 1993;53:721–724. [PubMed] [Google Scholar]
- 15.Yuan JM, Gao YT, Murphy SE, Carmella SG, Wang R, Zhong Y, Moy KA, Davis AB, Tao L, Chen M, Han S, Nelson HH, Yu MC, Hecht SS. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan JM, Butler LM, Stepanov I, Hecht SS. Cancer Res. 2014;74:401–411. doi: 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavvadias D, Scherer G, Cheung F, Errington G, Shepperd J, McEwan M. Biomarkers. 2009;14:547–553. doi: 10.3109/13547500903242883. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar M, Liu J, Koval T, Wang J, Feng S, Serafin R, Jin Y, Xie Y, Newland K, Roethig HJ. Nicotine Tob Res. 2010;12:105–116. doi: 10.1093/ntr/ntp183. [DOI] [PubMed] [Google Scholar]
- 20.Mirvish SS, Sams J, Hecht SS. J Natl Cancer Inst. 1977;59:1211–1213. doi: 10.1093/jnci/59.4.1211. [DOI] [PubMed] [Google Scholar]
- 21.Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I. Nicotine Tob Res. 2013;15:591–595. doi: 10.1093/ntr/nts172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanov I, Carmella SG, Han S, Pinto A, Strasser AA, Lerman C, Hecht SS. Nicotine Tob Res. 2009;11:99–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Shields PG, Murphy SE, Stepanov I, Hecht SS. Addiction. 2010;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatsukami DK, Stepanov I, Severson H, Jensen JA, Lindgren BR, Horn K, Khariwala SS, Martin J, Carmella SG, Murphy SE, Hecht SS. Cancer Prev Res (Phila) 2015;8:20–26. doi: 10.1158/1940-6207.CAPR-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orr MS. Tob Control. 2014;23(Suppl 2):ii18–ii22. doi: 10.1136/tobaccocontrol-2013-051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callahan-Lyon P. Tob Control. 2014;23(Suppl 2):ii36–ii40. doi: 10.1136/tobaccocontrol-2013-051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BWS, Vogel RI, Thompson E, Murphy SE, Hatsukami DK. Nicotine Tob Res. 2015;17:704–709. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirvish SS, Issenberg P, Sams JP. In: ACS Symposium Series 174: N-Nitroso Compounds. Scanlan RA, Tannenbaum SR, editors. 1981. pp. 181–191. [Google Scholar]
- 29.Kavvadias D, Scherer G, Urban M, Cheung F, Errington G, Shepperd J, McEwan M. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1185–1192. doi: 10.1016/j.jchromb.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, III, Benowitz N. Tob Control. 2013;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Shin HS. J Chromatogr A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Regul Toxicol Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Etter JF, Zather E, Svensson S. Addiction. 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]