Abstract
A number of pathogenic species of basidiomycete fungi are either life-threatening pathogens of humans or major economic pests for crop production. Sensing the host is a key aspect of pathogen proliferation during disease, and signal transduction pathways are critically important for detecting environmental conditions and facilitating adaptation. This review focuses on the contributions of the cAMP/protein kinase A (PKA) signaling pathway in Cryptococcus neoformans, a species that causes meningitis in humans, and Ustilago maydis, a model phytopathogen that causes a smut disease on maize. Environmental sensing by the cAMP/PKA pathway regulates the production of key virulence traits in C. neoformans including the polysaccharide capsule and melanin. For U. maydis, the pathway controls the dimorphic transition from budding growth to the filamentous cell type required for proliferation in plant tissue. We discuss recent advances in identifying new components of the cAMP/PKA pathway in these pathogens and highlight an emerging theme that pathway signaling influences iron acquisition.
Keywords: cAMP/PKA pathway, pathogenesis, iron homeostasis, pH signaling
Introduction
As with all organisms, fungal pathogens have evolved sophisticated signaling pathways to perceive environmental conditions and respond appropriately. For pathogens, it is particularly important to mount a rapid and fitting response during the transition from the environment to host tissue. That is, pathogens must accurately perceive host conditions and adapt to differences in nutrient availability, physical conditions such as pH, oxygen, and temperature, and challenges posed by the host immune response. Perception and adaptation generally involve mitogen activated protein kinase (MAPK) pathways as well as the cyclic AMP (cAMP)/protein kinase A (PKA) pathway that plays a key role in adaptation to nutrient availability.
The core components of the cAMP/PKA pathway have been defined in some detail in a number of fungi with considerable information from the model yeast Saccharomyces cerevisiae (Thevelein and De Winde, 1999). In general, the pathway is activated in response to nutrients in the environment including glucose and amino acids that are perceived at the cell surface by G-protein coupled receptors (GPCRs). Detection of the signal results in activation of guanine nucleotide-binding proteins (G-proteins) leading to an increase in intracellular cAMP levels upon stimulation of adenylyl cyclase. The impact of elevated cAMP is to activate cAMP-dependent protein kinase A (PKA), which then phosphorylates downstream target proteins. These proteins include enzymes, structural proteins and transcription factors that carry out a myriad of responses as a result of signaling through the pathway. In fungal pathogens of animals and plants, these responses include morphological changes and the deployment of virulence factors (Mitchell and Dean, 1995; D’Souza et al., 2001; Leberer et al., 2001; Liebmann et al., 2004).
The connections between the cAMP/PKA pathway, adaptation to the host, and regulation of virulence have been particularly well studied in two species of pathogenic fungi in the basidiomycete group, Cryptococcus neoformans and Ustilago maydis. C. neoformans has a worldwide distribution and is found in soil, in association with trees and in pigeon droppings (Mitchell and Perfect, 1995; Choi et al., 2010). Inhalation of fungal cells from these sources leads to pulmonary infection that can spread to the central nervous system in the absence of immune containment. This fungus is an opportunistic pathogen that generally attacks people with a suppressed immune system due to HIV infection, cancer or transplantation (Park et al., 2009). C. neoformans is able to cause disease in immunocompromised patients because: 1) it can resist the elevated body temperature of mammalian hosts; 2) it produces a polysaccharide capsule that has immunomodulatory properties and; 3) it secretes laccases to the cell wall to form protective melanin (Kronstad et al., 2011b). C. neoformans also secretes enzymes such as urease that contribute to virulence (Kronstad et al., 2011b). Interestingly, the major virulence traits of the pathogen are regulated by cAMP/PKA signaling, a process that involves activation of adenylyl cyclase via binding of the G-protein (Gpa1) leading to conversion of ATP to cAMP. The pathway responds to nutrients including glucose and amino acids (Xue et al., 2006). The elevated level of cAMP promotes dissociation of the regulatory subunit (Pkr1) from the catalytic subunit (Pka1) of PKA. Defects in signaling components such as the G-protein (Gpa1), adenylyl cyclase (Cac1) or the catalytic subunit of PKA (Pka1) result in attenuated capsule elaboration, melanin formation and virulence (Fig. 1) (Alspaugh et al., 1997; D’Souza et al., 2001). In contrast, deletion of the gene for the regulatory subunit of PKA (PKR1) results in an enlarged capsule and hypervirulence (D’Souza et al., 2001). The pathway also regulates the formation of enlarged cells called titan cells and the process of mating (D’Souza et al., 2001; Zaragoza et al., 2010; Okagaki et al., 2011). The impact of PKA on these processes was recently demonstrated with strains in which the expression of PKA1 and PKR1 was controlled by the glucose-repressed and galactose-activated GAL7 promoter (Choi et al., 2012). This study revealed that induction of PKA1 expression in galactose resulted in enlarged capsule, cell and vacuole size, as well as increased ploidy. Additionally, the regulated strains were used to demonstrate a positive impact for Pka1 on extracellular protease activity, a negative impact on urease activity, and a requirement for balanced PKA expression to properly regulate melanization (Choi et al., 2012).
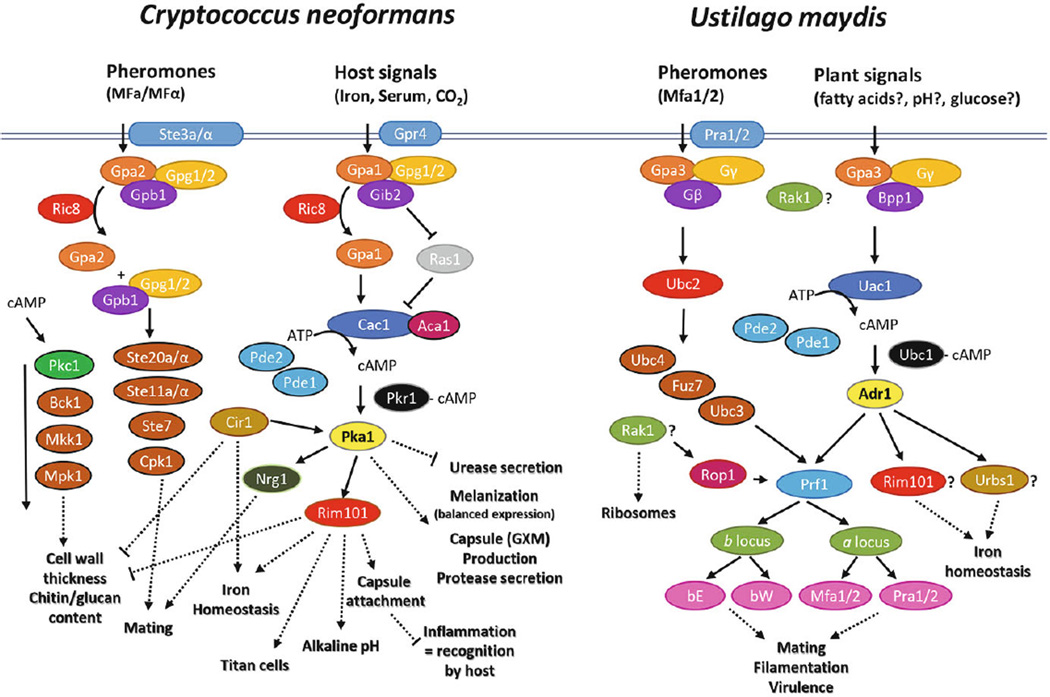
Fig. 1. The pheromone response MAPK and cAMP signaling pathways in C. neoformans and U. maydis.
The known input signals are indicated at the top of each pathway (at the level of the plasma membrane) and the key identified components of the pathways are shown below as ovals. The position of Rak1 in U. maydis is shown as influencing ribosomes and the transcription factor Rop1 (Wang et al., 2011). A speculative position for Rak1 at the level of G protein signaling is also shown for U. maydis and indicated with a question mark based on the findings in C. neoformans (Wang et al., 2014). In addition, Rim101 and Urbs1 are positioned as potential downstream targets of the protein kinase A catalytic subunit Adr1. Solid arrows indicate the flow of signals through the pathways with dotted arrows representing the influence on downstream target phenotypes. The representations of the signaling pathways are based on previous reviews and the recent findings (Regenfelder et al., 1997; Muller et al., 2004; Arechiga-Carvajal and Ruiz-Herrera, 2005; Klosterman et al., 2007; Kozubowski et al., 2009; Wang et al., 2011, 2014; Kronstad et al., 2011a; Kozubowski and Heitman, 2012; Donlin et al., 2014; Roach et al., 2014). Details on additional signaling components can be found in these reviews.
U. maydis is a pathogen of maize and teosinte, and infection of these hosts requires haploid, budding yeast cells of compatible mating types to fuse and establish dikaryotic filamentous cells that penetrate and colonize plant tissue (Banuett, 1995). Infection results in the formation of tumors at sites of infection along with stimulation of anthocyanin pigmentation. The dikaryon proliferates within tumor tissue and morphologically differentiates to form masses of abundant and highly melanized spores (teliospores). These spores disperse widely to allow colonization of new plants with subsequent germination to produce meiotic haploid progeny that can mate to reinitiate the disease cycle (Christensen, 1963; Snetselaar and Mims, 1992, 1994; Banuett and Herskowitz, 1996).
The morphological transition from budding to filamentous cells that underpins the virulence of U. maydis is regulated by mating and two conserved signaling pathways; a mitogen activated protein kinase (MAPK) signaling cascade and a cAMP/PKA pathway (Fig. 1) (Kronstad et al., 1998). These pathways sense environmental signals such as nutrient availability, the presence of lipids, air exposure, acidic pH, and the presence of cells of opposite mating types, to regulate the dimorphic transition (Bölker et al., 1995; Klose et al., 2004; Martínez-Espinoza et al., 2004). Mating in U. maydis is regulated by two unlinked mating loci, a and b (Bölker et al., 1992, 1995). The a locus encodes a pheromone (Mfa1/2) and a pheromone receptor (Pra1/2) for cell recognition involving a MAPK pathway (Fig. 1) (Bölker et al., 1992). The b locus encodes two homeodomain proteins (bE and bW) required for dikaryotic growth and completion of the life cycle (Kahmann et al., 1995; Kronstad and Staben, 1997). The MAPK pathway leads to the activation of the pheromone response factor Prf1 (Banuett and Herskowitz, 1994; Hartmann et al., 1996; Mayorga and Gold, 1999; Andrews et al., 2000). Prf1 is also regulated by the cAMP/PKA pathway thus highlighting the integration of signaling pathways and mating in the regulation of filamentous growth (Gold et al., 1994; Kaffarnik et al., 2003; Lee et al., 2003). For the cAMP/PKA pathway, the analysis of mutants with defects in signaling components indicates that high PKA activity leads to a budding phenotype, and low PKA activity results in filamentous growth.
In this review, we discuss recent findings related to the cAMP/PKA signaling pathway in C. neoformans and U. maydis, and point out similarities between the pathogens in the context of new components and shared downstream targets. In particular, there are intriguing connections between the cAMP/PKA pathway and other signaling pathways as well as shared regulatory outcomes involving iron acquisition. A number of excellent reviews are available to provide additional information on these pathogens and signaling mechanisms, as well as related topics (Kronstad et al., 2011a; Vollmeister et al., 2012; Coelho et al., 2014).
Association of RACK1-like proteins with the cAMP/PKA pathway
The Gib2 protein functions in cAMP signaling in Cryptococcus neoformans
Recent work revealed an interesting connection between a Gβ-like/RACK1 protein homolog designated Gib2 and cAMP/PKA signaling in C. neoformans. RACK1 (Receptor for Activated C Kinase 1) is a WD repeat protein that plays a large number of roles in eukaryotic cells including moving proteins within cells, anchoring proteins at specific locations and stabilizing protein activity (Adams et al., 2011). Additionally, RACK proteins interact with a large number of proteins including ribosomal proteins, proteins in the nucleus, and cell surface receptors. As mentioned above, transmembrane GPCRs transduce signals from outside the cell by binding ligands and causing activation of a canonical G-protein complex through dissociation of the Gα subunit from the Gαβγ complex. As a RACK1-like protein, Gib2 is a non-canonical Gβ-like protein that shares a seven WD-40 repeat motif and that can substitute for the Gβ subunit in the complex (Palmer et al., 2006; Adams et al., 2011). Like the conventional Gβ sub-unit (Gpb1), Gib2 interacts with Gα (Gpa1) and Gγ (Gpg1 and Gpg2) to form a complex (Fig. 1) (Palmer et al., 2006; Wang et al., 2014; Ero et al., 2015). Gpa1 is a key player in the cAMP/PKA signaling pathway which, as mentioned above, contributes to diverse phenotypes such as virulence, capsule formation, melanization, mating, titan cell formation, and secretion of specific enzymes such as protease and urease (Alspaugh et al., 1997; D’Souza et al., 2001; Alspaugh et al., 2002; Kronstad et al., 2011a). The defect in capsule formation and melanin production of the gpa1Δ mutant can be restored by addition of exogenous cAMP (Alspaugh et al., 1997).
The contribution of Gib2 to cAMP/PKA signaling in C. neoformans is complex. In addition to binding Gα (Gpa1) and Gγ (Gpg1 and Gpg2) proteins, Gib2 also interacts with a phosphodiesterase Pde2 and the small G-protein Ras1 (Fig. 1). Gpa1 and Ras1 both influence the activity of adenylyl cyclase (Cac1) to catalyze the synthesis of cAMP (Alspaugh et al., 2002). A cac1Δ mutant lacking adenylyl cyclase has the same phenotypes as a gpa1Δ mutant, while a ras1Δ mutant, in contrast, does not show defects in capsule formation and melanin production (Alspaugh et al., 2002). Ras1 does contribute to growth at 37°C (Alspaugh et al., 2000; Wang et al., 2014). In this context, a gib2Δ mutant also has a defect in growth at 37°C and is attenuated for virulence in mice, but the mutant is still able to produce capsule and melanin. Interestingly, Ras1 appears to negatively regulate the cAMP/PKA pathway in cells lacking Gpa1. Specifically, deletion of the RAS1 gene in the gpa1Δ mutant resulted in enhanced capsule formation. Furthermore, overexpression of GIB2 in the gpa1Δras1Δ double mutant allowed capsule formation. Adenylyl cyclase (Cac1) appears to be downstream of both Gib2 and Ras1 because disruption of CAC1 in strains lacking Gpa1, Gpa1 and Ras1, and Gpa1 and Ras1 with overexpression of GIB2 blocked both capsule and melanin formation. In general, the results support the novel finding that Ras1 plays a negative role in the cAMP/PKA pathway by regulating Cac1 activity (Wang et al., 2014). A negative role for Ras1 in the cAMP/PKA pathway was also suggested by the comparative transcriptional analysis of the impact of mutations in RAS1 and genes encoding components of the pathway (Maeng et al., 2010). Furthermore, Gib2 physically interacts with Ras1 and elevates cAMP through inhibition of the Ras1 influence on Cac1 (Wang et al., 2014). The scenario that emerges from these studies is one in which Gib2 causes elevated cAMP by preventing the negative influence of Ras1 on Cac1 when Gpa1 is absent. More recent work determined the structure of Gib2 and further characterized its role as an adaptor or scaffold protein that interacts with a large number of proteins (i.e., 55) in C. neoformans (Wang et al., 2014). These interactions include preferential association with ribosomal proteins and the translation machinery (Wang et al., 2014).
Recently, Gong et al. (2014) reported that the Ric8 protein (resistance to inhibitors of cholinesterase 8) is also involved in the cAMP/PKA pathway and pheromone signaling during mating in C. neoformans (Gong et al., 2014). Ric8 functions as a guanine nucleotide exchange factor (GEF) for activation of Gα (Gpa1) in the cAMP/PKA pathway and deletion of RIC8 results in capsule and melanin defects similar to those of the gpa1Δ mutant. A ric8Δ mutant is also attenuated for virulence in mice. Ric8 appears to function upstream of Gpa1 because expression of an activated allele of Gpa1 (GPA1Q284L) suppresses the phenotypes of the ric8Δ mutant (Fig. 1). Ric8 also interacts with the Gα protein Gpa2 that has a role in pheromone signaling and this is consistent with the ric8Δ mutant phenotype of reduced mating (Gong et al., 2014). In C. neoformans, pheromone exchange between mating partners activates the Gα protein Gpa2 by dissociating it from a Gβγ complex. The Gβ and Gγ subunits (Gpb1 and Gpg2) play central roles in mating because deletion of either gene causes sterility (Hsueh et al., 2007). The mating defect of the gpb1Δ mutant was not restored by addition of cAMP (Wang et al., 2000). In contrast, the mating defect of a gpa1Δ mutant can be suppressed by exogenous cAMP (Alspaugh et al., 1997; Wang et al., 2000). Activated Gpa2 relays the pheromone signal via the Ste20-mediated MAPK pathway to facilitate mating (Wang et al., 2000) (Fig. 1). Ric8 interacts with both Gpa1 and Gpa2 and establishes a connection between cAMP signaling via Gpa1 and pheromone signaling via Gpa1 and a MAP kinase pathway (Gong et al., 2014). A connection between cAMP signaling and another MAP kinase pathway for cell wall integrity has also been established recently in C. neoformans (Donlin et al., 2014) (Fig. 1). Overall, the recent studies with Gib2 and Ric8 add depth to our understanding of cAMP/PKA signaling at the level of the Gα protein Gpa1.
The Rak1 protein functions in cAMP and MAPK signaling in Ustilago maydis
A RACK1-like protein encoded by the RAK1 gene has also been characterized in U. maydis and found to indirectly interact with the cAMP/PKA pathway (Fig. 1) (Wang et al., 2011). The RAK1 gene was identified in a search of the U. maydis genome and found to complement the adhesive growth defect of an asc1Δ mutant lacking a RACK1 homolog in S. cerevisiae (Valerius et al., 2007; Coyle et al., 2009; Wang et al., 2011; Rachfall et al., 2013). Loss of RAK1 in U. maydis causes a variety of phenotypes including slow growth, sensitivity to cell wall stress, attenuated virulence and a reduction in the formation of dikaryotic hyphae upon mating. Rak1 influences the expression of genes involved in mating as revealed by a reduction in expression of the a and b mating-type genes in a rak1Δ mutant. A broader study of the impact of the RAK1 mutation on the transcriptome identified 164 up-regulated and 37 down-regulated genes relative to the wild-type strain. The down-regulated genes included components of a MAPK signaling pathway for mating including the pheromone gene MFA1, the pheromone receptor gene PRA1 and the genes for two high-mobility-group-domain transcription factors Prf1 and Rop1 (regulator of Prf1) (Kaffarnik et al., 2003; Brefort et al., 2005). Rop1 is a direct regulator of PRF1 expression and, interestingly, Prf1 is regulated at the post-transcriptional level by the cAMP pathway and the MAPK pathway. In particular, PKA phosphorylation pheromone-induced expression of the a and b mating-type genes (Kaffarnik et al., 2003). As in C. neoformans, Rak1 interacts with a large number (54) of proteins including 32 ribosomal proteins (Wang et al., 2011). Overall, a model emerges of Rak1 as a regulator that links the cAMP/PKA and MAPK pathways with a number of cellular processes including mating and translation in both fungi. Additional studies are needed to determine whether Rak1 participates in similar interactions with signaling pathway components as was found for Gib2 in C. neoformans.
Other roles for RACK1-like proteins in fungi: hints from S. cerevisiae
A RACK1-like protein, Asc1, was identified as a component of the 40S ribosomal subunit in S. cerevisiae and, as later found with Gib2 in C. neoformans, the protein interacts with adenylyl cyclase to reduce the production of cAMP upon glucose stimulation (Fig. 1) (Gerbasi et al., 2004; Valerius et al., 2007; Zeller et al., 2007). In this regard, it acts oppositely to the Gα protein Gpa2 in yeast that mediates the response to glucose by stimulating cAMP production (Colombo et al., 1998). Recent work examined the impact of an asc1Δ mutation on the proteome, transcriptome and various phenotypes (Rachfall et al., 2013). The proteome analysis revealed a substantial influence on proteins for energy metabolism including glycolysis, mitochondrial functions, oxidative stress and fermentation. Proteins for cell wall biogenesis and maintenance were also down-regulated in the asc1Δ mutant. The characterization of the transcriptome revealed Asc1 regulation of 80 genes that encoded functions for transposable elements and energy metabolism with the notable inclusion of proteins for glucose uptake and iron homeostasis. The latter group included functions for iron uptake such as the cell surface iron reductase Fre1 and several siderophore transporters. The phenotypes of the asc1Δ mutant are consistent with the proteome and transcriptome work because the mutant is susceptible to iron limitation, nitrosative stress, and agents that challenge cell-wall integrity. Asc1 also regulates the translation of the mRNAs for several transcription factors.
Given the collective information for S. cerevisiae, C. neoformans and U. maydis, the picture emerges of RACK1-like proteins as central regulators of signaling at the level of G proteins with an impact on signaling via the cAMP/PKA pathway and on downstream transcription factors. As discussed below, the connections with iron homeostasis revealed by the work on Asc1 may be relevant to virulence in fungal pathogens such as C. neoformans and U. maydis. In this regard, there is a need to test the role of Gib2 and Rak1 in iron homeostasis for these fungi.
Downstream targets of the cAMP/PKA pathway: Transcription factors and iron
The analysis of Asc1 in S. cerevisiae highlights connections between cAMP/PKA signaling and iron homeostasis that were previously identified by Robertson et al. (2000) in a transcriptome study of the role of each of the catalytic sub-units of PKA, Tpk1, Tpk2, and Tpk3. This analysis revealed that Tpk2 negatively regulates genes for iron uptake including ferric reductase genes, the iron permease and ferroxidase of the high affinity uptake system, siderophore transporters and other functions. For C. neoformans, there is a growing body of evidence that the cAMP signaling regulates a similar set of functions and that iron regulatory proteins may also influence expression of components of the cAMP/PKA pathway. This evidence is discussed below followed by information on similar connections in U. maydis.
Iron uptake and cAMP/PKA signaling in C. neoformans
A connection between cAMP signaling and iron regulation was initially identified in C. neoformans during a characterization of the major iron regulator, Cir1 (Jung et al., 2006). A transcriptome study that compared a cir1Δ mutant with the wild-type strain indicated that Cir1 exerted a positive regulatory influence on the GPCR Gpr4 under both low iron and iron-replete conditions. A further association between the cAMP/PKA pathway and iron uptake came from characterization of the SIT1 gene encoding a siderophore transporter in C. neoformans (Tangen et al., 2007). Deletion of the gene for the catalytic subunit of PKA resulted in elevated transcript levels for SIT1, a pattern reminiscent of the influence of Tpk2 on the expression of siderophore transporters in S. cerevisiae (Robertson et al., 2000; Tangen et al., 2007). In a further connection, the SIT1 gene is also regulated by the transcription factor Nrg1, a candidate phosphorylation target of PKA (Cramer et al., 2006). Similarly, the Alspaugh group discovered interactions between cAMP/PKA signaling, iron uptake and the response to pH as mediated by the transcription factor Rim101 (O’Meara et al., 2010).
The interaction of cAMP signaling and Rim101 provides an interesting view of the complexities of the regulation and integration of signals related to cryptococcal virulence (O’Meara et al., 2010). Rim101 is a Cys2His2 Zinc finger transcription factor that is activated by the alkaline pH-responsive Pal/Rim pathway in fungi to regulate adaptation to environmental conditions (Selvig and Alspaugh, 2011). Alkaline or neutral pH conditions result in activation of the pathway leading to proteolytic cleavage to activate Rim101. In C. neoformans, activation of Rim101 is required for elaboration of the polysaccharide capsule that is critical for virulence (O’Meara et al., 2010). Interestingly, the Rim101 protein contains a predicted consensus sequence for PKA phosphorylation and nuclear localization is influenced by both Pka1 and Rim20, a scaffold protein required for cleavage (O’Meara et al., 2010). Nuclear localization is a prerequisite for successful capsule formation. Further analysis revealed that Rim101 is specifically involved in capsule attachment to the cell wall rather than the production of capsule material (O’Meara et al., 2010, 2013). Therefore, part of the influence of PKA on capsule formation could be mediated by Rim101. In addition, Rim101 contributes to the formation of titan cells, a subpopulation of enlarged cells that is observed during pulmonary infection (Okagaki et al., 2011). Titan cell formation is also influenced by cAMP signaling (Choi et al., 2012; Zaragoza and Nielsen, 2013). Along with a larger size, typical titan cells exhibit a thick cell wall, and increased capsule size in wild-type cells, whereas the rim101Δ mutant exhibits a significant increase in cell wall thickness and altered composition without enlargement of the cells (O’Meara et al., 2013). This increase might enhance survival of the rim101Δ mutant within host tissue and, indeed, the mutant was found to be somewhat more virulent in mice when compared with wild type (O’Meara et al., 2010).
A role for Rim101 in the regulation of iron uptake and homeostasis was also revealed by the comparison of transcriptome profiles between a rim101Δ mutant and the wild-type strain (O’Meara et al., 2010). This experiment identified a subset of genes involved in iron uptake and homeostasis that showed down-regulated transcript levels in the rim101Δ mutant. The regulated genes encoded functions such as the cell wall mannoprotein Cig1, the siderophore transporter Sit1, the ferroxidase Cfo1, and the iron permease Cft1, which are responsible for heme uptake, siderophore uptake, and high-affinity iron uptake in C. neoformans, respectively. Reduced transcript levels of these genes were well correlated with the growth deficiency of the rim101Δ mutant in low-iron media (O’Meara et al., 2010). Furthermore, a series of recent studies showed that both the cig1Δ mutant and the rim101Δ mutant were defective in heme uptake and that Rim101 plays a role in heme utilization via the ESCRT machinery, although evidence for a Rim101-independent heme uptake pathway was also obtained (Cadieux et al., 2013; Hu et al ., 2015).
A recent comparative transcriptome analysis with pka1Δ and rim101Δ mutants also revealed that 1,077 genes are commonly regulated by Pka1 and Rim101, thus strengthening the position of Rim101 as a key downstream target of cAMP signaling (O’Meara et al., 2014). Transcripts for genes encoding cell wall biosynthesis and remodeling functions were prominent among the set regulated by both PKA and Rim101. In contrast, the transcript levels for some genes were distinctly regulated by only one factor. For example, the ENA1 gene encoding a sodium transporter was regulated specifically by Rim101 and this finding was consistent with the shared growth defects of ena1Δ and rim101Δ mutants on media with high salt or high pH (O’Meara et al., 2014). In this context, it has previously been shown that that the expression of ENA1 is induced in response to salt and osmotic stress, and that this response is dependent on the Hog1 MAPK pathway in C. neoformans (Jung et al., 2012). The transcriptome analysis of O’Meara et al. (2014) was also coupled with an examination of the binding of Rim101 to the promoters of regulated genes. These included genes for cell wall biosynthesis, the ENA1 gene, the HAPX gene (encoding a regulator of iron homeostasis), the CFT1 gene for the high-affinity iron permease, and the CTR4 gene encoding a copper transporter. Chromatin immunoprecipitation with a Gfp-Rim101 protein detected binding at the promoters of all of these genes.
Iron acquisition functions such as the high-affinity iron uptake system encoded by CFT1 and CFO1, and the heme uptake function of Cig1 are important for the virulence of C. neoformans in a mouse model of cryptococcosis (Jung and Kronstad, 2008; Jung et al., 2009; Han et al., 2012; Cadieux et al., 2013). A screen for functions needed for growth on heme revealed that proteins of the endosomal sorting complex required for transport (ESCRT) participate in iron acquisition, as well as in capsule formation and virulence (Hu et al., 2013, 2015). Interestingly, ESCRT functions are also required in fungi for the activation of Rim101 (Selvig and Alspaugh, 2011; Ost et al., 2015). In this context, mutants defective in ESCRT functions share a number of phenotypes with a rim101Δ mutant including impaired growth on heme as the sole iron source and a reduced capsule size (Hu et al., 2013, 2015). Most of the impact of the ESCRT machinery on capsule occurs through participation with PKA in the activation of Rim101. However, PKA appears to make a contribution to capsule elaboration in addition to its influence on Rim101 because expression of the N-terminal (activated) portion of the transcription factor only partially restores capsule in a mutant lacking both ESCRT function and the regulatory subunit of PKA. Also, the cAMP/PKA pathway regulates the expression of genes for capsule production (Pukkila-Worley et al., 2005). For iron acquisition, the ESCRT machinery make a contribution both through an influence of Rim101 activation and, independently, perhaps through endocytosis of heme (Hu et al., 2015).
Iron uptake and cAMP/PKA signaling in U. maydis
The analysis of the impact of cAMP/PKA signaling on iron uptake has not been studied to the same extent in U. maydis compared with C. neoformans. For example, Rim101 has been characterized in U. maydis but a functional analysis of the influence of the factor on iron uptake has not been performed (Arechiga-Carvajal and Ruiz-Herrera, 2005; Antonio et al., 2010; Franco-Frias et al., 2014). However, a transcriptome analysis of a rim101Δ mutant versus wild type does implicate Rim101 in the regulation of iron uptake functions (Franco-Frias et al., 2014). Specifically, the transcript level for the FER3 gene encoding a siderophore peptide synthetase was positively regulated by Rim101 while the transcripts for a siderophore transporter (FER7) and a ferric reductase (FRE4) were negatively regulated. In addition, the transcript for the GATA transcription factor Urbs1 that regulates siderophore biosynthesis was also positively regulated by Rim101 (2.5 fold). Urbs1 shows sequence similarity to the iron regulator Cir1 in C. neoformans (Jung et al., 2006). Thus, the connection between Rim101 and iron regulation that is observed in C. neoformans may also be present in U. maydis, although functional studies are needed to assess the ability of a rim101Δ mutant to grown under iron limited conditions.
Interestingly, the FER3 and FER7 genes that are regulated by Rim101 are found in one of three gene clusters that were previously shown to be regulated upon induction of the ADR1 gene encoding the catalytic subunit of protein kinase A (Eichhorn et al., 2006). The transcripts for the genes in the clusters were demonstrated to be down-regulated upon growth in iron-replete conditions, and a transcriptome analysis with an urbs1Δ mutant revealed an impact of the transcription factor on the transcript levels of the genes. Eichhorn et al. (2006) went on to assess the impact of a defect in the cAMP pathway (by deletion of the UAC1 gene encoding adenylyl cyclase) and exogenous cAMP on the expression of some of the genes in the clusters. Importantly, the expression analysis identified the FER2 gene, encoding a high-affinity iron uptake permease, as being completely dependent on an intact cAMP signaling pathway; deletion of this gene attenuated virulence in maize plants. Taken together, these studies link Rim101 to the regulation of iron uptake in U. maydis and reveal a definitive connection between cAMP signaling and iron in this pathogen. The results also suggest that the iron regulator Urbs1 may be a target of PKA phosphorylation (Eichhorn et al., 2006).
Conclusions
Recent studies on Gβ-like/RACK1 protein homologs in C. neoformans and U. maydis add depth to our understanding of the cAMP/PKA signaling pathway and its interconnections with MAPK signaling pathways. Importantly, detailed studies of the Gβ-like/RACK1 protein homolog Asc1 in S. cerevisiae support the linkage of the cAMP/PKA pathway with the control of iron uptake functions in the basidiomycete pathogens. This linkage is strengthened by emerging information on the pH-responsive transcription factor Rim101 and the iron regulators Cir1 and Urbs1 that may be targets of the cAMP/PKA pathway. These transcription factors integrate cAMP/PKA signaling with other functions including the pH response pathway and the MAPK pathways for pheromone response and cell wall integrity. There is a wealth of emerging systems biology information on the functions of transcription factors in C. neoformans and this work will likely support the identification of additional targets of cAMP/PKA signaling (Jung et al., 2015; Maier et al., 2015). Overall, these findings set the stage for future work to identify new signaling components including transcription factor targets as well the molecular mechanisms of regulation and cross talk.
Acknowledgements
Research in the authors’ laboratories is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research (CIHR), the National Institute of Allergy and Infectious Diseases (NIAID), and the National Research Foundation of Korea grant funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1010928 and NRF-2014-K2A2A2000677). J.W.K. is a Burroughs Wellcome Fund Scholar in Molecular Pathogenic Mycology.
References
- Adams DR, Ron D, Kiely PA. RACK1, a multifaceted scaffolding protein: Structure and function. Cell Commun. Signal. 2011;9:22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Cavallo LM, Perfect JR, Heitman J. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 2000;36:352–365. doi: 10.1046/j.1365-2958.2000.01852.x. [DOI] [PubMed] [Google Scholar]
- Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, Heitman J. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Crypto-coccus neoformans. Eukaryot. Cell. 2002;1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, Egan JD, Mayorga ME, Gold SE. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol. Plant-Microbe Interact. 2000;13:781–786. doi: 10.1094/MPMI.2000.13.7.781. [DOI] [PubMed] [Google Scholar]
- Antonio CJ, Lucila O, Miriam T, Scott G, José R. Functional analysis of the pH responsive pathway Pal/Rim in the phytopathogenic basidiomycete Ustilago maydis. Fungal Genet. Biol. 2010;47:446–457. doi: 10.1016/j.fgb.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Arechiga-Carvajal ET, Ruiz-Herrera J. The RIM101/pacC homologue from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot. Cell. 2005;4:999–1008. doi: 10.1128/EC.4.6.999-1008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu. Rev. Genet. 1995;29:179–208. doi: 10.1146/annurev.ge.29.120195.001143. [DOI] [PubMed] [Google Scholar]
- Banuett F, Herskowitz I. Identification of Fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 1994;8:1367–1378. doi: 10.1101/gad.8.12.1367. [DOI] [PubMed] [Google Scholar]
- Banuett F, Herskowitz I. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development. 1996;122:2965–2976. doi: 10.1242/dev.122.10.2965. [DOI] [PubMed] [Google Scholar]
- Bölker M, Genin S, Lehmler C, Kahmann R. Genetic regulation of mating and dimorphism in Ustilago maydis. Can. J. Bot. 1995;73:320–325. [Google Scholar]
- Bölker M, Urban M, Kahmann R. The a mating type locus of U. maydis specifies cell signaling components. Cell. 1992;68:441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- Brefort T, Muller P, Kahmann R. The high-mobility-group domain transcription factor Rop1 is a direct regulator of prf1 in Ustilago maydis. Eukaryot. Cell. 2005;4:379–391. doi: 10.1128/EC.4.2.379-391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux B, Lian T, Hu G, Wang J, Biondo C, Teti G, Liu V, Murphy ME, Creagh AL, Kronstad JW. The Mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus Cryptococcus neoformans. J. Infect. Dis. 2013;207:1339–1347. doi: 10.1093/infdis/jit029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, Carriconde F, Meyer W, Litvintseva AP, Lee WG, Shin JH. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res. 2010;10:769–778. doi: 10.1111/j.1567-1364.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Vogl AW, Kronstad JW. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Mol. Microbiol. 2012;85:700–715. doi: 10.1111/j.1365-2958.2012.08134.x. [DOI] [PubMed] [Google Scholar]
- Christensen JJ. Corn smut caused by Ustilago maydis. American Phytopathological Society; 1963. pp. 1–52. [Google Scholar]
- Coelho C, Bocca AL, Casadevall A. The tools for virulence of Cryptococcus neoformans. Adv. Appl. Microbiol. 2014;87:1–41. doi: 10.1016/B978-0-12-800261-2.00001-3. [DOI] [PubMed] [Google Scholar]
- Colombo S, Ma P, Cauwenberg L, Winderickx J, Crauwels M, Teunissen A, Nauwelaers D, de Winde JH, Gorwa M, Colavizza D. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 1998;17:3326–3341. doi: 10.1093/emboj/17.12.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle SM, Gilbert WV, Doudna JA. Direct link between RACK1 function and localization at the ribosome in vivo. Mol. Cell. Biol. 2009;29:1626–1634. doi: 10.1128/MCB.01718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KL, Gerrald QD, Nichols CB, Price MS, Alspaugh JA. Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans. Eukaryot. Cell. 2006;5:1147–1156. doi: 10.1128/EC.00145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlin MJ, Upadhya R, Gerik KJ, Lam W, VanArendonk LG, Specht CA, Sharma NK, Lodge JK. Cross talk between the cell wall integrity and cyclic AMP/protein kinase A pathways in Cryptococcus neoformans. MBio. 2014;5:e01573. doi: 10.1128/mBio.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, Heitman J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn H, Lessing F, Winterberg B, Schirawski J, Kamper J, Muller P, Kahmann R. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell. 2006;18:3332–3345. doi: 10.1105/tpc.106.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ero R, Dimitrova VT, Chen Y, Bu W, Feng S, Liu T, Wang P, Xue C, Tan SM, Gao Y. Crystal structure of Gib2, a signal-transducing protein scaffold associated with ribosomes in Cryptococcus neoformans. Sci. Rep. 2015;5:8688. doi: 10.1038/srep08688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Frias E, Ruiz-Herrera J, Arechiga-Carvajal ET. Transcriptomic analysis of the role of Rim101/PacC in the adaptation of Ustilago maydis to an alkaline environment. Microbiology. 2014;160:1985–1998. doi: 10.1099/mic.0.076216-0. [DOI] [PubMed] [Google Scholar]
- Gerbasi VR, Weaver CM, Hill S, Friedman DB, Link AJ. Yeast Asc1p and mammalian RACK1 are functionally orthologous core 40S ribosomal proteins that repress gene expression. Mol. Cell. Biol. 2004;24:8276–8287. doi: 10.1128/MCB.24.18.8276-8287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Duncan G, Barrett K, Kronstad J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994;8:2805–2816. doi: 10.1101/gad.8.23.2805. [DOI] [PubMed] [Google Scholar]
- Gong J, Grodsky JD, Zhang Z, Wang P. A Ric8/synembryn homolog promotes Gpa1 and Gpa2 activation to respectively regulate cyclic AMP and pheromone signaling in Cryptococcus neoformans. Eukaryot. Cell. 2014;13:1290–1299. doi: 10.1128/EC.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Do E, Jung WH. A human fungal pathogen Cryptococcus neoformans expresses three distinct iron permease homologs. J. Microbiol. Biotechnol. 2012;22:1644–1652. doi: 10.4014/jmb.1209.09019. [DOI] [PubMed] [Google Scholar]
- Hartmann HA, Kahmann R, Bolker M. The pheromone response factor coordinates filamentous growth and pathogenicity in Ustilago maydis. EMBO J. 1996;15:1632–1641. [PMC free article] [PubMed] [Google Scholar]
- Hsueh YP, Xue C, Heitman J. G protein signaling governing cell fate decisions involves opposing Gα subunits in Cryptococcus neoformans. Mol. Biol. Cell. 2007;18:3237–3249. doi: 10.1091/mbc.E07-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Caza M, Cadieux B, Bakkeren E, Do E, Jung WH, Kronstad JW. The endosomal sorting complex required for transport machinery influences haem uptake and capsule elaboration in Cryptococcus neoformans. Mol. Microbiol. 2015 doi: 10.1111/mmi.12985. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Caza M, Cadieux B, Chan V, Liu V, Kronstad J. Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, for capsule formation, and for virulence. Infect. Immun. 2013;81:292–302. doi: 10.1128/IAI.01037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Hu G, Kuo W, Kronstad JW. Role of ferroxidases in iron uptake and virulence of Cryptococcus neoformans. Eukaryot. Cell. 2009;8:1511–1520. doi: 10.1128/EC.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Kronstad JW. Iron and fungal pathogenesis: a case study with Cryptococcus neoformans. Cell. Microbiol. 2008;10:277–284. doi: 10.1111/j.1462-5822.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- Jung WH, Sham A, White R, Kronstad JW. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 2006;4:e410. doi: 10.1371/journal.pbio.0040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Strain AK, Nielsen K, Jung K, Bahn Y. Two cation transporters Ena1 and Nha1 cooperatively modulate ion homeostasis, antifungal drug resistance, and virulence of Cryptococcus neoformans via the HOG pathway. Fungal Genet. Biol. 2012;49:332–345. doi: 10.1016/j.fgb.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Yang D, Maeng S, Lee K, So Y, Hong J, Choi J, Byun H, Kim H, Bang S. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nat. Commun. 2015;6 doi: 10.1038/ncomms7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffarnik F, Müller P, Leibundgut M, Kahmann R, Feldbrügge M. PKA and MAPK phosphorylation of Prf1 allows promoter discrimination in Ustilago maydis. EMBO J. 2003;22:5817–5826. doi: 10.1093/emboj/cdg554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahmann R, Romeis T, Bölker M, Kämper J. Control of mating and development in Ustilago maydis. Curr. Opin. Genet. Dev. 1995;5:559–564. doi: 10.1016/0959-437x(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Klose J, De Sá MM, Kronstad JW. Lipid-induced filamentous growth in Ustilago maydis. Mol. Microbiol. 2004;52:823–835. doi: 10.1111/j.1365-2958.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- Klosterman SJ, Perlin MH, Garcia-Pedrajas M, Covert SF, Gold SE. Genetics of morphogenesis and pathogenic development of Ustilago maydis. Adv. Genet. 2007;57:1–47. doi: 10.1016/S0065-2660(06)57001-4. [DOI] [PubMed] [Google Scholar]
- Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol. Rev. 2012;36:78–94. doi: 10.1111/j.1574-6976.2011.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Lee SC, Heitman J. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 2009;11:370–380. doi: 10.1111/j.1462-5822.2008.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad JW, Attarian R, Cadieux B, Choi J, D’Souza CA, Griffiths EJ, Geddes JM, Hu G, Jung WH, Kretschmer M. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 2011a;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J, De Maria A, Funnell D, Laidlaw RD, Lee N, de Sá MM, Ramesh M. Signaling via cAMP in fungi: interconnections with mitogen-activated protein kinase pathways. Arch. Microbiol. 1998;170:395–404. doi: 10.1007/s002030050659. [DOI] [PubMed] [Google Scholar]
- Kronstad JW, Hu G, Choi J. The cAMP/protein kinase A pathway and virulence in Cryptococcus neoformans. Mycobiology. 2011b;39:143–150. doi: 10.5941/MYCO.2011.39.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J, Staben C. Mating type in filamentous fungi. Annu. Rev. Genet. 1997;31:245–276. doi: 10.1146/annurev.genet.31.1.245. [DOI] [PubMed] [Google Scholar]
- Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, Schröppel K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- Lee N, D’Souza CA, Kronstad JW. Of smuts, blasts, mildews, and blights: cAMP signaling in phytopathogenic fungi. Annu. Rev. Phytopathol. 2003;41:399–427. doi: 10.1146/annurev.phyto.41.052002.095728. [DOI] [PubMed] [Google Scholar]
- Liebmann B, Muller M, Braun A, Brakhage AA. The cyclic AMP-dependent protein kinase A network regulates development and virulence in Aspergillus fumigatus. Infect. Immun. 2004;72:5193–5203. doi: 10.1128/IAI.72.9.5193-5203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Ko YJ, Kim GB, Jung KW, Floyd A, Heitman J, Bahn YS. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot. Cell. 2010;9:360–378. doi: 10.1128/EC.00309-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EJ, Haynes BC, Gish SR, Wang ZA, Skowyra ML, Marulli AL, Doering TL, Brent MR. Model-driven mapping of transcriptional networks reveals the circuitry and dynamics of virulence regulation. Genome Res. 2015;25:690–700. doi: 10.1101/gr.184101.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Espinoza AD, Ruiz-Herrera J, León-Ramírez CG, Gold SE. MAP kinase and cAMP signaling pathways modulate the pH-induced yeast-to-mycelium dimorphic transition in the corn smut fungus Ustilago maydis. Curr. Microbiol. 2004;49:274–281. doi: 10.1007/s00284-004-4315-6. [DOI] [PubMed] [Google Scholar]
- Mayorga ME, Gold SE. A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 1999;34:485–497. doi: 10.1046/j.1365-2958.1999.01610.x. [DOI] [PubMed] [Google Scholar]
- Mitchell TK, Dean RA. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell. 1995;7:1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS-100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Leibbrandt A, Teunissen H, Cubasch S, Aichinger C, Kahmann R. The Gβ-subunit-encoding gene bpp1 controls cyclic-AMP signaling in Ustilago maydis. Eukaryot. Cell. 2004;3:806–814. doi: 10.1128/EC.3.3.806-814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki LH, Wang Y, Ballou ER, O’Meara TR, Bahn YS, Alspaugh JA, Xue C, Nielsen K. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot. Cell. 2011;10:1306–1316. doi: 10.1128/EC.05179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. MBio. 2013;4 doi: 10.1128/mBio.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Path. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara TR, Xu W, Selvig KM, O’Meara MJ, Mitchell AP, Alspaugh JA. The Cryptococcus neoformans Rim101 transcription factor directly regulates genes required for adaptation to the host. Mol. Cell. Biol. 2014;34:673–684. doi: 10.1128/MCB.01359-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost KS, O’Meara TR, Huda N, Esher SK, Alspaugh JA. The Cryptococcus neoformans alkaline response pathway: Identification of a novel Rim pathway activator. PLoS Genet. 2015;11:e1005159. doi: 10.1371/journal.pgen.1005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DA, Thompson JK, Li L, Prat A, Wang P. Gib2, a novel Gβ-like/RACK1 homolog, functions as a Gβ subunit in cAMP signaling and is essential in Cryptococcus neoformans. J. Biol. Chem. 2006;281:32596–32605. doi: 10.1074/jbc.M602768200. [DOI] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot. Cell. 2005;4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfall N, Schmitt K, Bandau S, Smolinski N, Ehrenreich A, Valerius O, Braus GH. RACK1/Asc1p, a ribosomal node in cellular signaling. Mol. Cell. Proteomics. 2013;12:87–105. doi: 10.1074/mcp.M112.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenfelder E, Spellig T, Hartmann A, Lauenstein S, Bölker M, Kahmann R. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 1997;16:1934–1942. doi: 10.1093/emboj/16.8.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach KC, Feretzaki M, Sun S, Heitman J. Unisexual reproduction. Adv. Genet. 2014;85:255–305. doi: 10.1016/B978-0-12-800271-1.00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LS, Causton HC, Young RA, Fink GR. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc. Natl. Acad. Sci. USA. 2000;97:5984–5988. doi: 10.1073/pnas.100113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvig K, Alspaugh JA. pH response pathways in fungi: adapting to host-derived and environmental signals. Mycobiology. 2011;39:249–256. doi: 10.5941/MYCO.2011.39.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snetselaar KM, Mims CW. Light and electron microscopy of Ustilago maydis hyphae in maize. Mycol. Res. 1994;98:347–355. [Google Scholar]
- Snetselaar KM, Mims CW. Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia. 1992;84:193–203. [Google Scholar]
- Tangen KL, Jung WH, Sham AP, Lian T, Kronstad JW. The iron- and cAMP-regulated gene SIT1 influences ferrioxamine B utilization, melanization and cell wall structure in Cryptococcus neoformans. Microbiology. 2007;153:29–41. doi: 10.1099/mic.0.2006/000927-0. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, De Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- Valerius O, Kleinschmidt M, Rachfall N, Schulze F, Lopez Marin S, Hoppert M, Streckfuss-Bomeke K, Fischer C, Braus GH. The Saccharomyces homolog of mammalian RACK1, Cpc2/Asc1p, is required for FLO11-dependent adhe sive growth and dimorphism. Mol. Cell. Proteomics. 2007;6:1968–1979. doi: 10.1074/mcp.M700184-MCP200. [DOI] [PubMed] [Google Scholar]
- Vollmeister E, Schipper K, Baumann S, Haag C, Pohlmann T, Stock J, Feldbrügge M. Fungal development of the plant pathogen Ustilago maydis. FEMS Microbiol. Rev. 2012;36:59–77. doi: 10.1111/j.1574-6976.2011.00296.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Berndt P, Xia X, Kahnt J, Kahmann R. A seven-WD40 protein related to human RACK1 regulates mating and virulence in Ustilago maydis. Mol. Microbiol. 2011;81:1484–1498. doi: 10.1111/j.1365-2958.2011.07783.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Perfect JR, Heitman J. The G-protein β sub-unit GPB1 is required for mating and haploid fruiting in Crypto-coccus neoformans. Mol. Cell. Biol. 2000;20:352–362. doi: 10.1128/mcb.20.1.352-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shen G, Gong J, Shen D, Whittington A, Qing J, Treloar J, Boisvert S, Zhang Z, Yang C, et al. Noncanonical Gβ Gib2 is a scaffolding protein promoting cAMP signaling through functions of Ras1 and Cac1 proteins in Crypto coccus neoformans. J. Biol. Chem. 2014;289:12202–12216. doi: 10.1074/jbc.M113.537183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Bahn YS, Cox GM, Heitman J. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol. Biol. Cell. 2006;17:667–679. doi: 10.1091/mbc.E05-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, García-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Path. 2010;6:e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr. Opin. Microbiol. 2013;16:409–413. doi: 10.1016/j.mib.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller CE, Parnell SC, Dohlman HG. The RACK1 ortholog Asc1 functions as a G-protein β subunit coupled to glucose responsiveness in yeast. J. Biol. Chem. 2007;282:25168–25176. doi: 10.1074/jbc.M702569200. [DOI] [PubMed] [Google Scholar]