Abstract
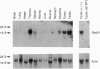
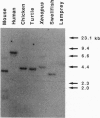
Analysis of mitotic and meiotic recombination in mammalian cells has been hampered by the complexity of the reactions involved as well as lack of mutants. Furthermore, none of the genes involved in the process has yet been identified. In budding yeast, Saccharomyces cerevisiae, the RAD51 gene is essential along with other genes of the RAD52 epistasis group for mitotic and meiotic recombination and DNA repair. The Rad51 protein is structurally similar to Escherichia coli RecA protein, which is required in homologous recombination and SOS responses in bacteria. Here we report the isolation of a mouse homolog of the yeast RAD51 gene. The amino acid sequence predicted from the gene shows 83% and 55% homology with those of the yeast RAD51 and the E. coli recA product, respectively. The mouse gene complemented a rad51 mutation of S. cerevisiae with sensitivity to methyl-methanesulfonate, which produces double-strand breaks of DNA. This gene is expressed in the thymus, testis, ovary, spleen, and intestine, suggesting that its product is involved in mitotic and meiotic recombination in addition to DNA repair.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Chanet R., Adjiri A., Fabre F. Semidominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA proteins. Mol Cell Biol. 1992 Jul;12(7):3224–3234. doi: 10.1128/mcb.12.7.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile G., Aker M., Mortimer R. K. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol Cell Biol. 1992 Jul;12(7):3235–3246. doi: 10.1128/mcb.12.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Park D., Xu L., Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992 May 1;69(3):439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Bradley A. The role and fate of DNA ends for homologous recombination in embryonic stem cells. Mol Cell Biol. 1992 Jun;12(6):2464–2474. doi: 10.1128/mcb.12.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth K., Roberds S., Poteet C., Tamkun M. Highly degenerate, inosine-containing primers specifically amplify rare cDNA using the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 25;16(22):10932–10932. doi: 10.1093/nar/16.22.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczykowski S. C. Biochemistry of genetic recombination: energetics and mechanism of DNA strand exchange. Annu Rev Biophys Biophys Chem. 1991;20:539–575. doi: 10.1146/annurev.bb.20.060191.002543. [DOI] [PubMed] [Google Scholar]
- Kubota H., Morita T., Nagata T., Takemoto Y., Nozaki M., Gachelin G., Matsushiro A. Nucleotide sequence of mouse Tcp-1a cDNA. Gene. 1991 Sep 15;105(2):269–273. doi: 10.1016/0378-1119(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Nagawa F., Okazaki K., Kingsbury L., Yoshida K., Müller U., Larue D. T., Winer J. A., Sakano H. Detection of somatic DNA recombination in the transgenic mouse brain. Science. 1991 Oct 4;254(5028):81–86. doi: 10.1126/science.1925563. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Shinohara A., Ogawa H., Tomizawa J. Functional structures of the recA protein found by chimera analysis. J Mol Biol. 1992 Aug 5;226(3):651–660. doi: 10.1016/0022-2836(92)90622-q. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992 May 1;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Valancius V., Smithies O. Double-strand gap repair in a mammalian gene targeting reaction. Mol Cell Biol. 1991 Sep;11(9):4389–4397. doi: 10.1128/mcb.11.9.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]