SUMMARY
SETTING
Multidrug-resistant tuberculosis (MDR-TB) treatment facility, Orel Oblast, Russian Federation.
OBJECTIVES
To determine factors associated with poor outcome and to document status of patients after recording of TB outcomes.
DESIGN
Retrospective review of prospective single cohort.
RESULTS
Among 192 patients, factors significantly associated with poor outcome in multivariate analysis include three or more treatment interruptions during the intensive phase of therapy and alcohol or drug addiction (adjusted OR [aOR] 2.1, 95%CI 1.0–4.3 and aOR 1.9, 95%CI 1.0–3.7). Previous treatment was associated with poor outcome, but only among smear-positive patients (aOR 3.1, 95%CI 1.3–7.3). Ten patients (5%) developed extensively drug-resistant TB (XDR-TB) during treatment; of 115 patients with at least 6 months of follow-up data after outcomes were recorded, 13 (11%) developed XDR-TB.
CONCLUSION
Interventions focused on supporting patient adherence during the intensive phase of treatment; the management of drug and alcohol addiction should be developed and studied. A substantial proportion of patients developed XDR-TB during and after treatment. Longer term follow-up data of patients treated for MDR-TB are needed to better inform programmatic policy.
Keywords: recurrence, default, alcohol
Treatment outcomes for multidrug-resistant tuberculosis (MDR-TB), defined as TB caused by Mycobacterium tuberculosis strains resistant to both isoniazid (INH) and rifampin (RMP), are significantly worse than for drug-susceptible TB, but have varied widely.1–4 Moreover, patients documented as having a good outcome do not always do well over time.5–8 However, few reports have documented longer term follow-up of patients uniformly treated with second-line agents. Extensively drug-resistant TB (XDR-TB), defined as MDR-TB that is additionally resistant to a fluoroquinolone and an injectable second-line drug (SLD), is even more difficult to treat than MDR-TB;9–13 however, relatively little has been documented about the risks of developing XDR-TB during and after the treatment of MDR-TB.14
Political change and economic difficulties in Russia in the 1990s contributed to difficulties obtaining medications, which increased the incidence of MDR-TB.15 By 2000, the prevalence of MDR-TB among newly treated patients in some oblasts exceeded 9%.16 Treatment success, and risk factors associated with outcome, among patients in MDR-TB treatment programs in countries of the former Soviet Union have varied.4,9,17,18 A better appreciation of these differences in treatment outcome may help elucidate reasons for treatment success or failure, and guide programmatic policy.
The Orel Oblast DOTS-Plus Tuberculosis Treatment Project was one of the first Green Light Committee (GLC) approved programs in the Russian Federation specifically designed to treat MDR-TB with individualized regimens. The first cohort began enrollment in 2002, and the program has been active since.
The purpose of this analysis was to investigate factors associated with poor treatment outcome in a cohort of MDR-TB cases enrolled in a DOTS-Plus project in Orel Oblast, Russia, and to describe cases with progression to XDR-TB during or after treatment.
STUDY POPULATION AND METHODS
Study setting
The DOTS-Plus program in Orel is a collaboration between the Central Tuberculosis Research Institute (CTRI) of the Russian Academy of Medical Sciences, the US Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO) office in Moscow, and the Orel Oblast Health Department and TB Dispensary. Patients in this analysis were hospitalized at a central reference hospital in Orel City, generally for the duration of the intensive phase of treatment; thereafter, treatment was continued at an in-patient or out-patient facility close to where the patient resided. Sputum smear microscopy was performed at TB clinics and dispensaries in each of the oblast’s raions (or districts), and cultures were performed at culture laboratories in the most populous raions. Drug susceptibility testing (DST) was performed for all drugs at the central TB laboratory at the Oblast TB Dispensary. External quality assurance and confirmatory testing were performed at CTRI and at the CDC TB laboratory in Atlanta.
Patients
Patients are referred to the treatment program from across the oblast. Between 2002 and 2005, 200 patients were enrolled into the first DOTS-Plus treatment program, composing the first treatment cohort. Many patients had received anti-tuberculosis treatment with available medications prior to enrollment.
For review of treatment outcomes, we included all patients who demonstrated resistance to at least RMP and INH. Patients were documented as prisoners if they were incarcerated at the time of enrollment, and as excessively using alcohol or drugs based on the determination of the clinician.
Procedures
Sputum was scheduled for collection from all patients at enrollment and then monthly throughout treatment. Sputum was processed using the modified Petroff method and concentrated by centrifugation at 3000× g. Smears were examined under regular light microscopy, and sediment was cultured on Löwenstein-Jensen (LJ) medium. Culture isolates were sent for DST using the absolute concentration method on LJ medium.
Initial DST was performed against INH, RMP, ethambutol (EMB) and streptomycin. Isolates resistant to INH and RMP were also tested for susceptibility to kanamycin (KM), capreomycin (CPM), ofloxacin (OFX), para-aminosalicylic acid, cycloserine (CS) and ethionamide.
Treatment regimens were based on the most recent DST prior to enrollment, and included pyrazinamide and any other first-line agents to which the organism was susceptible, a fluoroquinolone, an injectable agent, CS and prothionamide. Regimens were modified according to subsequent DST. All drugs were procured through the GLC.
Patients were actively followed during treatment, and default tracing using the local TB coordinators was used to locate and return patients to treatment. After outcomes were reported, patients were followed for relapse, but fewer resources were available for tracing, and follow-up data for those who relocated or died were often not documented.
Data collection and analysis
We abstracted clinical data from treatment forms in medical records and laboratory data from laboratory databases. We defined a clinically relevant treatment interruption as discontinuation of all prescribed drugs for a period of ≥4 days. Patient registration and treatment outcome definitions were those recommended by the WHO in 2008,19 with the exception that cure was documented on the basis of at least three (and not five) negative cultures in the final 12 months of treatment, with no cultures positive; failure was defined as any culture positive in the last 12 months of treatment. We further defined poor outcome as death from any cause, treatment failure or default; we defined favorable outcome as cure or treatment completion.
We calculated the risk of poor outcome associated with various factors, excluding from the analysis those who transferred out. We conducted a separate analysis to determine associations with default, comparing the risk of default to other outcomes among patients who were cured, completed treatment or failed treatment. In addition, we performed descriptive analyses of the patients who developed XDR-TB either during or after treatment.
Comparative analyses were conducted using the t-test and Wilcoxon rank-sum test for continuous data, and Pearson’s χ2 or Fisher’s exact test for categorical data, where appropriate. A predictive multivariate analysis was conducted using logistic regression to obtain adjusted odds ratios (ORs). We used a cut-off of P ≤ 0.25 in univariate analysis to include variables in the original multivariate model. Variables were assessed for collinearity and effect modification. Using backward elimination, we developed a final model by retaining variables that had P ≤ 0.3, and stratified results by variables that exhibited effect modification. All data were analyzed with SAS version 9.2 (SAS Institute Inc, Cary, NC, USA).
Ethical considerations
This project was reviewed by the US CDC and approved as evaluation of a public health surveillance program; this does not require human subjects review or informed consent.
RESULTS
The characteristics of the 200 patients enrolled in the DOTS-Plus program are displayed in Table 1. In total, 165 (85%) were male and the mean age was 42 years; 144 (72%) patients were previously treated for TB, 102 with second-line agents; 133 patients (67%) had isolates that exhibited resistance to EMB, including 30/54 (56%) new patients and 101/144 (70%) previously treated patients. Sixty-nine patients (35%) had isolates resistant to a second-line injectable agent, including 34 to KM alone, two to CPM alone and 33 to both agents. Two patients (1%), both previously treated, had initial isolates resistant to OFX; 198 patients had initial resistance to both INH and RMP and are included in subsequent analyses.
Table 1.
Patient characteristics at enrollment in a DOTS-Plus treatment program (N = 200)
| Patient characteristic | n (%)* |
|---|---|
| Sex | |
| Male | 165 (83) |
| Female | 35 (18) |
| Age, years [range] | 42 [16–75] |
| Prison status at treatment initiation | |
| Prisoner | 21 (11) |
| Non-prisoner | 179 (90) |
| Initial smear status† | |
| Positive | 139 (70) |
| Negative | 61 (31) |
| Initial culture status† | |
| Positive | 143 (72) |
| Negative | 57 (29) |
| Treatment category and resistance pattern at treatment initiation‡ | |
| New patients without SLD resistance | 42 (21) |
| New patients with SLD resistance | 12 (6) |
| Previously treated with FLDs without SLD resistance | 24 (12) |
| Previously treated with FLDs with SLD resistance | 18 (9) |
| Previously treated with SLDs without SLD resistance | 64 (32) |
| Previously treated with SLDs with SLD resistance | 38 (19) |
| Undetermined | 2 (1) |
| Excessive alcohol or drug use | |
| Yes | 69 (35) |
| No | 131 (66) |
| Cavitary disease | |
| Yes | 171 (86) |
| No | 28 (14) |
| Missing | 1 (1) |
| Bilateral disease | |
| Yes | 126 (63) |
| No | 73 (37) |
| Missing | 1 (1) |
| HIV infection | |
| Yes | 0 |
| No | 198 (99) |
| Missing | 2 (1) |
| Mean time from MDR-TB diagnosis to treatment, days | 446 |
Some frequencies may not add up to 100% due to rounding.
Initial documented positive culture or smear result within 30 days after enrollment into DOTS-Plus; some patients were being treated in a nonstandardized fashion and were culture- or smear-negative at enrollment.
Data from the first culture and DST obtained after enrollment, or, for those who were culture-negative at enrollment, from the most recent previous positive results.
SLD = second-line drug; FLD = first-line drug; HIV = human immunodeficiency virus; MDR-TB = multidrug-resistant tuberculosis; DST = drug susceptibility testing.
Among the 198 MDR-TB patients, cure was documented in 108 (55%) and 10 (5%) completed treatment; 18 (9%) patients died during treatment and treatment failed in 30 (15%); 26 (13%) defaulted and 6 (3%) transferred out of the region. Treatment was stopped for six patients for >2 months; these patients were clinically monitored, resumed treatment and had a reported outcome. We analyzed their outcomes as reported.
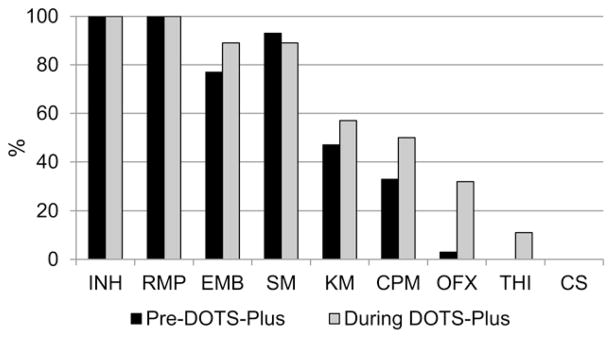
Of the 30 patients in whom treatment failed, 29 underwent DST during DOTS-Plus. By the end of treatment, the proportion of patients with resistance to SLDs had increased considerably: 20/29 (69%) had TB resistant to either KM or CPM and 9/29 (31%) had fluoroquinolone-resistant TB (Figure).
Figure.
Drug resistance among patients in whom treatment failed (n = 30). INH = isoniazid; RMP = rifampin; EMB = ethambutol; SM = streptomycin; KM = kanamycin; CPM = capreomycin; OFX = ofloxacin; THI = thioamide; CS = cycloserine.
Among those with favorable outcomes, the mean total treatment duration was 621 days (standard deviation [SD] 109), the mean intensive phase regimen was 242 days (SD 89) and the mean continuation phase regimen was 377 days (SD 71). The median number of treatment interruptions during the intensive phase was one (range 0–14), and the median number of treatment interruptions during the continuation phase was two (range 0–17).
Among patients in whom treatment failed, the mean total treatment duration was 465 days (SD 128). The median number of treatment interruptions during the intensive phase was two (range 0–26) and the median number of treatment interruptions during the continuation phase was four (range 0–12).
Excluding patients who transferred out of the region, factors significantly associated with poor outcome in univariate analyses of the remaining 192 patients were previous treatment, bilateral disease on initial chest radiograph, positive sputum smear at enrollment, three or more treatment interruptions during the intensive phase of treatment and addiction to alcohol or illicit drugs (Table 2). In multivariate analysis, smear status at enrollment modified the effect of previous treatment on outcome; the stratified results of the final model with the interaction are presented in Table 2. The strongest association with default was alcohol or drug addiction (OR 2.28, 95%CI 0.99–5.33, adjusted OR [aOR] 2.12, 95%CI 0.90–5.03).
Table 2.
Associations with poor outcome among MDR-TB patients, excluding patients who transferred out (n = 192)
| Patient characteristic | Poor outcome n/N (%) |
OR (95%CI) | Univariate P value |
aOR (95%CI)* |
|---|---|---|---|---|
| Previously treated† | 59/137 (43) | 2.33 (1.14–4.74) | 0.02 | Smear-positive at start: 2.75 (0.80–9.44) Smear-negative at start: 0.49 (0.12–2.03) |
| New† | 13/53 (25) | Reference | Smear-positive at start: 0.82 (0.20–3.33) Smear-negative at start: Reference |
|
| Sputum status | ||||
| Sputum-positive at start | 63/134 (47) | 3.79 (1.81–7.94) | <0.001 | See above |
| Sputum-negative at start | 11/58 (19) | Reference | See above | |
| Age, years | ||||
| ≥42 | 46/106 (43) | 1.49 (0.89–2.87) | 0.13 | — |
| <42 | 28/86 (33) | Reference | ||
| Sex | ||||
| Male | 64/159 (40) | 1.55 (0.69–3.47) | 0.29 | — |
| Female | 10/33 (30) | Reference | ||
| Bilateral disease‡ | ||||
| Bilateral | 54/122 (44) | 1.95 (1.04–3.66) | 0.04 | — |
| Unilateral | 20/69 (29) | Reference | ||
| Cavitary disease‡ | ||||
| Yes | 67/165 (41) | 1.86 (0.74–4.66) | 0.18 | — |
| No | 7/26 (27) | Reference | ||
| Initial resistance to | ||||
| FLD and an injectable agent | 30/65 (46) | 1.62 (0.88–2.97) | 0.12 | 1.67 (0.85–3.29) |
| FLD only | 44/127 (35) | Reference | Reference | |
| Time of enrollment | ||||
| Before 2004 | 39/104 (38) | 0.91 (0.51–1.63) | 0.75 | — |
| In or after 2004 | 35/88 (40) | Reference | ||
| Continuation phase | ||||
| In-patient | 4/16 (25) | Reference | 0.25 | — |
| Out-patient | 70/176 (40) | 1.98 (0.61–6.39) | ||
| Time from MDR-TB to treatment | ||||
| <446 days | 53/141 (38) | 0.86 (0.45–1.65) | 0.65 | — |
| ≥446 days | 21/51 (41) | Reference | ||
| Incarceration | ||||
| Yes | 8/19 (42) | 1.18 (0.45–3.08) | 0.73 | — |
| No | 66/173 (38) | Reference | ||
| Treatment interruptions during intensive phase | ||||
| ≥3 | 26/49 (53) | 2.24 (1.16–4.33) | 0.02 | 2.07 (1.001–4.27) |
| <3 | 48/143 (34) | Reference | Reference | |
| Alcohol or drug addiction | ||||
| Yes | 35/68 (52) | 2.31 (1.26–4.24) | 0.007 | 1.95 (0.99–3.82) |
| No | 39/124 (31) | Reference | Reference | |
| Surgical treatment | ||||
| Yes | 8/18 (44) | 1.31 (0.49–3.48) | 0.59 | — |
| No | 66/174 (38) | Reference | ||
Smear status at enrollment modified the effect of previous treatment on outcome, and these aORs are stratified. The referent for the stratified aORs is new patient, smear-negative.
Two previously treated patients with unknown regimens were excluded.
One patient with no radiology report was excluded.
MDR-TB = multidrug-resistant tuberculosis; aOR = adjusted odds ratio; CI = confidence interval; FLD = first-line drug.
Ten patients (5%) developed XDR-TB during the course of treatment. Of these, 4 died, treatment failed in 4, 1 transferred out and 1 was reported as cured. Among the 115 remaining patients for whom there were at least 6 months of follow-up culture data after the reported treatment outcome, an additional 13 (11%) developed XDR-TB. Of these, 10 (77%) used illicit drugs or alcohol excessively, 5 (38%) had defaulted during treatment, treatment had failed in 6 (46%) and 2 (15%) had been reported as cured (Table 3). Among 70 patients who had a favorable outcome and for whom there were at least 12 months of follow-up culture data after treatment outcome, 7 (10%) had a subsequent positive culture after treatment outcome was documented.
Table 3.
Characteristics of patients who developed XDR-TB and patients who did not after ≥ 6 months of follow-up after outcomes were reported
| Patients who developed XDR-TB during treatment (n = 10) n (%) |
Patients who developed XDR-TB after treatment (n = 13) n (%) |
Patients who did not develop XDR-TB after ≥6 months of follow-up (n = 102) n (%) |
|
|---|---|---|---|
| Male | 8 (80) | 12 (92) | 83 (81) |
| Prisoners | 1 (10) | 2 (15) | 5 (5) |
| Alcohol or drug addiction | 4 (40) | 10 (77) | 29 (28) |
| Patients treated with FLDs before MDR-TB treatment | 1 (10) | 1 (8) | 22 (22) |
| Patients treated with SLDs before MDR-TB treatment | 5 (50) | 9 (69) | 48 (47) |
| Outcome of DOTS-Plus program | |||
| Died | 4 (40) | NA | NA |
| Defaulted or transferred out | 1 (10) | 5 (38) | 16 (16) |
| Failed | 4 (40) | 6 (46) | 12 (12) |
| Cured | 1 (10) | 2 (15) | 69 (68) |
| Completed treatment | 0 | 0 | 5 (5) |
XDR-TB = extensively drug-resistant tuberculosis; FLD = first-line drug; MDR-TB = multidrug-resistant tuberculosis; SLD = second-line drug; NA = not applicable.
DISCUSSION
Our analysis suggests that previous treatment, smear positivity at initiation of MDR-TB treatment and treatment interruptions during the intensive phase were associated with poor outcome. Alcohol or drug addiction also appeared to be associated with both poor outcome, which included defaulting, as well as with defaulting alone, but these associations did not reach conventional statistical significance in our analysis.
Previous research has described factors associated with poor outcome in patients treated for MDR-TB in DOTS-Plus programs in countries of the former Soviet Union,17,18,20,21 and the outcomes for this cohort were similar to descriptions in other treatment cohorts.9,17,22 Default, while arguably distinct from death or treatment failure, was included in our definition of poor outcome to allow for a more complete program evaluation, consistent with previous analyses.18,20,22 As this was the first cohort of DOTS-Plus patients in the region, it is likely that this group was more sick than subsequent cohorts, with longer durations of disease and higher rates of previous treatment with, and consequent resistance to, second-line agents—both of which were associated with poor treatment outcome in univariate analysis. In this cohort, over half of the patients had previously been treated with second-line agents prior to enrollment, over a third of whom had resistance to a second-line agent. Equally worrying is the fact that 12/54 newly treated patients had resistance to second-line agents, suggesting transmission of resistant organisms in the community. Our data also suggest that previously treated patients with positive sputum smears at treatment initiation may have a particularly high association with poor outcomes, even after adjusting for initial resistance to an injectable agent, but the lack of precision limits our conclusions. These patients may harbor a higher burden of organisms or may be a population that is more heterogeneous and with variable drug susceptibility, and may require more deliberately aggressive therapy during the intensive phase of treatment.
Treatment interruptions and alcohol or drug addiction were identified risks for poor outcome that are potentially modifiable. A predictive rule to identify patients at risk for treatment interruption has been developed,23 and focused interventions to prevent interruptions should be developed and evaluated. Alcoholism and drug addiction are prevalent among patients with TB disease in Russia,24,25 and while not statistically significant, our analysis suggests association with both poor outcome and default. This has been previously documented in other Russian TB programs.18,22,26,27 Alcohol and drug addiction can be treated in the context of anti-tuberculosis treatment in many ways using pharmaceutical, behavioral and other interventions,28 and these interventions urgently need evaluation.
Our data demonstrate that 5% of MDR-TB patients in this cohort developed XDR-TB during the course of treatment, a proportion similar to that described in another review of outcomes in Russia.14 The number of patients who developed XDR-TB after completion of treatment is worrying. This concern is amplified by the fact that 13% of the patients in this cohort defaulted, and for many of them, outcomes and follow-up are not known. Given the potentially catastrophic consequence of XDR-TB transmission in a community,29 more detailed research into risk factors for increased resistance should be performed and interventions to lower default rates should be evaluated and implemented. The high recurrence among patients who were successfully treated and for whom follow-up data were available, raises concerns and questions about current definitions of treatment outcomes. Longer term and more complete follow-up of patients after treatment for MDR-TB is needed, and the use of genotyping to distinguish between re-infection and relapse may help better characterize these findings.
Our analysis has several limitations. First, this was a retrospective review with a limited number of participants, and we were unable to control for additional factors that may have confounded or modified our findings. Alcohol and drug addiction were identified by experienced clinicians, but the reliability of these determinations is not certain. The reliability and reproducibility of DST against SLDs is questionable, and our conclusions about DST may not be fully generalizable. The relatively high rate of patients with unknown outcomes may bias the association between these risk factors and outcome, and this limits the confidence of these findings. The limited follow-up data on patients from this cohort, and the significant proportion of patients who were lost to follow-up after outcomes were recorded, restricts the conclusions that can be drawn from these findings and introduces the possibility of bias. Finally, the precision of the findings and conclusions were limited by the relatively small size of this cohort. Data collection, however, was thorough and documentation on a wide variety of variables was available. We believe that in spite of these limitations, this analysis expands upon previous reports about MDR-TB treatment in this region and highlights areas where further research is needed to inform programmatic policy.
Acknowledgments
This work was supported by the US Agency for International Development and the US Centers for Disease Control and Prevention.
References
- 1.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 2.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan ED, Laurel V, Strand MJ, et al. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004;169:1103–1109. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 4.Nathanson E, Lambregts-van Weezenbeek C, Rich ML, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg Infect Dis. 2006;12:1389–1397. doi: 10.3201/eid1209.051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Lim H-J, Cho Y-J, et al. Recurrence after successful treatment among patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2011;15:1331–1333. doi: 10.5588/ijtld.11.0098. [DOI] [PubMed] [Google Scholar]
- 6.He GX, Xie YG, Wang LX, et al. Follow-up of patients with multidrug resistant tuberculosis four years after standardized first-line drug treatment. PLoS One. 2010;5:e10799. doi: 10.1371/journal.pone.0010799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migliori GB, Espinal M, Danilova ID, Punga VV, Grzemska M, Raviglione MC. Frequency of recurrence among MDR-TB cases ‘successfully’ treated with standardised short-course chemotherapy. Int J Tuberc Lung Dis. 2002;6:858–864. [PubMed] [Google Scholar]
- 8.Becerra MC, Appleton SC, Franke MF, et al. Recurrence after treatment for pulmonary multidrug-resistant tuberculosis. Clin Infect Dis. 2010;51:709–711. doi: 10.1086/655892. [DOI] [PubMed] [Google Scholar]
- 9.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–1409. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 10.Goldman RC, Plumley KV, Laughon BE. The evolution of extensively drug resistant tuberculosis (XDR-TB): history, status and issues for global control. Infect Disord Drug Targets. 2007;7:73–91. doi: 10.2174/187152607781001844. [DOI] [PubMed] [Google Scholar]
- 11.Caminero JA. Likelihood of generating MDR-TB and XDR-TB under adequate National Tuberculosis Control Programme implementation. Int J Tuberc Lung Dis. 2008;12:869–877. [PubMed] [Google Scholar]
- 12.Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008;21:587–595. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- 13.Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–626. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 14.Shin SS, Keshavjee S, Gelmanova IY, et al. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med. 2010;182:426–432. doi: 10.1164/rccm.200911-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toungoussova OS, Bjune G, Caugant DA. Epidemic of tuberculosis in the former Soviet Union: social and biological reasons. Tuberculosis (Edinb) 2006;86:1–10. doi: 10.1016/j.tube.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization/International Union Against Tuberculosis and Lung Disease. The WHO/ IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance. Geneva, Switzerland: WHO; 2000. Anti-tuberculosis drug resistance in the world. Report no. 2. Prevalence and trends; p. 253. [Google Scholar]
- 17.Cox HS, Kalon S, Allamuratova S, et al. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: treatment complexity and XDR-TB among treatment failures. PLoS One. 2007;2:e1126. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–408. [PubMed] [Google Scholar]
- 19.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. Geneva, Switzerland: WHO; 2008. WHO/HTM/TB/2008.402. [Google Scholar]
- 20.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 21.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–1384. [PubMed] [Google Scholar]
- 22.Gelmanova IY, Keshavjee S, Golubchikova VT, et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ. 2007;85:703–711. doi: 10.2471/BLT.06.038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belilovsky EM, Borisov SE, Cook EF, Shaykevich S, Jakubowiak WM, Kourbatova EV. Treatment interruptions among patients with tuberculosis in Russian TB hospitals. Int J Infect Dis. 2010;14:e698–703. doi: 10.1016/j.ijid.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Fleming MF, Krupitsky E, Tsoy M, et al. Alcohol and drug use disorders, HIV status and drug resistance in a sample of Russian TB patients. Int J Tuberc Lung Dis. 2006;10:565–570. [PMC free article] [PubMed] [Google Scholar]
- 25.Zaridze D, Brennan P, Boreham J, et al. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet. 2009;373:2201–2214. doi: 10.1016/S0140-6736(09)61034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewan PK, Arguin PM, Kiryanova H, et al. Risk factors for death during tuberculosis treatment in Orel, Russia. Int J Tuberc Lung Dis. 2004;8:598–602. [PubMed] [Google Scholar]
- 27.Jakubowiak WM, Bogorodskaya EM, Borisov ES, Danilova ID, Kourbatova EK. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]
- 28.Greenfield SF, Shields A, Connery HS, et al. Integrated management of physician-delivered alcohol care for tuberculosis patients: design and implementation. Alcohol Clin Exp Res. 2010;34:317–330. doi: 10.1111/j.1530-0277.2009.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raviglione MC, Smith IM. XDR tuberculosis—implications for global public health. N Engl J Med. 2007;356:656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]