Abstract
The mechanisms of hyperalgesia in alcoholics are not completely clear, and the development of animal models would therefore be necessary in investigating the underlying changes. Several studies including our own have demonstrated that the intermittent access to 20% ethanol two-bottle choice procedure (IA2BC) promotes escalation of drinking, and induces physical dependence in the Sprague-Dawley (SD) rat, one of the strains most commonly used in preclinical alcohol research. In this study, we investigated whether the IA2BC procedure could produce hyperalgesia in SD rats. We show here that, the SD rats in the IA2BC procedure significantly escalated their drinking within 8 weeks, which is consistent with other studies. Starting from 8 weeks of repeated chronic drinking, the mechanical and thermal sensitivity was significantly increased. During withdrawal, there were noticeable physical dependence signs, including tail stiffness and lower limb flexion, which started at 4 hours and lasted for more than 3 days after ethanol removal. Importantly, during withdrawal, the mechanical and thermal sensitivity was further increased, which started at 12 hours and lasted for more than seven days after ethanol removal. These results suggest that utilizing the SD rat under the IA2BC procedure could be a useful animal model with heuristic value for exploring the mechanisms underlying hyperalgesia induced by chronic alcohol abuse.
Keywords: Sprague-Dawley rat, pain, self-administration, physical dependence, withdrawal
Introduction
Each year, an estimated 116 million Americans suffer from persistent pain arising from a variety of sources [1]. Previous clinical and preclinical studies have found that excessive chronic alcohol exposure and withdrawal from it increases the sensitivity to noxious stimuli, or hyperalgesia [2-6]. However, the mechanisms underlying this hyperalgesia are not completely clear, and the development of animal models would therefore be useful in investigating the underlying changes. A better understanding of these changes would enable possible molecular and therapeutic interventions in order to prevent the development of alcoholism.
In order to mimic the progressive transition from low to moderate alcohol consumption to excessive alcohol consumption in alcoholics, many studies examining self-administration of ethanol in rodents have employed the oral route, using operant self-administration and intermittent access 2-bottle choice procedures (IA2BC), as a partial model of alcoholism in humans [7,8]. The IA2BC procedure consists of repeated cycles of drinking and abstinence, leading to a more rapid escalation in ethanol intake than the continuous access program [9-12]. Rodents in the IA2BC procedure can reliably drink alcohol to pharmacologically relevant levels [13,14].
The Sprague-Dawley (SD) rat is one of the strains most commonly used in preclinical alcohol research. However, unlike Long-Evans and Wistar rats or other alcohol-preferring rats that usually drink higher levels of ethanol, SD rats usually drink low to moderate levels [15,16]. Recently, several studies including our own have found that SD rats with the IA2BC procedure could drink excessive amounts of ethanol [10,17,18] and show signs of physical dependence characterized in distal limb flexion response, tail stiffness and abnormal body posture during alcohol withdrawal [18]. This observation suggests that the full potential of SD rats in studies examining voluntary ethanol consumption has yet to be realized [17].
However, it is unclear whether the IA2BC paradigm would affect the pain threshold in SD rats. Among a number of different methods for assessing alteration of pain threshold, the paw-withdrawal assay is one of the most common tests [19]. In the current study, we examined the effect of ethanol on pain thresholds in SD rats trained with the IA2BC procedure.
Material and method
Animals and housing
All animal handling procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of New Jersey Medical School at Rutgers University and were conducted according to specifications of the NIH as outlined in the Guide for the care and Use of Laboratory Animals. Experiments were done on adult male SD rats (Taconic Farm, NY, 250-350 g at the start of the experiments). The rats were individually housed in ventilated Plexiglas cages and were kept on a 12-hour light/dark cycle: lights on at 07:00 p.m., in a climate-controlled room (20-22°C). Food and water were available ad libitum unless as indicated.
Intermittent access to 20% ethanol in a 2-bottle choice procedure (IA2BC)
We used the IA2BC as described previously [10]. Briefly, after acclimation to the homecage environment, all animals had 24-hour concurrent access to two bottles, one with 20% ethanol (v/v) and another with water, starting on Monday afternoon. After 24 hours, the ethanol bottle was replaced with a second water bottle that was available for the next 24 hours. This pattern was repeated on Wednesdays and Fridays. On all other days the rats had unlimited access to two bottles of water. In each ethanol drinking session, the placement of the ethanol bottle was alternated to control for side preferences. Rats under this paradigm escalated their ethanol intake and preference [10]. Animals in the control group were allowed access to water and food without limitation. The mean body weight was 305 ± 11 g at the start of the experiments when rats were approximately 3 months old and 461 ± 16 g after 2 months’ chronic ethanol consumption. There was no significant difference in body weight between the control and the ethanol-drinking rats at the end of the experiments.
Mechanical and thermal pain test
To determine whether chronic ethanol consumption could alter sensory sensitivity, we measured the paw withdrawal threshold (PWT) in response to mechanical or paw withdrawal latency (PWL) to thermal stimuli in a double-blinded manner. We first measured the baseline before the rats were exposed to ethanol by taking the mean value of 5 continuous readings of the PWT or PWL. We then measured the PWT or PWL in rats after 4, 8 or 12 weeks of ethanol exposure in the IA2BC program (at the end of the 12th, 24th or 36th drinking session). To overcome the possible effect of increasing body weight on weight on PWT and PWL, control tests were performed on 25 age-matched ethanol naïve SD rats at each time point.
To determine the effect of withdrawal from chronic ethanol consumption, we measured the PWT or PWL on rats during a 1-week withdrawal period after 12 weeks of alcohol drinking, at the following time points: immediately (0 h) and 12 h, 1 d, 3 d and 7 d after the ethanol bottles were removed. The rats were introduced to the testing room immediately after ethanol bottle removal.
In the testing room, after at least 30 min acclimation on an elevated mesh screen, an experimenter who was blinded to the treatment of each group measured the PWT, as described [20]. Briefly, the unrestrained rat was placed in a Plexiglas chamber on an elevated mesh screen. A series of von Frey hairs in log increments of force (0.41, 0.69, 1.20, 2.04, 3.63, 5.50, 8.51, 15.14 g) were applied perpendicularly to the plantar surface of the hind paw for 3 s. The 2.041-g stimulus was applied first. A sharp withdrawal of the hind paw indicated a positive response. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next larger von Frey hair was used. The test ended when (1) a negative response was obtained with the 15.14-g hair and (2) 3 stimuli were applied after the first positive response. The PWT was determined by converting the pattern of positive and negative responses to the von Frey filament stimulation to a 50% threshold value with the formula provided by Dixon et al. [21]. The 50% PWT was determined by the formula Xf + kð, where Xf = last von Frey filament employed, k = Dixon value corresponding to response pattern, and ð = mean difference between stimuli. Baseline mechanical nociceptive thresholds were similar to those reported for the ages of rats employed in this study [20].
The PWL to thermal stimuli was conducted as previously described [22], by applying radiant heat (Model 336 Analgesia Meter, IITC Life Science, Woodland Hills, CA) by aiming a beam of light from the light box through the glass plate to the middle of the plantar surface of the right and left hind paws. When the animal lifted its foot, the light beam was shut off and the PWL was recorded. The PWL is defined as the length of time between the start of the light beam and the lift of the hind paw. Each trial was repeated five times for each paw. To avoid tissue damage, a 20 second cut-off time was used.
Evaluation of physical signs of ethanol withdrawal
At the end of the 36th ethanol drinking session (12 weeks), the ethanol bottle was removed while the food and water were available ad libitum for one week. We measured the physical signs of ethanol withdrawal from 4 hours to 7 days after alcohol removal as previously described [10]. On the test day, the rats were gently transferred from the homecage to an empty table and carefully observed for 3 min. All disturbing voices or movements were avoided during the observation. The criterion for each physical sign is as follows:
Tail stiffness: the observer gently slid the middle and index fingers on the ventral surface of the rostral end of the tail of the rat towards the caudal end of the tail and observed the following signs: A score of 0 showed that the tail laid flat on the ground or was very lowly suspended and there was no resistance with touch. A score of 1 showed the tail medially suspended, with increased muscle tone when touching the tail or the tip of the tail wrapped around the observer’s finger. A score of 2 showed the tail completely suspended in the air and with touch was rigid with no flexibility, along with the wrapping of the tip of the tail around the observer’s finger.
Limb flexion: each rat was gently grasped by the scruff of their neck and observed for retraction of the hind limbs from the body. A score of 0 showed the abduction of the hip joint and knee joint, with straight hind legs and paws. A score of 1 showed hind legs parallel to the ground with hind legs and ankles bent and abduction of the paws. A score of 2 was comparable to the score of 1 with the addition of the hind paws being upright with digits facing the head (typically the feet would be retracted to the abdominal surface).
Gait: observing the movement of rats assessed gait. A score of 0 was given to rats that showed a normal scurrying, which was directly compared to control rats. A score of 1 was given to rats with a slower movement accompanied by stumbling. A score of 2 was given to rats that were not mobile.
For each of the three physical signs, a subjective 0-2 point scale was utilized as follows: a score of 0 for undetectable withdrawal, 1 for moderately severe withdrawal and 2 for severe withdrawal. Summing the scores for all three signs and yielding a range from 0 to 6 determined the score of each rat. The same investigator, blind to the treatments, did the rating of physical signs in order to minimize experimental bias.
Statistical analysis
Statistical analysis was performed using SigmaPlot 12.5 (Systat Software Inc, San Jose, CA). All data are expressed as mean ± S.E.M. (standard error of the mean). The ethanol drinking data was analyzed using a one-way repeated measure ANOVA (RM ANOVA), followed by a Bonferroni or Tukey test for post hoc comparison. Comparisons of the EWS score and PWT or PWL tested among naïve control and IA2BC groups at different time points were performed using two-way ANOVA followed by a post hoc Bonferroni test. Significance was set at P < 0.05.
Results
The intermittent-access to 20% ethanol 2-bottle-choice paradigm (IA2BC) resulted in excessive ethanol consumption in SD rats
We first examined the intake of and the preference for ethanol of SD rats under the IA2BC paradigm. In keeping with our previous report [23], SD rats under this paradigm significantly escalated their ethanol intake (F (36, 30) = 2.941, P < 0.001, n = 30, Figure 1A). The ethanol intake reached a stable level of 4.38 ± 0.36 g/kg/24 h following the 25th drinking session. Thereafter the ethanol intake did not significantly increase. The elevation in ethanol intake was paralleled by a significant increase in preference for ethanol (F (36, 30) = 9.69, P < 0.001, n = 30, Figure 1B). Additionally, there was a significant decrease in water intake on the days when ethanol was presented (F (36, 30) = 5.867, P < 0.001, n = 30, Figure 1C). Notably, there was no significant difference in the total fluid intake during all the training sessions (F (36, 30) = 1.116, P = 0.294, n = 30, Figure 1D). Five rats, which drank less than 3 g/kg of ethanol in 24 hours by the end of the 25th session, were excluded from further study.
Figure 1.
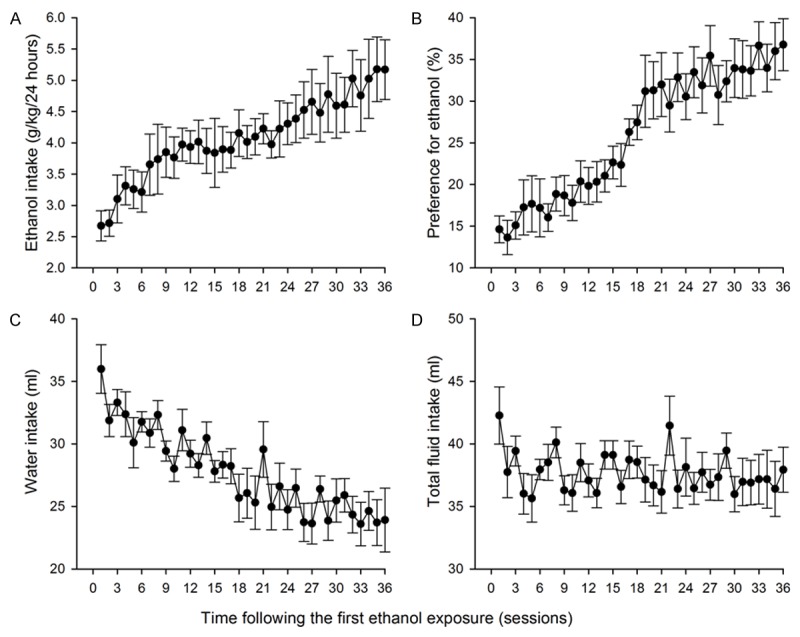
Sprague-Dawley rats in the intermittent access to 20% ethanol 2-bottle choice procedure escalated the intake (A) of and the preference (B) for ethanol, and decreased water intake (C), without altering the total fluid intake (ml/24 h) (D).
Physical dependence induced by withdrawal from chronic alcohol consumption
We next characterized the physical dependence signs during one-week ethanol removal in rats that had been in the IA2BC paradigm for 12 weeks. As shown in Figure 2A-D, compared with the alcohol naïve counterparts, the SD rats in the IA2BC procedure presented a significantly higher physical withdrawal score, which started at 4 h, peaked at 12 h and last for more than 3 d after alcohol removal (F (1, 48) = 111.493, P < 0.001, n = 25 rats for each group). A main effect of time (F (1, 240) = 20.399, P < 0.001) and an interaction between both ethanol and time (F (5, 240) = 12.064, P < 0.001) were shown. These results indicate mild to moderate physical dependence occurs in these rats (Figure 2E).
Figure 2.
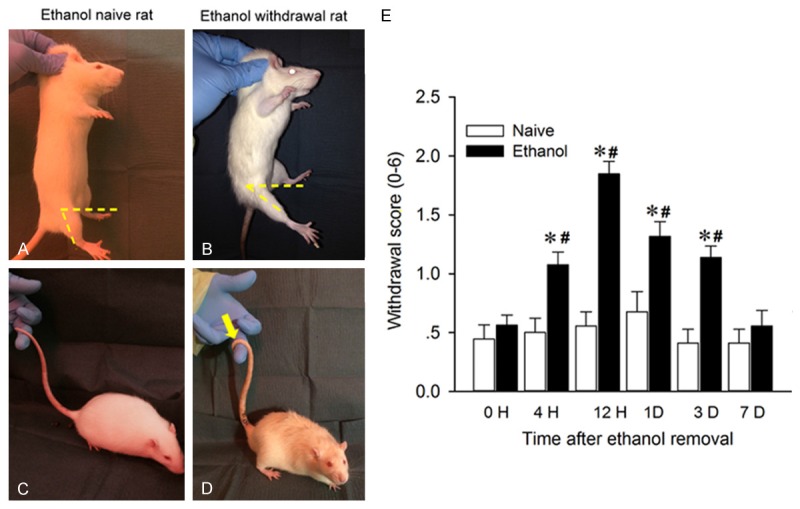
Physical dependence syndromes in SD rats withdrawn from chronic ethanol administration. After consuming ethanol intermittently for 12 weeks, the physical withdrawal syndrome was observed and quantified (on a scale of 0-2) during one week of alcohol removal. The left panels are representative photographs showing the increased score derived predominantly from positive signs of lower limb flexion and tail stiffness 12 h after alcohol removal, when comparing to ethanol naïve SD rats. Note that the smaller angle between horizontal axis and longitudinal axis of lower limb indicates an obvious flexion of the hip joint in withdrawn rats (B) compared to alcohol-naïve rats (A). An observation of the rat tail tip wrapped around the observer’s finger (yellow arrow in D) was found in withdrawn rats but not in alcohol-naïve rats (C). The right panel shows the withdrawal scores, determined by summing the scores for all three symptoms, significantly increased at 4 hr, peaked at 12 hr and lasted for 3 days, compared to alcohol naïve rats. (All P < 0.001, n = 25).
Hyperalgesia induced by chronic ethanol consumption
Withdrawal from chronic alcohol exposure often results in increased pain sensitivity in alcoholics [24]. To determine whether this also occurs in SD rats under the IA2BC procedure, we measured the PWT or PWL during alcohol withdrawal. The PWT in SD rats that were in the IA2BC procedure for 8 and 12 weeks, but not 4 weeks, was significantly lower than that of ethanol naïve counterparts (Figure 3A). A two-way RM ANOVA indicated a main effect of ethanol (F (1, 48) = 34.403, P < 0.001, n = 25 rats for each group), a main effect of time (F (3, 144) = 15.549, P < 0.001) and an interaction between both factors (F (3, 144) = 16.251, P < 0.001). Post-hoc analysis revealed that no significant difference of PWL was found among rats before access to ethanol (P = 0.759) or after 4-week ethanol exposure (P = 0.358).
Figure 3.
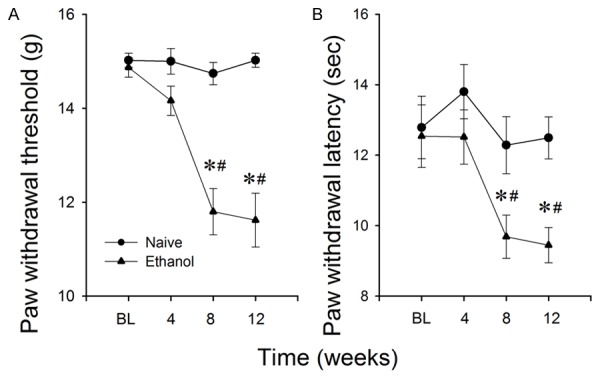
Repeated chronic ethanol drinking induces pain hypersensitivity. The paw withdrawal threshold to mechanical (A) and paw withdrawal latency to heat stimuli (B) in SD rats at 4, 8 and 12 weeks in the IA2BC program. The paw withdrawal threshold and paw withdrawal latency in rats that in IA2BC program for 8 and 12 weeks was significantly lower than that of ethanol-naïve counterparts. Data are presented as mean ± SEM. (*)P < 0.001 compared to naïve rats. (#)P < 0.001, compared to baseline (BL) or 4 weeks after ethanol exposure, n = 25 rats for each group.
Similarly, the PWL to the thermal stimulus in SD rats in the IA2BC program for 8 and 12 weeks was significantly lower than that of ethanol naïve counterparts (Figure 3B). A two-way RM ANOVA detected a main effect of ethanol (F (1, 48) = 17.972, P < 0.001, n = 25 rats for each group), a main effect of time (F (3, 144) = 10.898, P < 0.001) and an interaction between both factors (F (3, 144) = 3.163, P = 0.03). Post-hoc analysis revealed no significant difference was found among rats before access to ethanol (P = 0.552) or after 4-week ethanol exposure (P > 0.05).
To further characterize hyperalgesia induced by withdrawal from chronic ethanol administration, we next measured the pain threshold during one week after ethanol removal in rats that have been in the IA2BC procedure for 12 weeks. The PWT in ethanol group was significantly lower than that of ethanol naïve counterparts starting at 12 h and lasted for more than seven days after ethanol removal (all P < 0.001, n = 25 rats for each group, Figure 4A). A two-way RM ANOVA analysis revealed a main effect of ethanol (F (1, 48) = 305.834, P < 0.001) and a main effect of time (F (4, 192) = 5.618, P < 0.001) with an interaction between both factors (F (4, 192) = 5.618, P < 0.001). Similarly, the PWL in the ethanol group was significantly lower than the ethanol naïve counterparts starting at 4 h and lasting for seven days after ethanol removal (all P < 0.001, n = 25 rats for each group Figure 4B). A two-way RM ANOVA analysis revealed both main effects of ethanol (F (1, 48) = 63.709, P < 0.001) and time (F (4, 192) = 11.849, P < 0.001) with an interaction between both factors (F (4, 192) = 12.081, P < 0.001).
Figure 4.
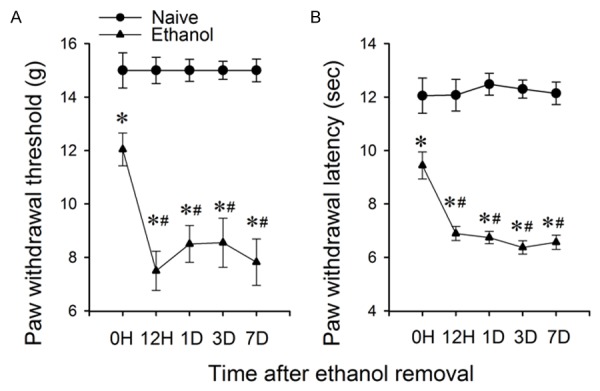
The mechanical and thermal pain thresholds further decreased during withdrawal in SD rats that were in IA2BC for 12 weeks. The paw withdrawal threshold (A) and paw withdrawal latency (B) significantly decreased starting at 12 hours after ethanol removal and lasted for 7 days. Data is presented as mean ± SEM. (*)P < 0.001 compared to naïve rats. (#)P < 0.001, compared to 0 h after ethanol removal, n = 25 rats for each group.
Discussion
The present study demonstrates that Sprague-Dawley rats, one of the strains most commonly used in preclinical alcohol research, could self-administer 20% ethanol to levels that could induce physical dependence and hyperalgesia.
In the present study, we observed that SD rats that chronically self-administered 20% ethanol under the IA2BC paradigm could consume excessive amounts of ethanol, in keeping with our previous report [23]. An advantage of this drinking paradigm is the repeated cycles of free-choice ethanol drinking and withdrawal over a period of several weeks, which leads to a gradual escalation of ethanol intake and preference that reaches a stable level after several weeks. These features may be a step closer to approximating human drinking patterns by mimicking the progressive transition from low or moderate social-like drinking to excessive alcohol consumption [7,25]. In the current study, the ethanol intake range varied from 3.14 to 12.82 g/kg/24 h, with an average of 5.33 g/kg/24 h. We observed also a higher preference to ethanol (36.78 ± 3.13% at 24 h). These results are consistent with previous studies including our own [17,18] and support the view that the full potential of SD rats in studies examining voluntary ethanol consumption has yet to be established [17]. Although we did not measure blood ethanol levels in the current study, previous studies including our own have reported that SD rats in the IA2BC paradigm consumed ethanol that reached pharmacologically relevant blood ethanol concentrations [26].
As with humans, the alcohol withdrawal signs in rodents appear as blood and brain alcohol levels drop, peak around the time when alcohol is completely eliminated from the body, and wane over the course of several days [27]. Rodents undergoing alcohol withdrawal demonstrate numerous indicators of impaired body function and motor activity. To validate the effect of withdrawal from chronic intermittent ethanol self-administration in SD rats, we measured three withdrawal signs (tail stiffness, limb flexion and gait) [28,29]. We noticed that tail stiffness is the earliest withdrawal sign, which started at 4 h and peaked at 6-8 h after alcohol removal. Whereas, the ventral distal limb flexion was peaked at 12-16 h after alcohol removal, in keeping with a previous report [30]. These results indicate that the severity of the ethanol withdrawal syndrome in rats is time related [28]. Our results are in general agreement with the view that signs of alcohol withdrawal syndrome in SD rats typically develop within 4~16 h of the last drink [28]. Interestingly, in the current study, we found the alcohol withdrawal syndrome lasted for 3 d, instead of 1 d [18]. The difference could be caused by longer ethanol exposure history (4 and 12 weeks of ethanol exposure was conducted in Li’s and current study respectively), in which more repeated ethanol withdrawal experiences may increase the severity of the withdrawal syndrome [31]. Notably, during withdrawal from chronic ethanol administration, the physical dependence signs lasted for only 3 days, whereas the hyperalgesia lasted for more than 7 days. While the mechanisms underlying this difference warrant further investigation, this observation suggests that the mechanisms underlying these two phenomena may be different. Previous animal studies have found that during alcohol withdrawal the γ-aminobutyric acid (GABA) central inhibition is down-regulation [32,33], whereas extracellular glutamate levels were increased [34]. This appears as one of the main causes of insufficient central inhibition during alcohol withdrawal, which leads to the symptoms of hyper-excitation. The mechanisms underlying hyperalgesia induced by withdrawal from chronic ethanol are less clear. The current study reports that chronic ethanol consumption under the IA2BC procedure induced hyperalgesia, a symptom that frequently occurs in alcoholics [35]. The mechanical hypersensitivity occurred 4 h after alcohol withdrawal and lasted for 7 d, consistent with previous studies in other rodent strains [36,37]. To our knowledge, this is the first report on mechanical and thermal hypersensitivity in SD rats during withdrawal from IA2BC drinking paradigm. Although it has been well documented that acute administration of ethanol can produce modest relief of pain [2,38,39], a tolerance to the anti-nociceptive effects after chronic administration has also been reported [2]. Continued exposure decreases the ability of ethanol to relieve pain and can eventually even increase sensitivity to noxious stimuli [40]. Previous studies have reported that hyperalgesia is a result of abnormal peripheral nociceptor function, including the decreased mechanical threshold of C-fibers and enhanced PKCε second messenger signaling in nociceptors [41]. Several studies suggested that this phenomenon might involve the hypothalamo-pituitary-adrenal system in the central nervous system [42,43], and the activation of mGlu5 receptors [44] or the opioidergic system [45]. Future studies should be performed to examine the possible mechanisms underlying alcoholic neuropathy. Additionally, we noted that the hypersensitivity to mechanical and thermal stimuli, as well as the escalation of alcohol intake occurs at the same period, suggesting that the emergence of hyperalgesia may relate to excessive ethanol intake [4]. Future studies should investigate if the relief of pain-induced alcohol withdrawal could attenuate the negative reinforcement and potentially stop alcohol relapse.
Conclusion
This study reveals that withdrawal from chronic ethanol drinking under intermittent-access to two-bottle choice drinking paradigm could induce mechanical and thermal hypersensitivity in Sprague-Dawley rats, implicating that withdrawal induced hyperalgesia may be negative reinforcement for future drinking.
Acknowledgements
This work was supported by grants (AA022292 and AA021657) from the National Institute of Health.
Disclosure of conflict of interest
None.
Authors’ contribution
RF and JHY were responsible for the study concept and design. DG and ZLP performed behavioral. RF and JHY analyzed the data and drafted the manuscript. JL helped on the data analysis; AB provided critical comments on the design. JHY supervised the study and provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- 1.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatch MB. Effects of benzodiazepines on acute and chronic ethanol-induced nociception in rats. Alcohol Clin Exp Res. 1999;23:1736–1743. [PubMed] [Google Scholar]
- 3.Dina OA, Gear RW, Messing RO, Levine JD. Severity of alcohol-induced painful peripheral neuropathy in female rats: role of estrogen and protein kinase (A and Cepsilon) Neuroscience. 2007;145:350–356. doi: 10.1016/j.neuroscience.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart SH, Finn PR, Pihl RO. A dose-response study of the effects of alcohol on the perceptions of pain and discomfort due to electric shock in men at high familial-genetic risk for alcoholism. Psychopharmacology (Berl) 1995;119:261–267. doi: 10.1007/BF02246289. [DOI] [PubMed] [Google Scholar]
- 6.Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333:1058–1065. doi: 10.1056/NEJM199510193331607. [DOI] [PubMed] [Google Scholar]
- 7.Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- 11.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009;33:1012–1024. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 14.Myers RD, Robinson DE, West MW, Biggs TA, McMillen BA. Genetics of alcoholism: rapid development of a new high-ethanol-preferring (HEP) strain of female and male rats. Alcohol. 1998;16:343–357. doi: 10.1016/s0741-8329(98)00031-7. [DOI] [PubMed] [Google Scholar]
- 15.Melchior CL, Myers RD. Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacol Biochem Behav. 1976;5:63–72. doi: 10.1016/0091-3057(76)90289-6. [DOI] [PubMed] [Google Scholar]
- 16.Linseman MA. Alcohol consumption in freefeeding rats: procedural, genetic and pharmacokinetic factors. Psychopharmacology (Berl) 1987;92:254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- 17.Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Zou Y, Ye JH. Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res Bull. 2011;86:428–434. doi: 10.1016/j.brainresbull.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 20.Li Z, Gu X, Sun L, Wu S, Liang L, Cao J, Lutz BM, Bekker A, Zhang W, Tao YX. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain. 2015;156:711–721. doi: 10.1097/j.pain.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Petralia RS, Takamiya K, Xia J, Li YQ, Huganir RL, Tao YX, Yaster M. Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C Kinase 1. Mol Pain. 2011;7:11. doi: 10.1186/1744-8069-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res. 2010;34:1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jochum T, Boettger MK, Burkhardt C, Juckel G, Bar KJ. Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain. 2010;14:713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Hwa LS, Chu A, Levinson SA, Kayyali TM, De-Bold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Sun Y, Ye JH. Electroacupuncture decreases excessive alcohol consumption involving reduction of FosB/DeltaFosB levels in reward-related brain regions. PLoS One. 2012;7:e40347. doi: 10.1371/journal.pone.0040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- 28.Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol selfadministration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 30.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- 32.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 33.Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- 34.Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- 35.Brain L, Walton J. Disorders of peripheral nerves: alcoholic polyneuritis. London: Oxford University Press; 1969. Brain’s Diseases of the Nervous System; pp. 817–819. [Google Scholar]
- 36.Kolik LG, Nadorova AV, Kozlovskaya MM. Efficacy of peptide anxiolytic selank during modeling of withdrawal syndrome in rats with stable alcoholic motivation. Bull Exp Biol Med. 2014;157:52–55. doi: 10.1007/s10517-014-2490-4. [DOI] [PubMed] [Google Scholar]
- 37.Shumilla JA, Liron T, Mochly-Rosen D, Kendig JJ, Sweitzer SM. Ethanol withdrawal-associated allodynia and hyperalgesia: age-dependent regulation by protein kinase C epsilon and gamma isoenzymes. J Pain. 2005;6:535–549. doi: 10.1016/j.jpain.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Gatch MB, Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–333. [PubMed] [Google Scholar]
- 39.Gameiro GH, Arthuri MT, Tambeli CH, de Arruda Veiga MC. Effects of ethanol on deep pain evoked by formalin injected in TMJ of rat. Life Sci. 2003;73:3351–3361. doi: 10.1016/j.lfs.2003.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Gatch MB. Ethanol withdrawal and hyperalgesia. Curr Drug Abuse Rev. 2009;2:41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- 41.Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000;20:8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res. 2003;27:410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- 43.Thayer JF, Hall M, Sollers JJ 3rd, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59:244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Miyoshi K, Narita M, Takatsu M, Suzuki T. mGlu5 receptor and protein kinase C implicated in the development and induction of neuropathic pain following chronic ethanol consumption. Eur J Pharmacol. 2007;562:208–211. doi: 10.1016/j.ejphar.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 45.Narita M, Miyoshi K, Narita M, Suzuki T. Functional reduction in mu-opioidergic system in the spinal cord under a neuropathic painlike state following chronic ethanol consumption in the rat. Neuroscience. 2007;144:777–782. doi: 10.1016/j.neuroscience.2006.10.028. [DOI] [PubMed] [Google Scholar]


