Abstract
This study was designed to illustrate the effects of electroacupuncture on cognitive function in rats with Parkinson’s disease (PD). The PD model was established by injecting 6-OHDA into the rat brain. Rats with PD were then subjected to electroacupuncture and levodopa treatment for 2 weeks. The level of choline acetyltransferase (ChAT) activity in rat brain homogenates was assessed, for the cerebral cholinergic system is a major chemical pathway consisting of cognitive functions. Immunohistochemistry was applied to observe ChAT expression in the rat hippocampus and corpus striatum. The effects of electroacupuncture on cognitive function were comprehensively assessed in PD rats using Y-maze test. Compared with model control group, electroacupuncture group were apparently improved in learning & memory abilities, and ChAT activity was elevated, and apoptosis was reduced in the rat hippocampus and corpus striatum. No significant differences in learning & memory abilities and ChAT activity were detected between electroacupuncture and levodopa groups. Electroacupuncture remarkably improved cognition in PD rats, and its mechanisms are possibly associated with protecting cholinergic neurons in the central nervous system and elevating ChAT activity, and also might suitable dosage of levodopa protect physiologically the cognitive function in PD rats.
Keywords: Electroacupuncture, Parkinson’s disease, cognitive function, Y-maze, choline acetyltransferase
Introduction
Parkinson’s disease (PD) is the most common motoric neurodegenerative disease of the central nervous system. The clinical symptoms include bradykinesia, resting tremor, muscle rigidity and posture disorder [1]. Besides the above-mentioned motor symptoms, cognitive dysfunction is one of the common symptoms in PD patients, and affects approximately 17%-59.5% of PD patients [2-4]. PD severely impacts the quality of life of patients and their family members. Levodopa (L-dopa) has been a clinical gold standard for PD treatment. Following prolonged use of L-dopa, approximately 75% patients experience motor complications such as dyskinesia and symptom fluctuation after the drug’s “honeymoon period” [5]. In order to overcome this pitfall, the current study tested electroaccupunture as an alternative strategy to improve the treatment efficiency of L-dopa in PD patients and thereby improve their quality of life.
Acupuncture is a key alternative treatment for PD. Evidence-based medicine has proved that acupuncture is an effective and safe alternative treatment for PD [6]. In PD patients supplemented with acupuncture treatment, improved motor skills were reported. However, the effect of acupuncture on cognitive function has not been clearly understood. The conclusions of clinical practice analysis are quite varied, and lack relevant experimental support [3,5]. The present study compares the changes in cognitive function after electroacupuncture and L-dopa administration in a rat model of PD. The investigation of the mechanism underlying improved cognitive function in PD following electroacupuncture will provide a theoretical basis for clinical practice.
Materials and methods
The reagents and equipment
The following reagents and equipment were used in this study-6-OHDA (Sigma), apomorphine hydrochloride (Sigma), L-dopa (Roche Compnay), choline acetyltransferase (ChAT) test kit (Nanjing Jiancheng, China), rabbit choline acetyltransferase polyclonal antibody (Abcam), strept avidin-biotin complex immunohistochemistry kit (Solarbio), enhanced HRP-DAB chromogenic substrate kit (Tiangen Company), rat brain stereotaxic apparatus (BAS Company), skull drill (BAS Company), UV/visible spectrophotometer (Bio-Rad), and tissue grinder (Bio-spec).
Experimental groups
A total of 26 male Sprague-Dawley rats weighing 260-350 g were provided by the Experimental Center, Shanghai First People’s Hospital in China. All experiments were performed in accordance with the IACUC. All rats were housed under the standard environment, allowed free access to food and water, in a 12-hour light/dark cycle. No abnormal rotation was found by behavioral test. The rats were randomly assigned to blank control group (n=4), and experimental series (then further divided as Model control group, Electroacupuncture group and L-dopa group, 4 rats each groups).
Model establishment
Ten mg 6-OHDA was dissolved in 2.5 ml physiological saline containing 0.2% ascorbic acid and stored at 4°C in the dark. Rats were intraperitoneally anesthetized with 400 mg/kg chloral hydrate, and mounted on the stereotaxic apparatus in a prone position. The stereotaxic co-ordinates for the two medial forebrain bundles are as follows: 2.8 mm posterior to anterior fontanelle, 1.95 mm lateral to the midline (right), 8.45 mm ventral to the anterior fontanelle; 2.8 mm posterior to anterior fontanelle, 1.95 mm lateral to the midline (right), 9.00 mm ventral to anterior fontanelle (Third edition of Paxinos & Watson rat brain atlas). A hole was drilled into the cranium on the right hemisphere of the brain to expose the dura in each of the co-ordinates mentioned above. Four µL 6-OHDA was injected in each target with a microsyringe at the rate of 0.5 µl/min. The needle was held in place for 5 minutes. The cranial hole was filled with gelatin sponge and the scalp was sutured. The rats were fed conventionally. After model induction, the rats were intraperitoneally injected with 200,000 U penicillin against infection.
On 7, 14, 28 and 35 days after 6-OHDA injection establishment, the rats were intraperitoneally injected with apomorphine hydrochloride (0.5 mg/kg) to induce rotational behavior. At 5 minutes after injection, rotational behavior appeared, circling to the healthy side, and the head and tail connected. Rotational behavior was observed and recorded for 10 minutes. Rats that had rotated for 7 cycles in a minute were considered as successful models. PD rats were randomly divided into model control group (n=4), electroacupuncture group (n=4) and L-dopa group (n=4). PD rats, together with rats in the blank control group, were used in following experiments.
Treatment
Electroacupuncture was performed on two locations, namely, Dazhui (DU14) and Baihui (DU20) [7]. According to rat accupoint atlas, Baihui (DU20) and Dazhui (DU14) are easily to locate, and the effect of acupuncture is considerable. Thus, these two accupoints were selected in this study. Needle handle was connected to the electroacupuncture device, and continuous wave was switched on to the point that rat head shook, with the frequency of 100 Hz, each for 20 minutes, once a day, for 2 consecutive weeks. L-dopa 15 mg was daily given orally to rats in the L-dopa group for 2 consecutive weeks. An equal volume of physiological saline was daily given in the blank control group and model control group, and rats in the above-mentioned groups were given food and water conventionally.
Learning & memory test
Y-shaped maze has three arms at a 120° angle from each other. The bottom of the maze is a metal gate that can be energized to stimulate rats. There is a small bulb on the top of each arm. No current passes through the base arm when the light is ON, which is considered as a safety zone. However, current passes through other arms when lights off. After treatment, all rats underwent behavioral test in the Y-maze. Before the test, rats were acclimatize in the Y-maze for 5 minutes. Subsequently, with switch “ON”, the current was adjusted until the rats responded. The safety zone was changed randomly. When the rat reached the safety zone, the light lasted for 10 seconds, which was considered as a test end. The arm where the rat stayed was considered as the test start. The interval between two tests was 30 seconds and the test was repeated 20 times. Correct reaction is that the rat jumped from the start zone to the safety zone after switching ON. In ten tests, nine corrections to find the safety zone were considered as the learned standard. Each rat was tested at a fixed time daily, 20 times a day. Training frequency (N1) and reaction time (T1) before reaching the learned standard in each rat were recorded and considered as the indicators to measure the learning ability of rats. The Y-maze was cleaned with ethanol after testing each rat.
At 24 hours after learning test, rats were tested by the same method. The frequency of reaching the standard (N2) and reaction time (T2) were recorded. The data of memory of rats was compared and analyzed.
ChAT activity in the rat brain
After learning & memory tests, two rats from Blank control group, two rats from Model control group, four rats from Electroacupuncture group, and four rats from the L-dopa group were obtained and decapitated on the ice. Their brains were obtained, washed with 4°C pre-cooled physiological saline, and dried by the filter paper. The brain was weighed. 9× volume of ice-cold normal solution (NS) was added and 10% homogenate was prepared with a tissue homogenizer. In accordance with ChAT activity kit instructions, reagents were added and centrifuged at 4,000 r/min for 10 minutes. Absorbance values of each tube were measured at 324 nm. ChAT activity in the rat brain was calculated by ChAT expression and the degree of ChAT expression according to the instruction of the kit.
Immunohistochemical staining for ChAT in the hippocampus and corpus striatum
The remaining rats were intraperitoneally injected with chloral hydrate. The heart was exposed. Half ml heparin sodium was injected into the ventricle for anticoagulation. Aortic cannulation was conducted through the left ventricle. The right atrium was cut and persistently washed with physiological saline. After washing, the rat was perfused with preheated 4% paraformaldehyde. Subsequently, the rat was decapitated, and the brain was obtained, post-fixed in 4% paraformaldehyde, dehydrated through a graded alcohol series, permeabilized, and embedded in paraffin. Samples were sliced into 4 µm-thick coronal sections. Sections were dewaxed, immersed in citrate buffer solution for antigen retrival, blocked with 5% bovine serum albumin and incubated with the primary antibody at 4°C overnight. On the next day, sections were washed with PBS, visualized with 3,3’-diaminobenzidine, counter stained with hematoxylin, dehydrated through a graded alcohol series, permeabilized, and embedded in neutral resin.
After staining, the left and right hemispheres of each rat were quantified. ChAT-positive fibers in the hippocampus and corpus striatum were observed under the low-power lens. The number of ChAT-positive fibers was quantified in three fields of the hippocampus and corpus striatum using the high-power lens (10×40).
Experimental results
Among 22 rats for model establishment, the 16 successful models were screened out after 4 weeks of intraperitoneal injection with apomorphine to induce rotational behaviors. The other 6 rats were excluded. The successful rate of model induction was 72%.
Learning & memory abilities
After 6-OHDA injection, rotational behavior was analyzed. Following model induction, Y-maze test was carried out. (1) Learning ability: compared with the blank control group, learning frequency was significantly increased and learning time was significantly longer in the model group (P < 0.05), which indicated that learning ability was remarkably reduced in PD rats compared with normal rats. Compared with the model group, learning frequency was reduced and learning time was shorter in the electroacupuncture and L-dopa groups (P < 0.05) (Figure 1), suggesting that learning ability of PD rats was improved after electroacupuncture and L-dopa treatment. No significant differences in learning frequency (P=0.16) and learning time (P=0.70) were detectable between the electroacupuncture group and L-dopa group. (2) Memory ability: no significant difference in memory frequency (P=0.16) and memory reaction time (P=0.25) was found between the model control group and blank control group (Table 1).
Figure 1.
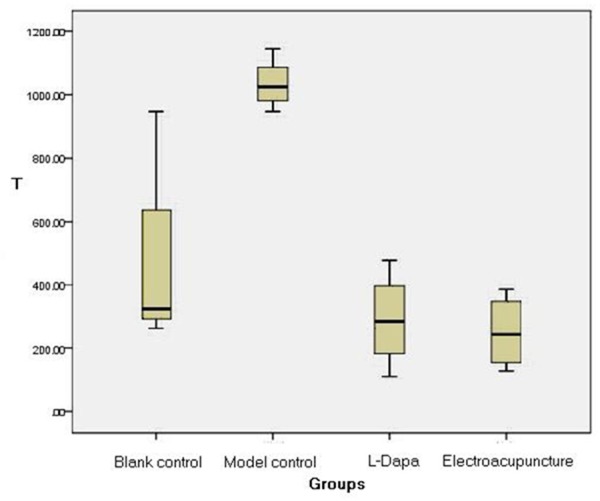
T indicates learning time (unit: second) in four groups of rats. The histograms indicate that learning frequency was more significantly reduced and learning time was significantly shorter in Electroacupuncture and L-dopa groups, than Model group.
Table 1.
Results of learning and memory abilities in Y-maze in rats
| Groups | Learning frequency/times | Learning time(s) | Memory frequency/times | Memory time(s) |
|---|---|---|---|---|
| Blank control | 39.00±14.98 | 465.00±322.03 | 26.33±2.51 | 285.75±48.04 |
| Model control | 64.67±9.57* | 1034.25±82.01* | 36.00±1.41 | 1040.33±99.33 |
| Electro-acupuncture | 23.75±8.85**,○ | 251.50±118.18**,□ | 12.33±10.15 | 70.20±45.15 |
| L-dopa | 33.50±8.35** | 289.75±151.07** | 13.75±7.23 | 77.50±41.40 |
P < 0.05, Electroacupuncture vs. Blank control group;
P < 0.05, vs. Model control group.
The table lists that Model group’ learning frequency was significantly increased and learning time was significantly longer than Blank control group; Electroacupuncture and L-dopa groups’ learning frequency was significantly reduced and the related learning time was significantly shorter. No signifcant differences in learning frequency ○ (P=0.16) and learning time □ (P=0.70) were detectable between the electroacupuncture group and L-dopa group.
ChAT activity in the brain
Rat brains from each group were collected to analyze ChAT activity. Results revealed that ChAT activity was significantly lower in the model control group than in the blank control group (P < 0.01), indicating that ChAT activity was apparently decreased in PD rats. ChAT activity was significantly greater in the electroacupuncture and L-dopa groups than in the model control group (P < 0.01), suggesting that electroacupuncture and L-dopa administration promoted the recovery of ChAT activity in the brain. No significant difference in ChAT activity was observed between the electroacupuncture and drug administration groups (P=0.86). These findings demonstrated that no significant difference in the improvement of cognitive function was observed between electroacupuncture and L-dopa treatment (Table 2).
Table 2.
ChAT activity in the rat brain
| Groups | ChAT activity |
|---|---|
| Blank control | 14.67±2.93 |
| Model control | 2.24±2.39* |
| Electroacupuncture | 17.39±4.58** |
| L-dopa | 18.21±7.96** |
P < 0.01, vs. Blank control group;
P < 0.01, vs. Model control group.
Lists of Groups that showed changes of ChAT activity: significantly lower in Model control group than in Blank control group; significantly greater in the Electroacupuncture and L-dopa groups than in Model control group.
Number of ChAT-positive cells in the hippocampus and corpus striatum
The comparison of ChAT-positive cells was done between the left and right hemispheres. Results showed that the number of ChAT-positive cells was 28.22±18.71 in the left hemisphere and 32.86±14.30 in the right hemisphere (P < 0.05), suggesting that the disease severely impacted the affected hemisphere. Under the low-power microscope, lesser number of neurons were detected which were sparsely arranged in the hippocampus and corpus striatum of the right hemisphere in model control group compared with other groups. After treatment with L-dopa and electroacupuncture, the number of ChAT-positive cells increased, and these cells had complete structure and arranged densely. ChAT expression noticeably improved after treatment with electroacupuncture (Figure 2). These results confirmed that electroacupuncture had a protective effect on nerve cells in the hippocampus and corpus striatum. Under the high-power microscope, the number of ChAT-positive cells was significantly more in the blank control group than in the model control group (P < 0.01), which indicated that ChAT expression obviously diminished in the brain of PD rats. The number of ChAT-positive cells was significantly higher in the electroacupuncture and L-dopa groups than in the model control group (P < 0.01). There was no significant difference in ChAT expression between the corpus striatum (P=0.45) and hippocampus (P=0.48) (Table 3).
Figure 2.
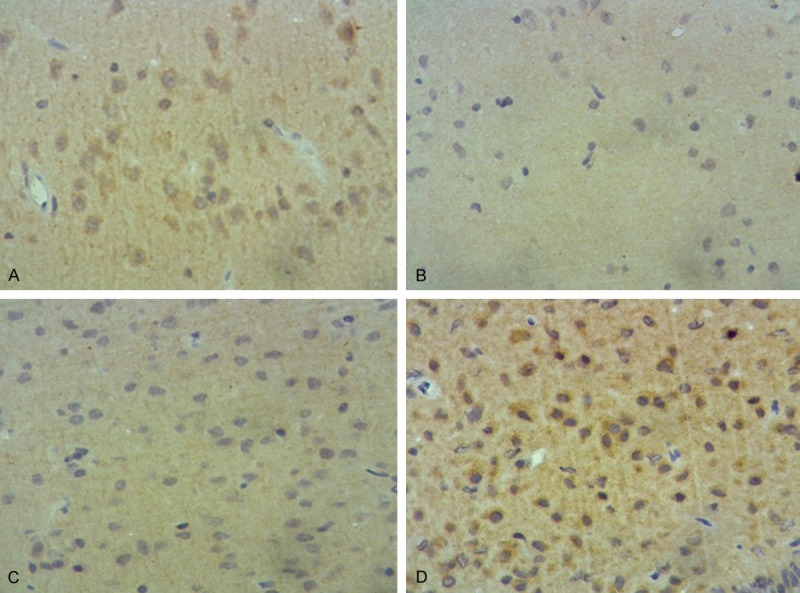
Results of ChAT immunohistochemical staining in rat hippocampus: after treatment by L-dopa and Electroacupuncture, the number of ChAT-positive cells increased. A. Blank control group; B. Model control group; C. L-dopa group; D. Electroacupuncture group.
Table 3.
Number of ChAT-positive cells in the hippocampus and corpus striatum
| Groups | Hippocampus | Corpus striatum |
|---|---|---|
| Blank control | 45.50±5.94 | 43.17±8.76 |
| Model control | 18.67±6.54* | 24.00±3.02* |
| Electroacupuncture | 42.67±9.57** | 35.25±7.22** |
| L-dopa | 40.25±6.82** | 37.25±5.64** |
P < 0.01, vs. Blank control group;
P < 0.01, vs. Model control group.
The number of ChAT-positive cells was significantly higher in the electroacupuncture and L-dopa groups than in the model control group. There was no significant difference in ChAT expression between the corpus striatum and hippocampus.
Discussion
Cognitive function includes learning, memory, spatial recognition, attention and exploration, and associates with human normal work and life. Cognitive decline mainly presents as a decrease in spatial recognition ability, exploration, learning and memory, and ability to execute, barrier to information extraction [8]. Central cholinergic system is a major pathway consisting of learning and memory functions, and exists in many regions and nuclei of the brain. Basal forebrain sends out a large number of cholinergic fibers that projects extensively into the central nervous system, including the cortex, hippocampus, corpus striatum, amygdala and thalamus. Experiments verified that decline in cognitive function correlated with the decreased ChAT activity and loss of cholinergic neurons, which results in the damage to the central cholinergic system and the reduction in spatial memory ability [9]. The occurrence of cognitive impairment in PD patients is strongly associated with the changes in neurotransmitters such as acetylcholine, dopamine and serotonin induced by extensive atrophy and neurodegneration in the frontal cortex, hippocampus and basal ganglia [10].
According to Traditional Chinese Medicine (TCM), the “tremor sign” seen during PD is considered to be an “endogenous wind-evil”. TCM classifies PD into four types: phlegm-heat and stirring of wind, Qi-stagnancy and blood stasis, insufficiency of vital energy and blood, and hepatic and renal Yin deficiency. At present, the points of acupuncture contain Baihui (DU20), Dazhui (DU14) and Hegu (LI4) [11].
The Y-maze test was used to determine rat’s spatial orientation, reaction time and memory retrieval, which are indicators to assess cognitive function. The above-mentioned is similar to the cognitive dysfunction identified in PD patients in a clinical setting. Changes in the level of ChAT expression in the brain can be explored at the molecular and cellular levels by measuring ChAT activity and the number of ChAT-positive fibers in the different treatment groups. In this study, intracranial injection of 6-OHDA was used to simulate the symptoms that occur during medial to advanced stages of PD pathology. The alterations in cognitive ability were comprehensively evaluated by Y-maze test, ChAT activity detection and determination of ChAT-positive fibers.
Experimental results exhibited that cognitive ability was decreased in the hippocampus and corpus striatum and ChAT activity was reduced in the central nervous system in the model control group (P < 0.01). The number of ChAT-positive cells was significantly diminished in the model control group (P < 0.01). After treatment with either electroacupuncture or L-dopa, cognitive ability was significantly improved in PD rats; i.e., learning frequency was diminished and learning time was shortened in the Y-maze test (P < 0.01); simultaneously, ChAT expression was increased (P < 0.01), and ChAT activity was elevated (P < 0.01). However, it is considered that treatment improved the behavioral ability in PD rats. Thus, rats can have a fast reaction to electric shock in the Y-maze test. There was no significant difference in cognitive ability, ChAT expression and the degree of ChAT expression in the brain between the electroacupuncture and L-dopa groups, indicating that the effects of electroacupuncture and conventional L-dopa treatment are possibly identical on improving cognitive function in PD. It has been shown that a physiologically suitable dosage of L-dopa, protects cellular function in PD rats. Interesting for some decades, a few Chinese PD experts have liked to follow a levodopa treatment dose rule: a small yet steady stream (Wang XD) [12].
The motor symptom is frequently severe in PD patients at the middle and advanced stages, and the cognitive function is apparently damaged [13]. Long-term administration of L-dopa may accompany motor complications. In accordance with experimental findings and taking into account the clinical practice, comprehensive assessment, appropriate use of electroacupuncture and L-dopa substitution can reduce motor complications, improve cognitive ability and elevate the quality of life in PD patients with middle and advanced stages. However, due to the specific nature of cognitive damage in PD model, memory impairment may take a long time to show clear performance, hence the related problems could be investigated in future studies [14-18].
Acknowledgements
Many thanks to the contribution of Dr HAN Lu. This research was supported by National Natural Science Foundation of China (No 8141038009, to XPW).
Disclosure of conflict of interest
None.
References
- 1.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 2.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 3.Gu SH, Chen J, Mei SJ, et al. Related factors and nursing intervention on cognitive impairment for patients with Parkinson’s disease. Nursing and Rehabilitation. 2012;11:118–120. [Google Scholar]
- 4.Emre MD. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2:229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 5.Wu B, Han L, Sun BM, Hu XW, Wang XP. Influence of deep brain stimulation of the subthalamic nucleus on cognitive function in patients with Parkinson’s disease. Neurosci Bull. 2014;30:153–161. doi: 10.1007/s12264-013-1389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam YC, Kum WF, Durairajan SS, Lu JH, Man SC, Xu M, Zhang XF, Huang XZ, Li M. Efficacy and safety of acupuncture for idiopathic Parkinson’s disease: a systematic review. J Altern Complement Med. 2008;14:663–671. doi: 10.1089/acm.2007.0011. [DOI] [PubMed] [Google Scholar]
- 7.Hua XB, Li CR, Zhou HL. Development of Rat Acupoint Atlas. Laboratory Animal and Animal Experiments. 1991;1:1–5. [Google Scholar]
- 8.Saur R, Maier C, Milian M, Riedel E, Berg D, Liepelt-Scarfone I, Leyhe T. Clock test deficits related to the globel cognitive state in Alzheimer’s and Parkinson’s disease. Dement Geriatr Cogn Disord. 2012;33:59–72. doi: 10.1159/000336598. [DOI] [PubMed] [Google Scholar]
- 9.Bueters T, von Euler M, Bendel O, von Euler G. Degeneration of newly formed CA1 neurons following global ischemia in the rat. Exp Neurol. 2008;209:114–124. doi: 10.1016/j.expneurol.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Nagano-Saito A, Washimi Y, Hrahaha Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K. Cerebral atrophy and its relation to cognitive impairment in Parkinson’s disease. Neurology. 2005;64:224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 11.Jiang YP, Liu H, Xu P, Wang Y, Lu GH. Effect of electro-acupuncture intervention on cognition attention bias in heroin addiction abstinence-a dot-probe-based event-related potential study. Chin J Integr Med. 2011;17:267–271. doi: 10.1007/s11655-011-0698-y. [DOI] [PubMed] [Google Scholar]
- 12.Wang XD. Levodopa in treatment of Parkinson’s disease: review and Prospect. Chin J Gerontol Med. 2004;2:129–131. [Google Scholar]
- 13.Wang XP, Sun BM, Ding HL. Changes of procedural learning in Chinese patients with nondemented Parkinson disease. Neurosci Lett. 2009;449:161–163. doi: 10.1016/j.neulet.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 14.Xiao JJ, Yin M, Wang ZJ, Wang XP. Transplanted Neural Stem Cells: Playing a Neuroprotective Role by Ceruloplasmin in the Substantia Nigra of PD Model Rats? Oxid Med Cell Longev. 2015;2015:618631. doi: 10.1155/2015/618631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei SB. Cross interaction of dopaminergic and adrenergic systems in neural modulation. Int J Physiol Pathophysiol Pharmacol. 2014;6:137–142. [PMC free article] [PubMed] [Google Scholar]
- 16.Arankalle DV, Nair PM. Effect of electroacupuncture on function and quality of life in Parkinson’s disease: a case report. Acupunct Med. 2013;31:235–23. doi: 10.1136/acupmed-2012-010285. [DOI] [PubMed] [Google Scholar]
- 17.Ghaffari BD, Kluger B. Mechanisms for alternative treatments in Parkinson’s disease: acupuncture, tai chi, and other treatments. Curr Neurol Neurosci Rep. 2014;14:451. doi: 10.1007/s11910-014-0451-y. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G, Batya SS. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]


