Abstract
Colorectal cancer is the third most common human cancer with frequent overexpression of the cGMP-specific phosphodiesterase 5 (PDE5). In the present study, we investigated that the anticancer effect of sildenafil on human colorectal cancer in vitro and in vivo, which is a potent and selective inhibitor of PDE5 for the treatment of erectile dysfunction and pulmonary arterial hypertension in the clinic. Sildenafil significantly induced cell growth inhibition, cell cycle arrest and apoptosis of human colorectal cancer with increased intracellular reactive oxidative specie (ROS) levels, which were accompanied by obvious alterations of related proteins such as CDKs, Cyclins and PARP etc. Pretreatment with ROS scavenger N-acetyl-L-cysteine could reverse sildenafil-induced ROS accumulation and cell apoptosis. Inhibition of the activity of protein kinase G with KT-5823 could enhance sildenafil-induced apoptosis. Furthermore, sildenafil caused the reduction of xenograft models of human colorectal cancer in nude mice. Overall, these findings suggest that sildenafil has the potential to be used for treatment of human colorectal cancer.
Keywords: Sildenafil, colorectal cancer, PDE5, PKG
Introduction
Colorectal cancer currently is the third most common human cancer with more than 1 million new cases every year in the world [1]. Treatments for colorectal cancer include surgery, chemotherapy, radiation therapy and immunotherapy [2]. Despite progress in decreasing the death rates of colorectal cancer has been accelerated by improving the diagnosis and combinational treatment, the five-year survival rate for colorectal cancer is less than 60% [3]. Therefore, it is urgent to develop novel therapeutic strategies against colorectal cancer.
Sildenafil (Viagra®), a potent and selective inhibitor of cGMP-specific phosphodiesterase type 5 (PDE5), is used to treat erectile dysfunction (ED) and pulmonary arterial hypertension (PAH) in the clinic. Sildenafil competitively binds to PDE5 with cGMP due to their similar structures, which enhances the levels of cGMP to activate protein kinase G (PKG) resulting in the relaxation of vascular smooth muscle and the increase of blood inflow [4]. PDE5 is widely expressed in the vascular, pulmonary, visceral smooth muscles, platelets, gastrointestinal epithelial cells, and cerebellum, etc [5]. Overexpression of PDE5 is detected in multiple types of cancer [6-9], which has also been confirmed in various cell lines originating from breast cancer (MCF-7, HTB-26, MDA-MB-468), prostate cancer (LNCAP, PC3), bladder cancer (HTB-76, HT1376) and colorectal cancer (HT29, HCT116, SW480, T84) [10]. These findings suggest that PDE5 may play an important role in tumorigenesis and that targeting PDE5 may be a promising anticancer method. Actually, the anticancer effect of sildenafil and other PDE5 inhibitors have been evaluated by several groups. It has been reported that sildenafil and vardenafil could suppress tumor cell growth and induce caspase-dependent apoptosis of B-cell chronic lymphocytic leukemia cells in vitro [11]. The non-specific PDE5 inhibitor exisulind and its analogues selectively induced the apoptosis of various human prostate, colon and breast cancer cells, which was owing to their inhibitory effects on PDE5 expression, thereby enhancing the cGMP-induced activation of PKG [12-21]. In the present study, we investigated that anticancer effect of sildenafil in human colorectal cancer in vitro and in vivo.
Material and methods
Cell lines, cell culture, and reagents
Human colorectal cancer cell lines (HT-29, SW480, SW620, HCT116, SW1116) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 ng/ml) in a humidified incubator at 37°C with 5% CO2. Sildenafil from ApexBio technology were dissolved as a stock solution 10mM in DMSO and stored at -20°C. N-acetly-L-cysteine (NAC) and dihydroethidium (DHE) were purchased from Sigma-Aldrich. Anti-PARP (9542) and Anti-β-Catenin (9582) antibodies were from Cell Signaling Technologies. Anti-CDK2 (SC-163), Anti-CDK4 (SC-260), Anti-Cycin A (SC-596), Anti-Cycin D1 (SC-718), Anti-Cyclin E (SC-481) and Anti-Hsp90 (SC-13119) antibodies were from Santa Cruz Biotechnology. Anti-CDK6 (3524-1) antibodies were from Abcam. Anti-GAPDH (LK9002T) antibodies were from Tianjin Sungene Biotech.
Cell viability assay
Cells were firstly seeded into a 96-well plate at a density of 5000 cells per well, and incubated with drugs in three parallel wells for 72 h. Then MTT was added to each well at a final concentration of 0.5 mg/ml. After incubation for 4 h, formazan crystals were dissolved in 100 μl of DMSO, and absorbance at 570 nm was measured by plate reader. The concentrations required to inhibit growth by 50% (IC50) were calculated from survival curves using the Bliss method [22,23].
Cell cycle analysis
Cells were harvested and washed twice with cold phosphate-buffered saline (PBS), then fixed with ice-cold 70% ethanol for 30 min at 4°C. After centrifugation at 200 × g for 10 min, cells were washed twice with PBS and resuspended with 0.5 ml PBS containing PI (50 μg/ml), 0.1% Triton X-100, 0.1% sodium citrate, and DNase-free RNase (100 μg/ml), and detected by FCM after 15 min incubation at room temperature in the dark. Fluorescence was measured at an excitation wavelength of 480 nm through a FL-2filter (585 nm). Data were analyzed using ModFit LT 3.0 software (Becton Dickinson) [24,25].
Apoptosis assay
Cell apoptosis was evaluated with flow cytometry (FCM) assay. Briefly, cells were harvested and washed twice with PBS, stained with Annexin V-FITC and propidium iodide (PI) in the binding buffer, and detected by FACS Calibur FCM (BD, CA, USA) after 15 min incubation at room temperature in the dark. Fluorescence was measured at an excitation wave length of 480 nm through FL-1 (530 nm) and FL-2 filters (585 nm). The early apoptotic cells (Annexin V positive only) and late apoptotic cells (Annexin V and PI positive) were quantified [26,27].
Reactive oxygen species (ROS) assay
Cells were incubated with 10 µM of DHE for 30 min at 37°C, washed twice with PBS and immediately photographed under fluorescent microscope (Olympus, Japan). For each well, 5 fields were taken randomly [28,29].
Western blot analysis
Cells were harvested and washed twice with cold PBS, then resuspended and lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 ng/ml PMSF, 0.03% aprotinin, 1 μM sodium orthovanadate) at 4°C for 30 min. Lysates were centrifuged for 10 min at 14,000 × g and supernatants were stored at -80°C as whole cell extracts. Total protein concentrations were determined with Bradford assay. Proteins were separated on 12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% BSA and incubated with the indicated primary antibodies. Corresponding horseradish peroxidase-conjugated secondary antibodies were used against each primary antibody. Proteins were detected using the chemiluminescent detection reagents and films [30,31].
Nude mice xenograft tumor assay
Balb/c nude mice were obtained from the Guangdong Medical Laboratory Animal Center and maintained with sterilized food and water. Six female nude mice with 5 weeks old and 20 g weight were used for each group. Each mouse was injected subcutaneously with SW480 or HCT116 cells (3 × 106 in 200 μl of medium) under the shoulder. When the subcutaneous tumors were approximately 0.4 × 0.4 cm2 (two perpendicular diameters) in size, mice were randomized into three groups, and were taken orally with vehicle alone (0.9% saline), sildenafil (50 mg/kg) and sildenafil (150 mg/kg) every two days. The body weights of mice and the two perpendicular diameters (A and B) of tumors were recorded. The tumor volume (V) was calculated according to the formula:
v = (π/6){(A+B)/2)}3
The mice were anaesthetized after experiment, and tumor tissue was excised from the mice and weighted. The rate of inhibition (IR) was calculated according to the formula [32,33]:
IR = 1-(Mean tumor weight of experimental group)/(Mean tumor weight of control group)×100%
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis of the difference between treated and untreated groups was performed with Student’s t-test. Values of P < 0.05 were considered as significant differences.
Results
Sildenafil inhibited the growth of human colorectal cancer cells in vitro
To evaluate the effect of sildenafil on colorectal cancer cells, cell survival was detected by MTT assay. Five human colorectal cancer cell lines HT-29, SW480, SW620, HCT116 and SW1116 were treated with increasing concentrations of sildenafil range from 10 μM to 1 mM for 72 h. As shown in Figure 1, the results showed that the growth of five human cancer cell lines were similarly inhibited by sildenafil in a concentration-dependent manner with the IC50 values range from 190 to 271 μM.
Figure 1.
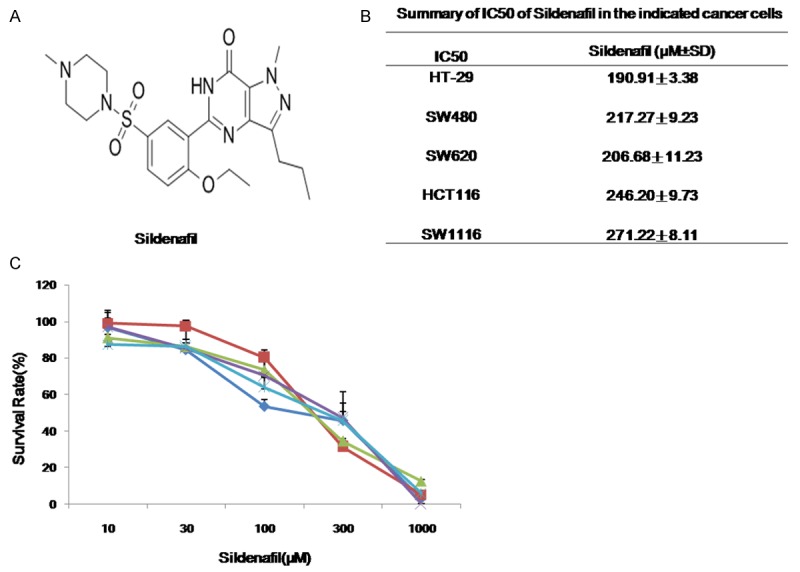
Sildenafil inhibited the growth of human colorectal cancer cells in vitro. Cells were treated with the indicated concentrations of sildenafil for 72 h, and cell survival was measured by MTT assay. The chemical structure of sildenafil (A), summary IC50 values (B) and survival curves (C) in the indicated cancer cells were shown. Data were mean ± SD of three independent experiments.
Sildenafil induced G1 cell cycle arrest in human colorectal cancer cells
To explore whether the growth inhibition of colorectal cancer cells by sildenafil is due to cell cycle arrest, cell cycle distribution was assessed after sildenafil treatment. Colorectal cancer cells SW480 and HCT116 were treated with sildenafil (100, 200, 300 μM) for 24 h and 48 h, stained with PI and examined by FCM. The cell cycle distribution was analyzed with ModFit LT 3.0 software. As shown in Figure 2A and 2B, compared with the control groups, sildenafil induced the accumulation of G0/G1 phase in SW480 and HCT116 cells. To further investigate the molecular mechanism of cell cycle arrest by sildenafil, the cell cycle related proteins were detected by Western blot. The results showed that the protein levels of Cyclin A, Cyclin D1, Cyclin E, CDK2, CDK4 and CDK6 were down-regulated in the time- and dose-dependent manners in SW480 and HCT116 cells after sildenafil treatment (Figure 2C).
Figure 2.
Sildenafil induced G1 cell cycle arrest in human colorectal cancer cells. SW480 (A) and HCT116 (B) cells were treated with sildenafil at the indicated concentrations and time-points. The distribution of cell cycle was detected by FCM with PI staining. The percentages of subG1, G0/G1, S, G2/M phase were calculated by using ModFit LT 3.0 software. The protein expression was examined by Western blot, and GAPDH was used as loading control. The representative charts, q uantified results and Western blot results (C) of three independent experiments were shown. *P < 0.05 and **P < 0.01 versus corresponding control.
Sildenafil induced apoptosis in human colorectal cancer cells
In addition to the evaluation of sildenafil induced growth inhibition and cell cycle arrest, its effects on apoptosis were analyzed by FCM. Both SW480 and HCT116 cells were treated with the different concentrations of sildenafil (100, 200, 300 μM) for 48 h, stained with Annexin V/PI, and examined by FCM. As shown in Figure 3A and 3B, sildenafil treatment significantly induced apoptosis in both SW480 and HCT116 cells, and both proportions of Annexin V+/PI- (early stage of apoptosis) and Annexin V+/PI+ (late stage of apoptosis) cells were increased with the elevated sildenafil concentrations. To further investigate the molecular mechanism of cell apoptosis by sildenafil, the apoptotic related proteins were detected by Western blot. After treatment with sildenafil, the protein levels of full-length PARP, β-Catenin and Hsp90 were time- and dose-dependent decreased in both SW480 and HCT116 cells (Figure 3C).
Figure 3.

Sildenafil induced apoptosis in human colorectal cancer cells. SW480 (A) and HCT116 (B) cells were treated with sildenafil at the indicated concentrations and time-points. The apoptosis was detected by FCM Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells indicated the early and late stage of apoptosis. The protein expression was examined by Western blot, and GAPDH was used as loading control. The representative charts, quantified results and Western blot results (C) of three independent experiments were shown. *P < 0.05 and **P < 0.01 versus corresponding control.
ROS generation was critical for sildenafil-induced apoptosis in human colorectal cancer cells
Many chemotherapeutic agents induce the death of cancer cells through elevating the level of intracellular ROS [34]. To explore the role of ROS in the anticancer effect of sildenafil in colorectal cancer cells, we used dihydroethidium (DHE) as ROS fluorescent probe, which can be oxidized by ROS to oxide ethidium that binds with DNA to emit the detectable red fluorescence [29]. As shown in Figure 4A and 4B, sildenafil exposure resulted in a time- and concentration-dependent ROS accumulation in both SW480 and HCT116 cells. To further explore the relationship between sildenafil induced apoptosis and ROS generation, both cells were treated with sildenafil for 48 h in the presence or absence of the anti-oxidative agent NAC pretreatment for 1 h and stained with DHE. As predicted, the sildenafil-induced ROS accumulation was greatly reduced by NAC due to its ability to elevate the intracellular glutathione to prevent the production of ROS (Figure 5A and 5B). Moreover, cell apoptosis was detected by FCM with Annexin V/PI staining. The sildenafil-induced apoptosis were partially blocked by NAC in both SW480 and HCT116 cells (Figure 5C and 5D). Taken together, these data suggested that ROS is critical for sildenafil-induced apoptosis in colorectal cancer cells.
Figure 4.

Sildenafil induced ROS accumulation in human colorectal cancer cells. SW480 and HCT116 cells were treated with sildenafil at the indicated concentrations and time-points, stained with DHE and photographed under microscope. The representative micrographs (A) and quantified results (B) of three independent experiments were shown. *P < 0.05 and **P < 0.01 versus corresponding control.
Figure 5.

ROS generation was critical for sildenafil-induced apoptosis in human colorectal cancer cells. SW480 and HCT116 cells were treated with sildenafil at 300 μM for 48 h in the presence or absence of 5 mM NAC pretreatment for 1 h, stained with DHE and photographed under microscope. The representative micrographs (A) and quantified results (B) of three independent experiments were shown. The apoptosis was detected by FCM with Annexin V/PI staining. The representative charts (C) and quantified results (D) of three independent experiments were shown. Sil: Sildenafil. *P < 0.05 and **P < 0.01 versus corresponding control.
Inhibition of PKG enhanced sildenafil-induced apoptosis in human colorectal cancer cells
PDE5 inhibitor exerts their biological effects mainly through the PKG pathway [35]. To evaluate the function of PKG on sildenafil-induced apoptosis in colorectal cancer cells, both SW480 and HCT116 cells were treated with sildenafil at 100 μM and a specific PKG inhibitor KT-5823 at the nontoxic concentration of 2 μM for 48 h, stained with Annexin V/PI, and examined by FCM. As shown in Figure 6A and 6B, KT-5823 alone did not significantly induce apoptosis, but obviously promoted sildenafil-induced apoptosis in both SW480 and HCT116 cells, suggesting inhibition of PKG could enhance sildenafil-induced apoptosis in colorectal cancer cells.
Figure 6.

Inhibition of PKG enhanced sildenafil-induced apoptosis in human colorectal cancer cells. SW480 and HCT116 cells were treated with sildenafil at 100 μM and KT-5823 at 2 μM alone or combination for 48 h. The apoptosis was detected by FCM with Annexin V/PI staining. The representative charts (A) and quantified results (B) of three independent experiments were shown. Sil: Sildenafil. *P < 0.05 and **P < 0.01 versus corresponding control.
Sildenafil inhibited the subcutaneous xenograft growth of human colorectal cancer in nude mice
To investigate the anticancer effect of sildenafil on colorectal cancer in vivo, the nude mice with subcutaneous xenografts of colorectal cancer cells SW480 and HCT116 were treated with two doses of sildenafil. As shown in Figure 7A-C, compared with control group, sildenafil clearly inhibited the tumors growth by reducing the volume and weight of SW480 and HCT116 xenografts. The inhibition rates of sildenafil at the doses of 50 and 150 mg/kg were 40.1% and 57.8% in SW480 xenografts as well as 13.3% and 61.4% in HCT116 xenografts, respectively (Figure 7D). In addition, the weight of mice were equal between before and after experiments in each group, suggesting treatment of sildenafil at the indicated doses did not cause significant side effects in mice (Figure 7D).
Figure 7.

Sildenafil inhibited the subcutaneous xenograft growth of human colorectal cancer in nude mice. Each nude mouse was injected subcutaneously with SW480 or HCT116 cells (3 × 106 in 200 μl of medium) under the shoulder. When the subcutaneous tumors were approximately 0.4 × 0.4 cm2 (two perpendicular diameters) in size, the nude mice were randomly divided into three groups (n = 6 mice/group), and taken orally with vehicle alone (0.9% saline), sildenafil (50 mg/kg), sildenafil (150 mg/kg) every two days. The body weights of mice and tumor volume were recorded. The mice were anaesthetized after experiment, and tumor tissue was excised from the mice and weighted. The original tumors (A), tumor volumes (B), tumor weights (C) and summary data (D) were shown. Sil: Sildenafil. *P < 0.05 and **P < 0.01 versus corresponding control.
Disscussion
Recently, the anticancer effect of sildenafil has been evaluated by several groups. Sildenafil could directly trigger caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells in vitro [11], and enhance endogenous antitumor immunity by inhibiting the function of myeloid-derived suppressor cells [36]. In a rat brain tumor model, sildenafil improved the efficacy of doxorubicin by increasing the permeability of blood-brain tumor barrier [37]. Moreover, sildenafil was reported to increase the chemotherapeutic efficacy of doxorubicin in prostate cancer and breast cancer cells without amplifying its toxicity [38,39]. Sildenafil could also enhance the effects of other chemotherapeutic agents including mitomycin C, doxorubicin, cisplatin, and gemcitabine in bladder and pancreatic cancer cells [40]. It has been reported that sildenafil could enhance the cytotoxicity of celecoxib in tumor cells including colorectal cancer, hepatoma, glioblastoma and medulloblastoma in an NOS-dependent activation of CD95 induced cell death [41]. Additionally, co-delivery of sildenafil and crizotinib with PEG-PLA micellar carriers showed synergistic and improved anti-tumoral therapy in MCF-7 breast cancer cells [42], and combinatorial delivery of sildenafil-crizotinib-palbociclib using TPGS-PLA micelles could improve cancer treatment in A549 non-small lung cancer cells [43]. Our and others previous data have shown that sildenafil is an inhibitor of ABCB1/P-gp, ABCG2/BCRP, ABCC4/MRP4, ABCC5/MRP5, and ABCC10/MRP7 [44-48]. However, the in vivo studies suggested that sildenafil is not sufficient to cause a meaningful interaction with the drug transporters ABCB1/P-gp and ABCG2/BCRP [49].
In this report, we firstly showed that sildenafil had anti-growth effect on human colorectal cancer cells in vitro and in vivo. Treatment with sildenafil induced cell arrest at G1 phase and apoptosis with the increasing accumulation of intracellular ROS in the concentration- and time-dependent manners. Sildenafil-induced apoptosis was partially inhibited by ROS inhibitor NAC but enhanced by PKG inhibitor KT-5823. PKG as the downstream kinase of cGMP normally activates specific proteins which control the contractile activity of smooth muscle cells. Interestingly, accumulating evidences indicate that cancer cells express the lower levels of PKG in comparison with normal tissue [50,51]. The increased activation of PKG by cGMP induced apoptosis and growth arrest in breast cancer cells [52], and the increased expression of PKG decreased invasiveness and tumor growth of human colon cancer xenograft models in nude mice [51]. PDE inhibitor sulindac sulfide specifically inhibited cGMP hydrolysis and activated PKG to produce apoptosis in breast cancer cells, but had no effect on normal human mammary epithelial cells [12]. However, inhibition of cGMP-induced PKG activation by PKG inhibitor KT-5823 also suppressed the growth of breast cancer cells through induction of cell apoptosis [52]. This is consistent with our results that inhibition of PKG could enhance sildenafil-induced apoptosis. It is possible that the anticancer action of PDE5 inhibitors including sildenafil may be mainly attributed to cGMP/PKG regulated downstream pathways. CGMP-activated PKG could decrease the expression of Wnt/β-catenin [18] and cyclin D1 [53] to induce caspase-dependent apoptosis and cell growth arrest, which was also detected in our study.
In summary, our data demonstrated that sildenafil significantly inhibited the growth of human colorectal cancer cells in vitro and in vivo by inducing cell cycle arrest and apoptosis with the increasing intracellular ROS levels. Sildenafil-induced apoptosis was partially reversed by inhibition of ROS generation but enhanced by inhibition of PKG activity. These results suggest that sildenafil may be a promising anticancer agent against colorectal cancer.
Acknowledgements
This work was supported by funds from the Chinese National Natural Science Foundation No. 31271444 and No. 81201726 (Z. S.), the Specialized Research Fund for the Doctoral Program of Higher Education No. 20124401120007 (Z. S.), the Guangdong Natural Science Funds for Distinguished Young Scholar No. 2014A030306001 (Z. S.), and the Science and Technology Program of Guangzhou No. 2014J4100009 (Z. S.).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 4.Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 5.Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–280. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 6.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 7.Karami-Tehrani F, Moeinifard M, Aghaei M, Atri M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch Med Res. 2012;43:470–475. doi: 10.1016/j.arcmed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Eggen T, Sager G, Berg T, Nergaard B, Moe BT, Orbo A. Increased gene expression of the ABCC5 transporter without distinct changes in the expression of PDE5 in human cervical cancer cells during growth. Anticancer Res. 2012;32:3055–3061. [PubMed] [Google Scholar]
- 9.Zhang X, Yan G, Ji J, Wu J, Sun X, Shen J, Jiang H, Wang H. PDE5 inhibitor promotes melanin synthesis through the PKG pathway in B16 melanoma cells. J Cell Biochem. 2012;113:2738–2743. doi: 10.1002/jcb.24147. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B, Strada SJ. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr Top Med Chem. 2007;7:437–454. doi: 10.2174/156802607779941198. [DOI] [PubMed] [Google Scholar]
- 11.Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, Binet JL, Delic J, Merle-Beral H. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–269. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 12.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu B, Vemavarapu L, Thompson WJ, Strada SJ. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem. 2005;94:336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, Zhao TL, Piazza G, Klein-Szanto AJ, Pamukcu R, Alila H, Bunn PA Jr, Thompson WJ. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–488. [PubMed] [Google Scholar]
- 15.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, Fetter JR, Gresh WE Jr, Klein-Szanto AJ, Farnell DR, Eto I, Grubbs CJ. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 16.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 17.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, Keeton AB, Xi Y, Abadi AH, Grizzle WE, Piazza GA. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res (Phila) 2012;5:822–833. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/beta-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 2011;4:1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed HA, Girgis NM, Wilcken R, Bauer MR, Tinsley HN, Gary BD, Piazza GA, Boeckler FM, Abadi AH. Synthesis and molecular modeling of novel tetrahydro-beta-carboline derivatives with phosphodiesterase 5 inhibitory and anticancer properties. J Med Chem. 2011;54:495–509. doi: 10.1021/jm100842v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, Maxuitenko YY, Keeton AB, Piazza GA. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal antiinflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3:1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abadi AH, Gary BD, Tinsley HN, Piazza GA, Abdel-Halim M. Synthesis, molecular modeling and biological evaluation of novel tadalafil analogues as phosphodiesterase 5 and colon tumor cell growth inhibitors, new stereochemical perspective. Eur J Med Chem. 2010;45:1278–1286. doi: 10.1016/j.ejmech.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Jain S, Kim IW, Peng XX, Abraham I, Youssef DT, Fu LW, El Sayed K, Ambudkar SV, Chen ZS. Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007;98:1373–1380. doi: 10.1111/j.1349-7006.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, Fu LW, Ambudkar SV, Chen ZS. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–793. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Ouyang Y, Zhang X, Zhou W, Wang F, Huang Z, Wang X, Chen Y, Zhang H, Fu L. Effect of HM910, a novel camptothecin derivative, on the inhibition of multiple myeloma cell growth in vitro and in vivo. Am J Cancer Res. 2015;5:1000–1016. [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao D, Tang S, Aslam S, Ahmad M, To KK, Wang F, Huang Z, Cai J, Fu L. UMMS-4 enhanced sensitivity of chemotherapeutic agents to ABCB1-overexpressing cells via inhibiting function of ABCB1 transporter. Am J Cancer Res. 2014;4:148–160. [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Z, Park HR, Du Y, Li Z, Cheng K, Sun SY, Fu H, Khuri FR. Cables1 complex couples survival signaling to the cell death machinery. Cancer Res. 2015;75:147–158. doi: 10.1158/0008-5472.CAN-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Z, Li Z, Li ZJ, Cheng K, Du Y, Fu H, Khuri FR. Cables1 controls p21/Cip1 protein stability by antagonizing proteasome subunit alpha type 3. Oncogene. 2015;34:2538–2545. doi: 10.1038/onc.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XX, Xie FF, Zhu XJ, Lin F, Pan SS, Gong LH, Qiu JG, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Shi Z, Yan XJ. Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer. Oncotarget. 2015;6:14926–39. doi: 10.18632/oncotarget.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid Med Cell Longev. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Liang YJ, Chen ZS, Wang XH, Ding Y, Chen LM, Fu LW. Overexpression of Survivin and XIAP in MDR cancer cells unrelated to P-glycoprotein. Oncol Rep. 2007;17:969–976. [PubMed] [Google Scholar]
- 31.Shi Z, Parmar S, Peng XX, Shen T, Robey RW, Bates SE, Fu LW, Shao Y, Chen YM, Zang F, Chen ZS. The epidermal growth factor tyrosine kinase inhibitor AG1478 and erlotinib reverse ABCG2-mediated drug resistance. Oncol Rep. 2009;21:483–489. [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu JG, Zhang YJ, Li Y, Zhao JM, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Shi Z. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget. 2015;6:15494–509. doi: 10.18632/oncotarget.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Z, Liang YJ, Chen ZS, Wang XW, Wang XH, Ding Y, Chen LM, Yang XP, Fu LW. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39–47. doi: 10.4161/cbt.5.1.2236. [DOI] [PubMed] [Google Scholar]
- 34.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, Weill B, Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- 35.Reffelmann T, Kloner RA. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation. 2003;108:239–244. doi: 10.1161/01.CIR.0000081166.87607.E2. [DOI] [PubMed] [Google Scholar]
- 36.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, Ko MK, Bayan JA, Sacapano MR, Espinoza A, Irvin DK, Shu Y. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di X, Gennings C, Bear HD, Graham LJ, Sheth CM, White KL Jr, Gewirtz DA. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat. 2010;124:349–60. doi: 10.1007/s10549-010-0765-7. [DOI] [PubMed] [Google Scholar]
- 39.Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, Kukreja RC. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107:18202–18207. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth L, Roberts JL, Cruickshanks N, Conley A, Durrant DE, Das A, Fisher PB, Kukreja RC, Grant S, Poklepovic A, Dent P. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol. 2014;85:408–419. doi: 10.1124/mol.113.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth L, Roberts JL, Cruickshanks N, Tavallai S, Webb T, Samuel P, Conley A, Binion B, Young HF, Poklepovic A, Spiegel S, Dent P. PDE5 Inhibitors Enhance Celecoxib Killing in Multiple Tumor Types. J Cell Physiol. 2015;230:1115–1127. doi: 10.1002/jcp.24843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marques JG, Gaspar VM, Markl D, Costa EC, Gallardo E, Correia IJ. Co-delivery of Sildenafil (Viagra(®)) and Crizotinib for synergistic and improved anti-tumoral therapy. Pharm Res. 2014;31:2516–2528. doi: 10.1007/s11095-014-1347-x. [DOI] [PubMed] [Google Scholar]
- 43.de Melo-Diogo D, Gaspar VM, Costa EC, Moreira AF, Oppolzer D, Gallardo E, Correia IJ. Combinatorial delivery of Crizotinib-Palbociclib-Sildenafil using TPGS-PLA micelles for improved cancer treatment. Eur J Pharm Biopharm. 2014;88:718–729. doi: 10.1016/j.ejpb.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Shi Z, Tiwari AK, Patel AS, Fu LW, Chen ZS. Roles of sildenafil in enhancing drug sensitivity in cancer. Cancer Res. 2011;71:3735–3738. doi: 10.1158/0008-5472.CAN-11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 46.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, Talele TT, Fu LW, Chen ZS. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 48.Chen JJ, Sun YL, Tiwari AK, Xiao ZJ, Sodani K, Yang DH, Vispute SG, Jiang WQ, Chen SD, Chen ZS. PDE5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding Cassette C10) transporter. Cancer Sci. 2012;103:1531–1537. doi: 10.1111/j.1349-7006.2012.02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin F, Hoogendijk L, Buil L, Beijnen JH, van Tellingen O. Sildenafil is not a useful modulator of ABCB1 and ABCG2 mediated drug resistance in vivo. Eur J Cancer. 2013;49:2059–2064. doi: 10.1016/j.ejca.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 50.Hou Y, Gupta N, Schoenlein P, Wong E, Martindale R, Ganapathy V, Browning D. An anti-tumor role for cGMP-dependent protein kinase. Cancer Lett. 2006;240:60–68. doi: 10.1016/j.canlet.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Yamanaka Y, Mammoto T, Kirita T, Mukai M, Mashimo T, Sugimura M, Kishi Y, Nakamura H. Epinephrine inhibits invasion of oral squamous carcinoma cells by modulating intracellular cAMP. Cancer Lett. 2002;176:143–148. doi: 10.1016/s0304-3835(01)00764-9. [DOI] [PubMed] [Google Scholar]
- 52.Fallahian F, Karami-Tehrani F, Salami S, Aghaei M. Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and -negative breast cancer cell lines. FEBS J. 2011;278:3360–3369. doi: 10.1111/j.1742-4658.2011.08260.x. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Liu L, David ML, Whitehead CM, Chen M, Fetter JR, Sperl GJ, Pamukcu R, Thompson WJ. Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve beta-catenin and cyclin D1 down-regulation. Biochem Pharmacol. 2002;64:1325–1336. doi: 10.1016/s0006-2952(02)01345-x. [DOI] [PubMed] [Google Scholar]



