Abstract
Melanoma is a highly aggressive form of skin cancer and a leading cause of death from skin diseases mainly due to its propensity to metastasis. Due to metastatic tendency, melanoma is often associated with activation of Wnt/β-catenin signaling mechanism. Blocking β-catenin activation may be a good strategy to block melanoma-associated mortality. We have shown earlier that grape seed proanthocyanidins (GSPs) inhibit melanoma cell migration via targeting cyclooxygenase-2 (COX-2) overexpression. Here we explored further whether inhibition of inflammatory mediators-mediated activation of β-catenin by GSPs is associated with the inhibition of melanoma cell migration. Our study revealed that PGE2 receptors (EP2 and EP4) agonists promote melanoma cell migration while PGE2 receptor antagonist suppressed the migration capacity of melanoma cells. GSPs treatment inhibit butaprost (EP2 agonist) or Cay10580 (EP4 agonist) induced migration of melanoma cells. Western blot analysis revealed that GSPs reduced cellular accumulation of β-catenin, and decreased the expressions of matrix metalloproteinase (MMP)-2, MMP-9 and MITF, downstream targets of β-catenin in melanoma cells. GSPs also reduced the protein expressions of PI3K and p-Akt in the same set of experiment. To verify that β-catenin is a specific molecular target of GSPs, we compared the effect of GSPs on cell migration of β-catenin-activated (Mel1241) and β-catenin-inactivated (Mel1011) melanoma cells. GSPs inhibit cell migration of Mel1241 cells but not of Mel1011 cells. Additionally, in vivo bioluminescence imaging data indicate that dietary administration of GSPs (0.5%, w/w) in supplementation with AIN76A control diet inhibited the migration/extravasation of intravenously injected melanoma cells in lungs of immune-compromised nude mice, and that this effect of GSPs was associated with an inhibitory effect on the activation of β-catenin and its downstream targets, such as MMPs, in lungs as a target organ.
Keywords: Grape seed proanthocyanidins, melanoma, cell migration, prostaglandin E2, PGE2 receptors, β-catenin, bioluminescence imaging, metastasis
Introduction
Melanoma is the cancer of pigment forming melanocytes of the skin. The overall incidence of melanoma worldwide is increasing than that of any other cancer. It is an aggressive cancer with high propensity for metastasis. Melanoma is less common than other types of skin cancer, however, it causes the majority of skin cancer-related deaths. Once, diagnosed with metastatic melanoma, most patients will ultimately die of their disease within 2 years [1,2]. Exposure of the skin to solar ultraviolet (UV) radiation is considered as a risk factor for melanoma [3]. UV radiation exposure induces inflammatory mediators, such as upregulation of inducible cyclooxygenase-2 (COX-2) expression and production of prostaglandin (PG) metabolites that have been recognized as potential risk factors for the development of skin cancers [4,5]. These inflammatory mediators play crucial roles in multiple events involved in cancer promotion, progression, tumor cell invasion and metastasis [4,6,7]. Since, melanoma cancer cells possess potent capacity to metastasize distantly, an approach that can block or inhibit its metastatic capacity may facilitate the development of an effective strategy for its treatment and/or prevention, and thus ultimately can reduce the melanoma related deaths.
Inflammatory mediators affect several signaling mechanisms responsible for tumor progression and tumor cell metastasis. One of such molecular targets is β-catenin and its signaling mechanism [8]. β-Catenin is an important component of cell-cell adhesions, where it forms a dynamic link between E-cadherin and cytoskeleton [9,10], and also a critical component of Wnt signaling. The β-catenin regulates expression of various target genes that mediate cellular processes including proliferation and migration of cells [11]. In the canonical model of Wnt signaling, β-catenin is phosphorylated at certain key residues by glycogen synthase kinase-3β (GSK-3β) and casein kinase 1α (CK1α) leading to its ubiquitination and subsequent degradation [11,12]. Like cancers of other organs, the regulation of β-catenin is lost in melanoma [13-15] that leads to nuclear accumulation of β-catenin and subsequent activation of downstream target genes, which includes the genes of cell proliferation and cell invasion [16-18]. Interestingly, COX-2 and β-catenin have been linked in colon cancer and UV-induced skin cancer [19,20], however, there is little information available about the association between inflammatory mediators and β-catenin in melanoma metastasis, and therefore requires further exploration.
Proanthocyanidins have been shown to have anti-carcinogenic activity in some murine tumor models and exhibit no apparent toxicity in vivo animals [21,22]. Seeds of grapes are the major source of proanthocyanidins. Grape seed proanthocyanidins (GSPs) contain primarily proanthocyanidins (89%), which constitute dimers, trimers, tetramers, and oligomers of monomeric catechins and/or (-)-epicatechins, as described previously [22]. Proanthocyanidins are readily available as an extract of grape seeds and have been examined as an anti-carcinogenic agent against some forms of cancers [21]. It is believed that at least some of the constituents present in the proanthocyanidins fraction may act synergistically and thus this product may be more effective than any single constituent. Our previous report suggests that GSPs inhibit melanoma cell migration by inhibiting the expression levels of inflammatory mediators and epithelial-to-mesenchymal transition in melanoma cells [23]. However, it is unclear how the inflammatory mediators act to stimulate the migration capacity of melanoma cells? What is the mechanism and whether there is any relationship between inflammatory mediators and β-catenin signaling which stimulates tumor cell migration and/or metastasis? Therefore, in the present study, we determined and verified the effect of inflammatory mediators on β-catenin signaling molecules and then determined the effect of GSPs on the expression levels of β-catenin in human melanoma cells (A375 and Hs294t). To verify whether inhibition of melanoma cell migration by GSPs is mediated through β-catenin signaling, we used Mel1241, which constitutes activation of Wnt/β-catenin signaling and Mel1011 cell line which is β-catenin-deficient. Finally, the anti-metastatic potential of GSPs on melanoma cell migration was determined in vivo nude mouse model using bioluminescence imaging.
Materials and methods
Source and composition of grape seed proanthocyanidins, and dietary administration
Proanthocyanidins fraction of grape seeds are commercially available from Kikkoman Corporation (Noda, Japan). Quality control of GSPs is maintained by the company on lot-to-lot basis. The chemical composition of GSPs has been detailed previously [22,24]. Briefly, GSPs contain approximately 89% proanthocyanidins, with dimers (6.6%), trimers (5.0%), tetramers (2.9%) and oligomers (74.8%), and are stable for at least two years when refrigerated at 4°C. Experimental diets containing GSPs (0.5%, w/w) were commercially prepared in pellet form in the AIN76A powdered control diet by TestDiet (Richmond, IN) using the GSPs that we provide for this purpose.
Cell lines and cell culture medium
The human melanoma cells lines, A375 and Hs294t, were purchased from the American Type Culture Collection (Manassas, VA), while melanoma cells Mel1241 and Mel1011 were obtained from Dr. Paul Robbins (Center of Cancer Research, National Cancer Institute (Bethesda, MD). The cell lines were cultured as monolayers in DMEM culture medium supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 100 μg/mL penicillin and 100 μg/mL streptomycin and maintained in cell culture incubator. For treatment of the cells, GSPs were dissolved in a small amount of dimethylsulfoxide (DMSO, 100 µL) which was added to the complete cell culture medium and then added to sub-confluent cells (60-70% confluent). Cells treated with DMSO only served as a vehicle control. DMSO concentration in cell culture media was not more than 0.1% (v/v).
Chemicals and antibodies
Celecoxib and EP2 agonist (butaprost) were purchased from Sigma Chemical Co. (St. Louis, MO). EP2 antagonist (AH6809) and EP4 agonist (cay10580) were obtained from Cayman Chemicals (Ann Arbor, MI). β-catenin inhibitor (CCT0364772) was obtained from Enzo life Science (Farmingdale, NY). The antibodies were obtained as follows: antibodies specific for β-catenin were purchased from R&D Biosystems (Minneapolis, MN), while antibodies for phospho β-catenin, CK1α, GSK-3β, PI3K (110), PI3K (85), p-Akt, and total Akt were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against matrix metalloproteinase (MMP)-2 and MMP-9 were obtained from Sigma Chemical Co. (St. Louis, MO), while antibodies for Histone H3, EP2, EP4 and the secondary antibodies conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Boyden Chambers and polycarbonate membranes (8 μm pore size) for cell migration assays were obtained from Neuroprobe (Gaithersburg, MD).
Cell migration assay
The migration capacity of melanoma cancer cells was determined in vitro using Boyden Chambers (Neuroprobe, Inc., Gaithersburg, MD) fitted with Millipore membranes (8 μm pore size), as detailed previously [23,25]. Migratory cells on the membrane were detected after staining with crystal violet dye. The membranes were examined microscopically and cellular migration per treatment group was determined by counting the number of stained cells under microscopic field in at least three to four randomly selected fields using an Olympus BX41 microscope. Data are presented as mean number of the migrating cells ± SD per microscopic field. Representative photomicrographs were obtained using a Qcolor5 digital camera system fitted to an Olympus BX41 microscope.
Western blot analysis
Following treatment of melanoma cells for the indicated time periods with or without GSPs or any other agent, the cells were harvested, washed with cold PBS and lysed with ice-cold lysis buffer supplemented with protease inhibitors, as detailed previously [23]. Similar to cell lysates, lung tissue lysates were also prepared after homogenizing the tissues in lysis buffer for western blot analysis. Lung samples were pooled from 2 animals and lysates were prepared. Thus, three samples were run for gel electrophoresis in each group of animals, n = 6/group. The proteins of interest were identified and detected using western blot analysis, as detailed previously [23,25]. The equal protein loading on the gels were verified after stripping the membrane and re-probed with anti-β actin antibody.
PI3K (110)-siRNA transfection of melanoma cells
Human-specific PI3K (110) siRNA was transfected into A375 and Hs294t cells using siRNA Transfection Reagent Kit (Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) according to the manufacturer’s protocol. Briefly, 2×105 cells/well were seeded in a 6-well plate and allowed to grow to 70% confluency. The PI3K (110) siRNA mix with transfection reagents was overlaid on the cells for approximately 6 h at 37°C and transferred into 2× growth medium for about 18-20 h. At 24 h post-transfection, fresh medium was added to the cells and the cells were incubated for an additional 48 h. Thereafter, cells were harvested and lysates were subjected to western blotting.
Generation of EGFP/luciferase reporter melanoma cells
To facilitate detection of experimental metastasis in vivo, A375 cells were transduced with a vesicular stomatits virus G envelope (VSV-G) pseudotyped lentiviral vector for constitutive expression of both firefly luciferase and enhanced green fluorescence protein (EGFP). The vector (designated K2947) comprised the mouse CMV promoter, followed by fire fly luciferase, the encephalomyocarditis internal ribosomal entry site (IRES), a puromycin resistance gene and the enhanced green fluorescence protein (EGFP), wherein puromycin and EGFP were fused in-frame at their 3’ and 5’ ends, respectively, with the “self-cleaving” T2A peptide-coding sequence (CMV-luciferase-IRES-puro.T2A.EGFP) [26]. The lentiviral vector, the packaging construct, and the VSV-G plasmid DNAs were co-transfected into 293T human embryo kidney cells to create infectious, replication defective, lentiviral vector-containing particles as described previously [27]. A375 cells were transduced with the vector using a multiplicity of infection of approximately 5. Stable, expression-positive A375 cells were selected by supplementing the culture medium with 5 µg/mL of puromycin for 5 days.
Detection of metastatic melanoma cells in lungs of nude mice using bioluminescence imaging
Migration of melanoma cells was determined by intravenous injection of A375 cells (2.5×106) constitutively expressing both luciferase and green fluorescent protein (GFP) into nude mice. Four-five weeks old female athymic nude mice were purchased from the National Cancer Institute (Bethesda, MD) and housed in the animal resource facility in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. Mice were divided into three groups of 6 animals/group. Mice were given AIN76A control diet with or without supplementing with GSPs (0.5%, w/w). After seven weeks, the mice were sacrificed and lungs were harvested. The presence of EGFP/luciferase reporter A375 melanoma cells in lungs were detected by bioluminescence imaging after spraying D-luciferin using Xenogen IVIS200 imaging system, following an established protocol [28,29].
Statistical analysis
For cell migration assays, the data from GSPs or any other agent treated group were compared with control group separately using one-way analysis of variance (ANOVA) followed by post hoc Dunn’s test using GraphPad Prism version 4.00 for Windows, GraphPad Software, (San Diego, CA), USA, www.graphpad.com. In each case P<0.05 was considered statistically significant.
Results
GSPs decrease expression levels of PGE2 receptors in melanoma cells
We have shown earlier that GSPs treatment decreases the levels of PGE2 in melanoma cells which has been associated with the melanoma cell migration [23]. As PGE2 has been shown to manifest its biological activity via four well-known G-protein-coupled receptors (i.e., EP1-EP4) [6,30], we examined the effect of GSPs on PGE2 receptors in melanoma cells. A375 and Hs294t human melanoma cells were treated with various concentrations of GSPs (0, 10, 20 and 40 µg/mL) for 24 h, and cell lysates were subjected to the analysis of EP1, EP2, EP3 and EP4 using western blot analysis. Western blot analysis revealed that GSPs treatment decreased the levels of EP2 and EP4 in melanoma cells in a dose-dependent manner (Figure 1A). The inhibitory effect of GSPs was also observed on EP1 and EP3 but was less prominent than the effect on EP2 and EP4 (data not shown).
Figure 1.
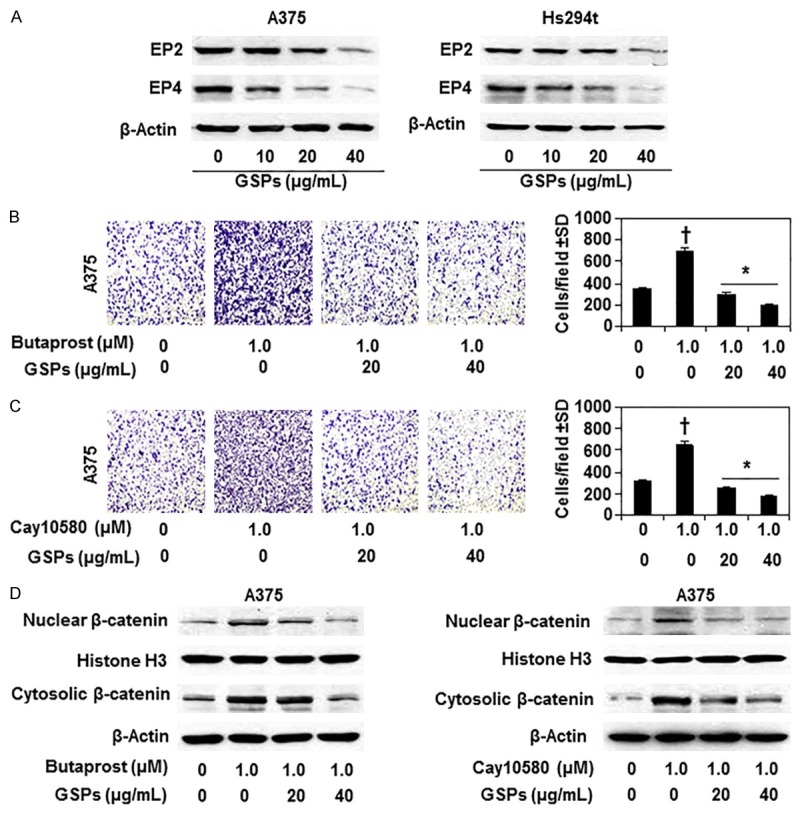
Effect of GSPs on PGE2 receptors and PGE2 receptors agonist-mediated effect on melanoma cell migration. A. Treatment of A375 and Hs294t cells with GSPs (0, 10, 20 and 40 µg/mL) for 24 h decreases the expression levels of PGE2 receptors (EP2 and EP4). Cell lysates were subjected to western blot analysis to determine the levels of EP2 and EP4. B. Treatment of GSPs inhibits EP2 agonist (Butaprost, 1.0 µM)-induced migration of A375 melanoma cells (left panel) and data of cell migration in each treatment group are summarized as a mean number of migratory cells ± SD/microscopic field, n = 3 (right panel). C. Treatment of GSPs inhibits EP4 agonist (Cay10580, 1.0 µM)-induced migration of A375 melanoma cells. Representative photomicrographs of cell migration are shown (left panels). The data on cell migration are summarized as a mean number of migratory cells ± SD/microscopic field, n = 3 (right panel). Significant inhibition versus non-GSPs-treated control group, *P<0.001; Significant increase versus non-EP2 agonist-treated or non-EP4 agonist-treated controls, †P<0.001. D. GSPs treatment decrease EP2- and EP4- agonist induced expression levels of nuclear and cytosolic β-catenin in A375 cells.
GSPs inhibit EP2 and EP4 agonist induced migration of melanoma cells
To verify the role of PGE2 receptors (EP2 and EP4) in melanoma cell migration, A375 cells were treated with EP2 and EP4 agonists separately for 24 h and cell migration was determined. As shown in Figure 1B, treatment of butaprost (EP2 agonist) significantly enhanced (P<0.001) the migration capacity of A375 melanoma cells compared to non-butaprost-treated control cells. Similar effect was also found when A375 cells were treated with EP4 agonist (Cay10580) for 24 h (Figure 1C). Next, we determined the effect of GSPs on EP2 agonist and EP4 agonist induced melanoma cell migration in same set of experiment. Treatment of A375 melanoma cells with GSPs (20 and 40 µg/mL) for 24 h decreased butaprost- and Cay10580-induced cell migration significantly (P<0.001) and by almost 100% compared to butaprost alone- or Cay10580 alone-treated cells (Figure 1B, 1C). The data on cell migration in each set of experiment are summarized in right panels (Figure 1B, 1C). These data suggest that GSPs inhibition of the melanoma cell migration is mediated, at least in part, by decreasing the levels of EP2 and EP4 receptors of PGE2.
GSPs inhibit EP2 and EP4 agonist induced expression levels of β-catenin
If GSPs treatment inhibit EP2 and EP4 agonist induced migration of melanoma cell, then in further studies we checked whether EP2 and EP4 agonists have any effect on the expression levels of β-catenin in melanoma cells, and whether GSPs decrease agonists-induced enhancement of β-catenin expression in these cells. A375 melanoma cells were identically treated with EP2 and EP4 agonists with and without the treatment with GSPs as in previous experiments. Cell lysates were subjected to western blot analysis. As shown in Figure 1D, western blot analysis revealed that treatment of A375 melanoma cells with EP2 agonist (butaprost) or EP4 agonist (Cay10580) resulted in increased accumulation of β-catenin in both the cytosolic and nuclear compartments of A375 cells. The increased accumulation of cytoplasmic and nuclear β-catenin, however, was inhibited when cells were treated with GSPs plus butaprost compared with the cells treated only with butaprost (Figure 1D, left panel). Similar results were obtained when cells were treated with GSPs and EP4 agonist (Figure 1D, right panel). These results suggest that GSPs inhibit β-catenin accumulation in A375 cells via reducing the expressions of PGE2 receptors (EP2 and EP4).
Effect of EP2 antagonist (AH6809) on melanoma cell migration and β-catenin levels
To further verify the role of PGE2 receptors in melanoma cell migration and its inhibition by GSPs, A375 and Hs294t melanoma cells were treated with AH6809 (EP2 antagonist) for 24 h and its effect on cell migration was determined. Cell migration analysis indicated that treatment of AH6809 inhibited the migration capacity of melanoma cells as reflected in the photomicrographs in terms of migrating cells density (Figure 2A and 2B, left panels). Migrating cell numbers were counted on the membranes under microscope and the numbers of migrating cells in each cell line and each group are summarized as mean ± SD per field in right panels (Figure 2A and 2B). Significant inhibition (P<0.05 and P<0.001) of cell migration was observed in A375 (20% to 67%) and Hs294T (24% to 65%) cells after the treatment of cells with AH6809. We also determined the levels of β-catenin in different treatment groups of both melanoma cell lines using western blotting. As expected, treatment of AH6809 decreased the accumulation of β-catenin in both nuclear and cytosolic compartments of both melanoma cell lines (Figure 2C, 2D).
Figure 2.
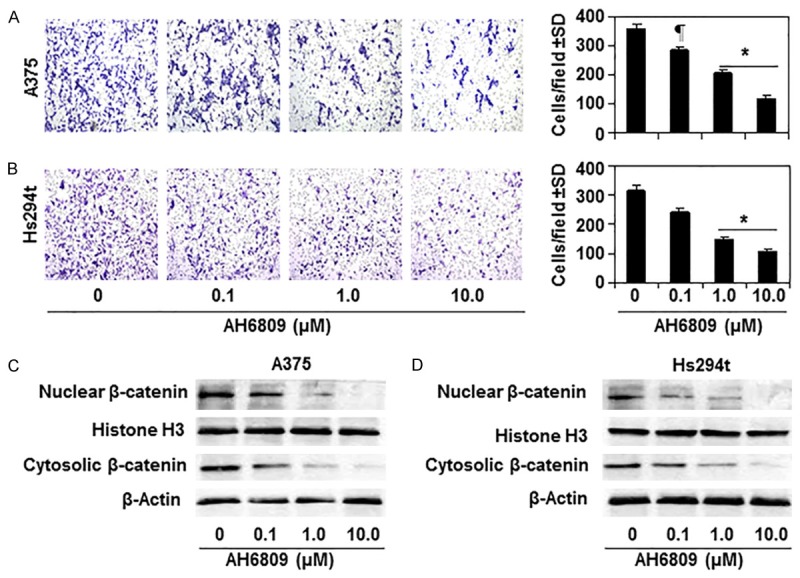
Effect of EP2 antagonist (AH6809) on the capacity of melanoma cell migration and expression levels of β-catenin in melanoma cells. Treatment of AH6809 for 24 h inhibits the capacity of A375 (A) and Hs294t (B) melanoma cell migration in a dose-dependent manner. Representative photomicrographs of cell migration are shown. Migrated cells are shown in purple-blue. Numbers of migratory cells/microscopic field are summarized as a mean ± SD in right panels, n = 3. Significant inhibition versus non-treated control cells, ¶P<0.05, *P<0.001. (C & D) Treatment of EP2 antagonist decreases the cytosolic as well nuclear levels of β-catenin in melanoma cells in a dose-dependent manner. A375 and Hs294t cells were treated with indicated doses of AH6809 for 24 h, cells were harvested, and nuclear and cytosolic protein fractions were subjected to the analysis of β-catenin using western blot analysis.
Celecoxib, an inhibitor of COX-2, reduced the levels of β-catenin and its downstream targets in melanoma cells
Earlier, we have shown that GSPs inhibit the migration capacity of melanoma cells through reduction in the endogenous expression levels of COX-2 [23]. Additionally, celecoxib, an inhibitor of COX-2, treatment also inhibits the migration capacity of melanoma cells [25]. Therefore, we were interested in determining whether GSPs inhibit β-catenin accumulation in melanoma cells via inhibition of COX-2 expression. In this context, we checked the effect of celecoxib on the expression levels of β-catenin in melanoma cell lines. Both A375 and Hs294t melanoma cell lines were treated with various concentrations of celecoxib (0, 5, 10 and 20 µM) for 24 h. After treatment, cells were harvested, lysates prepared and subjected to western blot analysis. We found that treatment of celecoxib resulted in decrease in the levels of nuclear as well as cytosolic β-catenin in both melanoma cell lines, and it was dose-dependent (Figure 3A, 3B). In the same set of experiment, we also checked the effect of celecoxib on the expression levels of MMP-2 and MMP-9, which are the downstream targets of β-catenin and have a role in cancer cell migration. Celecoxib treatment reduced the levels of MMP-2 and MMP-9 in both A375 and Hs294t human melanoma cell lines in a dose-dependent manner (Figure 3A and 3B).
Figure 3.
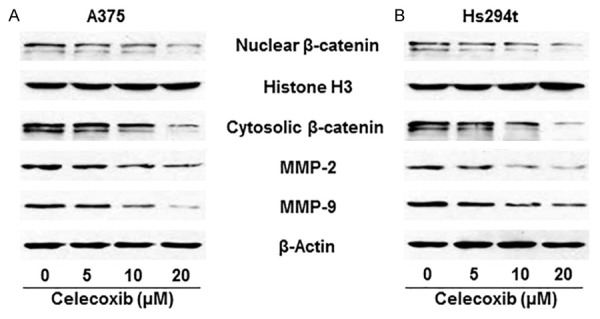
Effect of celecoxib, a COX-2 inhibitor, on the expression levels of β-catenin and downstream targets of β-catenin, MMP-2 and MMP-9, in A375 and Hs294t melanoma cells. A375 (A) and Hs294t (B) cells were treated with celecoxib (0, 5, 10, and 20 µM) for 24 h, then cells were harvested, cytoplasmic and nuclear fractions isolated and subjected to western blot analysis. Treatment of celecoxib inhibits the expression levels of β-catenin, MMP-2 and MMP-9 in both A375 and Hs294t melanoma cell lines in a dose-dependent manner.
GSPs reduce the expression levels of β-catenin and its downstream targets MMPs and MITF
The β-catenin has been implicated in migration and metastasis of cancer cells including melanoma [31,32]. Therefore, we determined the effect of GSPs on the levels of β-catenin and its downstream signaling molecules in human melanoma cells (A375 and Hs294t) using western blot analysis. For this purpose, cells were treated with GSPs (0, 10, 20 and 40 µg/mL). After 24 h, cells were harvested and nuclear and cytosolic fractions were prepared and subjected to western blot analysis. The data from western blot analysis revealed that treatment of A375 and Hs294t cells with GSPs resulted in reduction of β-catenin levels in both the cytosol and nucleus of the melanoma cells and the effect was dose-dependent (Figure 4A, 4B). As MMP-2, MMP-9 and MITF are the downstream targets of β-catenin [31,33,34], we also determined the effect of GSPs on the levels of these proteins. There was a reduction in expression levels of MMP-2, MMP-9 and MITF by GSPs in both A375 and Hs294t cells in a dose-dependent manner (Figure 4C, 4D).
Figure 4.
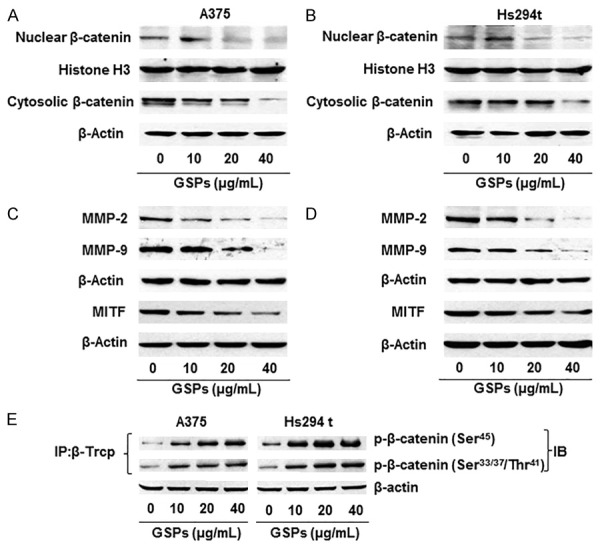
Effect of GSPs on β-catenin and its downstream molecular targets in human melanoma cell lines. A & B. Effect of GSPs on the cytosolic and nuclear levels of β-catenin in A375 and Hs294t cells. Cells were treated with GSPs, and cytosolic and nuclear fractions were subjected to western blot analysis to determine the levels of β-catenin. C & D. Effect of GSPs on the downstream molecular targets of β-catenin, such as MMP-2, MMP-9 and MITF, in melanoma cells. E. Treatment of A375 and Hs294t melanoma cells with GSPs enhances binding of β-TrCP with phospho forms of β-catenin. Cell lysates were used from the experiment explained under Panels A & B. In binding assay, β-TrCP was immunoprecipitated using specific antibody from total protein lysates followed by western blot analysis for phospho forms of β-catenin, as detailed in Materials and methods. IP, immunoprecipitation; IB, immunoblotting.
GSPs promote binding of β-catenin with β-TrCP
It has been shown that β-transduction repeat-containing proteins (β-TrCP) are components of the ubiquitin ligase complex targeting β-catenin for proteasomal degradation and are thus a negative regulator of Wnt/β-catenin signaling [35,36]. Therefore, we checked whether GSPs have any effect on the levels of β-TrCP in our melanoma cell migration model. A375 melanoma cells were treated with GSPs for 24 h, cell lysates were prepared, and β-TrCP was immunoprecipitated for detection of its binding with the phospho forms of β-catenin. Western blot analysis showed that treatment of A375 cells with GSPs enhanced the binding of β-TrCP with phospho forms of β-catenin in a dose-dependent manner (Figure 4E). Similar observations were noted when Hs294t cells were treated with GSPs. These data suggest that GSPs may have inactivated β-catenin by enhancing the proteasomal degradation of the β-catenin after its binding with β-TrCP.
β-catenin is the target of GSPs in melanoma cells: use of β-catenin-proficient and β-catenin-deficient melanoma cell lines
To further verify that blocking of melanoma cell migration by GSPs is mediated through inhibition of β-catenin, we used two different melanoma cell lines Mel1241 and Mel1011. Mel1241 cells possess constitutively active Wnt/β-catenin signaling, whereas Mel1011 cells lack activated β-catenin. We compared therapeutic effect of GSPs on the migration capacity of Mel1241 and Mel1011 cells. For this purpose, both Mel1241 and Mel1011 cell lines were treated with GSPs (0, 10, 20 and 40 µg/mL concentrations) for 8 h and subjected to cell migration assay. As the migration capacity of Mel1241 cells is several fold higher than A375 and Hs294t cells, 8 h time point was selected for migration analysis. Photomicrographs show the density of migrated cells in each treatment group (Figure 5A). We observed that: (i) migration capacity of Mel1241 cells was higher than Mel1011 cells. The numbers of migrating Mel1241 cells were 550 ± 50 cells/microscopic filed whereas the numbers of migrating Mel1011 cells were 26 ± 5 cells/microscopic field, as summarized in Figure 5B, n = 3. (ii) GSPs exerted their significant inhibitory effect on migration of Mel1241 cells (P<0.05-0.001), while there was no significant inhibitory effect of GSPs on the migration capacity of Mel1011 cells that are deficient or lack of activated β-catenin. The effect of GSPs on migration of melanoma cells on both cell lines are summarized in Figure 5B in terms of mean number of migrating cells ± SD/microscopic field (n = 3) for different treatment groups.
Figure 5.
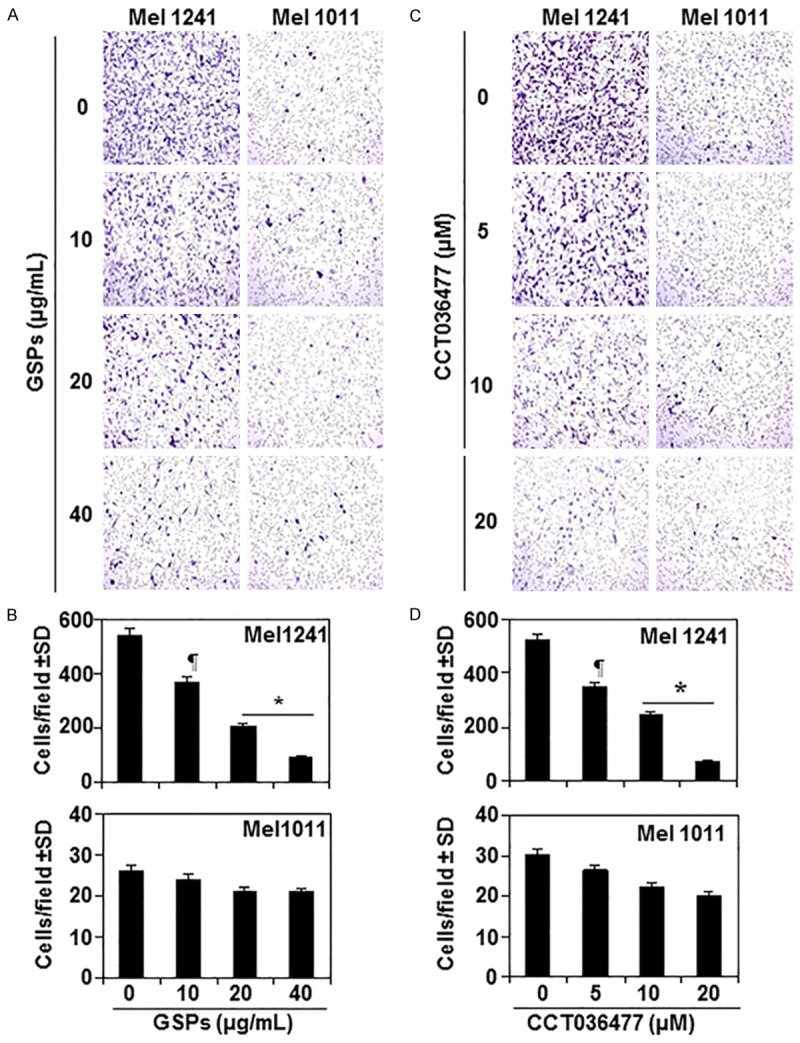
Inhibition of melanoma cell migration by GSPs is β-catenin dependent. A. Treatment of Mel1241 cells (β-catenin-activated) with GSPs inhibits cell migration capacity in a dose-dependent manner, while GSPs were failed to inhibit the migration of Mel1011 cells, which are β-catenin-inactivated. B. Migratory cells were counted on the membrane under microscope and results are expressed as the mean number of migratory cells ± SD per microscopic field (n = 3). C. Treatment of Mel1241 cells with CCT036477, a β-catenin inhibitor, inhibits cell migration capacity in a dose-dependent manner, while CCT036477 was failed to significantly inhibit the migration of Mel1011 cells. Representative photomicrographs are shown. D. Migratory cells were counted on the membrane under microscope and results are expressed as the mean number of migratory cells ± SD per microscopic field (n = 3). Significant inhibition in Mel1241 cells versus non-GSPs-treated or non-CCT036477-treated control cells, ¶P<0.05; *P<0.001.
In another experiment, Mel1241 and Mel1011 cell lines were treated with various concentrations of CCT036477, a β-catenin inhibitor, (0, 5, 10 and 20 µM) for 8 h and cell migration was determined. CCT036477 has unique ability to inhibit Wnt/β-catenin pathway [37]. As shown in Figure 5C, treatment of CCT036477 inhibited the migration capacity of Mel1241 cells in a dose-dependent manner (33-86%, P<0.05-0.001). In contrast, CCT036477 did not significantly inhibit the migration capacity of Mel1011 cells, which possess inactivated β-catenin status. Number of migrating cells on the membranes was counted under microscope at 3 different microscopic fields. Resultant data are summarized in Figure 5D. These observations indicate that GSPs inhibit cell migration by targeting β-catenin in melanoma cells.
Therapeutic intervention of GSPs on β-catenin in melanoma cell migration affects PI3K/Akt signaling mechanism
In resting cells, β-catenin is recruited to a destruction complex that consists of APC, Axin, and GSK-3β. This destruction complex facilitates phosphorylation of β-catenin by GSK-3β, which leads to its ubiquitinization and ultimately proteasomal degradation [12]. In cancer cells, however, binding of PGE2 to EP2 receptor activates G protein coupled to EP2 receptor that activates PI3K/Akt signaling, which leads to phosphorylation of GSK-3β at Ser9. Phosphorylation of GSK-3β at Ser9 inhibits its kinase activity, thereby preventing GSK-3β from being able to phosphorylate β-catenin in the phosphorylation/destruction complex. This leads to accumulation of β-catenin in cells. Therefore to check if GSPs inhibit inactivation of GSK-3β, we determined the effect of GSPs on expression level of components of PI3K/Akt signaling pathway. To examine this effect, A375 and Hs294t cells were treated with GSPs (0, 10, 20 and 40 µg/mL) for 24 h, cell lysates were prepared and subjected to western blot analysis for determining the levels of PI3K, phosphorylation of Akt at Ser473 and phosphorylation of GSK-3β. The results revealed that treatment of GSPs decreased the levels of both the regulatory (p85) and catalytic (p110) subunits of PI3K and reduced the phosphorylation level of Akt at Ser473 in a concentration-dependent manner (Figure 6A). A decreased phosphorylation level of GSK-3β at Ser9 was also observed. To verify that phosphorylation of GSK-3β at Ser9 is regulated by PI3K in human melanoma cells, we knocked-down PI3K (110) in A375 and Hs294t cells using PI3K siRNA kit (Santa Cruz Biotechnology, Inc.) following the manufacturer’s instructions. As shown in Figure 6B, western blot data revealed that siRNA knockdown of PI3K resulted in marked reduction in the levels of p-Akt and p-GSK-3β in both A375 and Hs294t cell lines.
Figure 6.
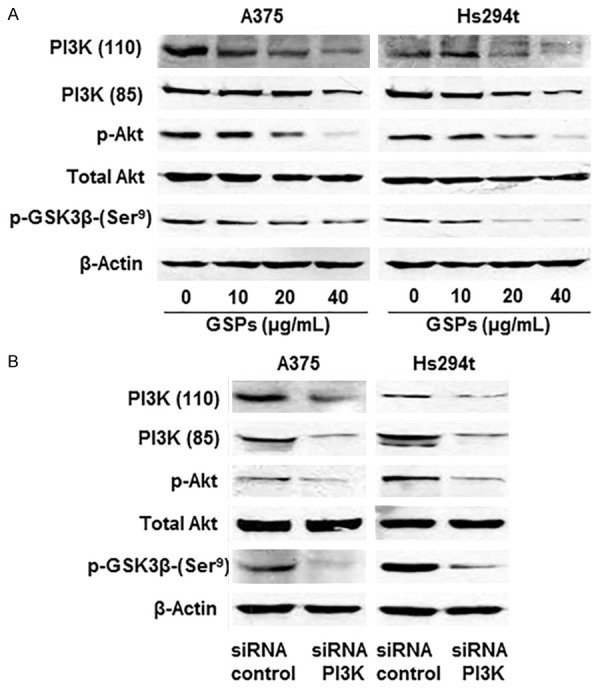
Effect of GSPs on PI3K/Akt cell survival signaling molecules in melanoma cells during migration. A. Treatment of GSPs for 24 h inhibits the levels of PI3K proteins, phospho Akt and p-GSK-3β in A375 and Hs294t cell lines in a dose-dependent manner. B. Effect of PI3K knockdown on cell survival signaling molecules in melanoma cells. PI3K (110) was knocked own using siRNA kit following the manufacturer’s protocol, and the levels of different proteins were determined using western blot analysis.
Dietary administration of GSPs inhibits the extravasation capacity of melanoma cells in nude mice
To further verify the inhibitory effect of GSPs on melanoma cell migration, in vivo experiments were conducted using athymic nude mice. Melanoma cells were i.v. injected into the tail vein of mice that were either not treated or were treated with GSPs-supplemented AIN76A control diet. Seven weeks after injection of melanoma cells, mice were sacrificed and lungs were harvested, and subjected to image analysis, as detailed in Materials and methods. Bioluminescence image analysis detected the presence of abundant melanoma cells in lungs (Figure 7A). Dietary administration of GSPs blocked migration capacity of melanoma cells in lungs compared to the lungs from non-GSPs-treated control animals. The levels of β-catenin and associated signaling molecules were determined in lung tissues using western blot analysis. As shown in Figure 7B, the levels of nuclear and cytosolic β-catenin and MITF were increased in lung tissues, while the levels of p-β-catenin (Ser45), GSK-3β and CK-1α were decreased in the mice which were injected melanoma cells compared to the lung tissues from the mice which were not injected melanoma cells or normal control group of mice. Western blot analysis further suggested that administration of dietary GSPs inhibited the levels of nuclear and cytosolic β-catenin and MITF in lung tissues, while increased the levels of p-β-catenin, GSK-3β and CK-1α compared to lung samples from non-GSPs-treated and melanoma cells injected mice.
Figure 7.
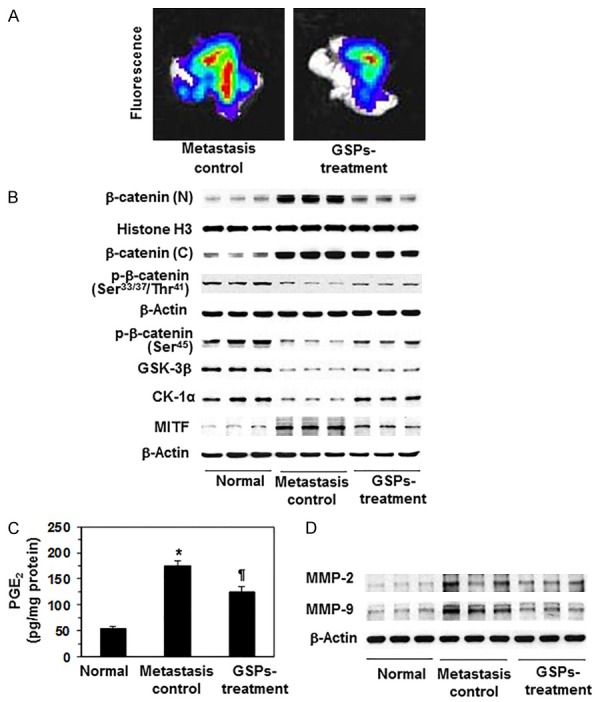
Effect of dietary administration of GSPs on melanoma cells invasion in vivo in athymic nude mice and associated proteins of β-catenin signaling molecules in internal body organ, such as lung. A. Mice were injected through the tail vein with A375 melanoma cells (2.5×106/mouse) constitutively expressing luciferase and EGFP. GSPs was given to mice in diet after supplementation with AIN76A control diet, as detailed in Materials and methods, n = 6/group. Seven weeks after melanoma cell injection, mice were sacrificed, and lungs were harvested as a target organ and subjected to bioluminescence imaging for the detection of melanoma cells using Xenogen IVIS200 imaging system. Red color indicates higher or intense accumulation or density of melanoma cells. B. Administration of dietary GSPs inhibits the cytosolic and nuclear levels of β-catenin, and MITF protein in lungs compared to the lungs from the non-GSPs-treated group of control mice, whereas the levels of p-β-catenin, GSK-3β and CK-1α were elevated or restored. Western blot data are shown in triplicate/group. Each sample was prepared from the pooled lung tissues from 2 animals. C. Dietary GSPs reduce the levels of PGE2 in lungs compared to the lungs from the control mice. PGE2 analysis in lung tissue samples was done using the Cayman PGE2 Enzyme Immunoassay Kit (Ann Arbor, MI) following the manufacturer’s instructions. D. GSPs decrease the levels of MMP-2 and MMP-9 in lungs compared to the lungs from the non-GSPs-treated control mice. At the termination of the experiment, lungs were harvested and lysates were prepared for the analysis of MMPs using western blot analysis, n = 6. Significant elevation of PGE2 (*P<0.001) compared to normal lung samples, while significant inhibition (¶P<0.01) versus metastasis-specific control group.
In the same set of experiment and lung samples, the levels of PGE2 and MMPs were also analyzed. Analysis of PGE2 was done using PGE2 immunoassay kit (Ann Arbor, MI). Analysis indicated that the levels of PGE2 were significantly higher (P<0.001) in metastatic lung tissues compared to lungs of normal mice, however, dietary GSPs significantly decreased (P<0.01) the levels of PGE2 in lungs compared to non-GSPs-treated control mice (Figure 7C). Similarly, dietary GSPs also inhibited the levels of MMP-2 and MMP-9 in lung tissues compared to lungs from non-GSPs-treated but melanoma cells injected mice as analyzed and detected by western blotting (Figure 7D).
Discussion
Studies have implicated the role of active Wnt/β-catenin signaling in tumor progression and tumor cell migration, and particularly in melanoma [8,13,15,17]. β-catenin is a dual function protein and is an important component of cell-cell adhesion [6,30]. This cell-to-cell adhesion may prevent the migration of cells. However, the breaking of cell-to-cell adhesion due to activation of β-catenin and its nuclear accumulation may increase the migration capacity of cancer cells. Thus nuclear/cytoplasmic ratio of β-catenin in the cells determines their migration activity. Our results show that GSPs inhibit melanoma cell migration by targeting β-catenin. Importantly, GSPs target or reduce accumulation of β-catenin in melanoma cells by decreasing the inflammatory mediators, such as COX-2 overexpression and PGE2 production [23]. In addition to COX-2 and PGE2 overexpression, the inflammatory mediators include PGE2 receptors and more importantly EP2 and EP4. Treatment of melanoma cells (A375 and Hs294t) with EP2-agonist or EP4-agonist stimulates the migration ability of cells while GSPs inhibit the migration capacity of melanoma cells enhanced by either EP2 agonist or EP4 agonist. Celecoxib, an anti-inflammatory agent and an inhibitor of COX-2, also reduced the cytoplasmic and nuclear levels of β-catenin and its downstream targets (MMPs) in melanoma cells suggesting that the anti-cell migration activity of GSPs is due to its anti-inflammatory effect.
Multiple diverse molecular events are integrated in the metastasis of tumor cells. In tumor cells, mechanisms that inhibit GSK-3β-induced phosphorylation of β-catenin block its interaction with the E3 ubiquitin ligase receptor, β-TrCP, which prevents β-catenin ubiquitination and degradation, and ultimately leads to β-catenin activation [24,25]. A major regulator of β-catenin stability and activity is the β-TrCP. In this study, we sought to determine whether the inactivation or degradation of β-catenin in melanoma cells by GSPs treatment is affected by the expression of its regulator, the β-TrCP. We found that GSPs enhanced the binding of β-TrCP to phospho forms of β-catenin, which suggests β-TrCP-mediated ubiquitination and degradation of β-catenin [25,31]. Thus, our finding provides evidence that GSPs inhibit melanoma cell migration by targeting β-catenin stability.
Further, in an attempt to confirm the role of GSPs on prevention or inhibition of migratory capacity of melanoma cell through inactivation of β-catenin signaling, we used two distinct melanoma cell lines, namely Mel1241 and Mel1011. The two cell lines differ in status of constitutive activation of Wnt/β-catenin. Our data show that Mel1241 melanoma cells which possess constitutively active Wnt/β-catenin are highly invasive and the capacity of cell migration is multiple-fold higher than Mel1011 cells. Treatment of Mel1241 cells with GSPs resulted in significant inhibition of cell migration while under identical experimental conditions, these effects of GSPs or β-catenin inhibitor (CCT036477) were not found in the Mel1011 cell line, which lacks constitutively active β-catenin.
The inhibitory effect of GSPs on melanoma cell migration was further verified using an in vivo immune-compromised nude mouse model. A375 melanoma cells were intravenously injected through the tail vein and their migration and tumor cell accumulation in lungs was detected and determined using bioluminescence imaging system. Simultaneously, we examined the inhibitory effect of dietary GSPs, if any, on the migration capacity of melanoma cells in vivo. Although this model system does not recapitulate the concept of tumor cell metastasis, as there was no intravasation phase of tumor cell metastasis, this approach does help to understand how the melanoma cells pass through the extravasation phase and reach internal vital organs such as lungs and stay there for tumor growth. Bioluminescence imaging detected the presence of large numbers of melanoma cells in lungs (Figure 7). Dietary administration of GSPs inhibited or blocked the migration capacity of melanoma cells as is evident by the presence of lesser numbers or density of melanoma cells in lungs compared to non-GSPs-treated control mice. To verify whether this inhibitory effect on the migration of melanoma cells in vivo is associated with the inhibitory effect of GSPs on β-catenin and its downstream molecular targets, lungs were harvested and analyzed for these biomarkers. Administration of GSPs decreased the levels of both cytosolic and nuclear β-catenin, while increased the levels of p-β-catenin (Ser45 and Ser33/37/Thr41), GSK-3β and CK-1α, which are responsible for inactivation or degradation of β-catenin. These results provide evidence that GSPs induced β-catenin degradation or inactivation in melanoma cells is associated with the up-regulation of CK1α and GSK-3β. Liu et al. [12] have identified CK1α as an essential component that controls β-catenin phosphorylation degradation in Drosophila. Inhibition of PGE2 levels in lung tissues by GSPs suggest that these effects of GSPs are mediated through inhibition of inflammatory mediators in melanoma cells. These observations explain the possible mechanism of inhibitory effects of GSPs against the migration of melanoma cells in in vivo model.
Together, the outcome of this study suggests that GSPs have the ability to block or inhibit the migration capacity of melanoma cells, and this anti-tumor cell migration ability of GSPs is mediated through inactivation of β-catenin. Thus therapeutic intervention strategies targeting key molecules of the Wnt/β-catenin pathway may represent promising approaches to inhibit metastasis of melanoma cells. This new insight on the anti-melanoma cell migration activity of GSPs could serve as the basis for therapeutic approach against malignant melanoma in high risk individuals.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH, CA166883) and the Veterans Administration Merit Review Award (1I01BX001410) to S.K.K. This research also used the facilities supported by of the Virology and Genetic Sequencing cores of the UAB Center for AIDS Research (CFAR), P30-AI-27767. The funding agencies had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.American Cancer Society, Cancer facts and figures. Available: http://www.cancer.org/. Accessed 2015.
- 2.Hall HI, Miller DR, Rogers JD, Bewerse B. Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol. 1999;40:35–42. doi: 10.1016/s0190-9622(99)70562-1. [DOI] [PubMed] [Google Scholar]
- 3.Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008;84:528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SD, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm Res. 2010;27:1092–1102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- 6.Riedl K, Krysan K, Põld M, Dalwadi H, Heuze-Vourc’h N, Dohadwala M, Liu M, Cui X, Figlin R, Mao JT, Strieter R, Sharma S, Dubinett SM. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat. 2004;7:169–184. doi: 10.1016/j.drup.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM. Autocrine/ paracrine prostaglandin E2 production by nonsmall cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia N, Spiegelman VS. Activation of Wnt/beta-catenin/Tcf signaling in mouse skin carcinogenesis. Mol Carcinog. 2005;42:213–221. doi: 10.1002/mc.20077. [DOI] [PubMed] [Google Scholar]
- 9.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth AI, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of betacatenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 13.Demunter A, Libbrecht L, Degreef H, De Wolf-Peeters C, Van den Oord JJ. Loss of membranous expression of beta-catenin is associated with tumor progression in cutaneous melanoma and rarely caused by exon 3 mutations. Mod Pathol. 2002;15:454–461. doi: 10.1038/modpathol.3880546. [DOI] [PubMed] [Google Scholar]
- 14.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of betacatenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 15.Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi J, Chen N, Wang J, Siu CH. Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell. 2005;16:4386–4397. doi: 10.1091/mbc.E05-03-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowy AM, Clements WM, Bishop J, Kong L, Bonney T, Sisco K, Aronow B, Fenoglio-Preiser C, Groden J. β-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res. 2006;66:4734–4741. doi: 10.1158/0008-5472.CAN-05-4268. [DOI] [PubMed] [Google Scholar]
- 18.Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gsaxin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 20.Smith KA, Tong X, Abu-Yousif AO, Mikulec CC, Gottardi CJ, Fischer SM, Pelling JC. UVB radiation-induced β-catenin signaling is enhanced by COX-2 expression in keratinocytes. Mol Carcinog. 2012;51:734–745. doi: 10.1002/mc.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandakumar V, Singh T, Katiyar SK. Multitargeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 23.Vaid M, Singh T, Katiyar SK. Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS One. 2011;6:e21539. doi: 10.1371/journal.pone.0021539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Meeran SM, Vaid M, Punathil T, Katiyar SK. Dietary grape seed proanthocyanidins inhibit 12-O-tetradecanoyl phorbol-13-acetatecaused skin tumor promotion in 7,12-dimethylbenz(a)anthracene-initiated mouse skin, which is associated with the inhibition of inflammatory responses. Carcinogenesis. 2009;30:520–528. doi: 10.1093/carcin/bgp019. [DOI] [PubMed] [Google Scholar]
- 25.Singh T, Katiyar SK. Green tea catechins reduce invasive potential of human melanoma cells by targeting COX-2, PGE2 receptors and epithelial-to-mesenchymal transition. PLoS One. 2011;6:e25224. doi: 10.1371/journal.pone.0025224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘selfcleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 27.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 28.Henriques C, Henriques-Pons A, Meuser-Batista M, Ribeiro AS, de Souza W. In vivo imaging of mice infected with bioluminescent Trypanosoma cruzi unveils novel sites of infection. Parasit Vectors. 2014;7:89. doi: 10.1186/1756-3305-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewals B, Myster F, Palmeira L, Gillet L, Ackermann M, Vanderplasschen A. Ex vivo bioluminescence detection of alcelaphine herpesvirus 1 infection during malignant catarrhal fever. J Virol. 2011;85:6941–6954. doi: 10.1128/JVI.00286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Brabletz T, Jung A, Dag S, Reu S, Kirchner T. β-catenin induces invasive growth by activating matrix metalloproteinases in colorectal carcinoma. Verh Dtsch Ges Pathol. 2000;84:175–181. [PubMed] [Google Scholar]
- 32.Vaid M, Prasad R, Sun Q, Katiyar SK. Silymarin targets β-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS One. 2011;6:e23000. doi: 10.1371/journal.pone.0023000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T. Beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer. 2004;108:321–326. doi: 10.1002/ijc.11522. [DOI] [PubMed] [Google Scholar]
- 34.Kolligs FT, Bommer G, Goke B. Wnt/betacatenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66:131–144. doi: 10.1159/000066755. [DOI] [PubMed] [Google Scholar]
- 35.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The Fbox protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 36.Ougolkov A, Zhang B, Yamashita K, Bilim V, Mai M, Fuchs SY, Minamoto T. Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer Inst. 2004;96:1161–1170. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 37.Ewan K, Pajak B, Stubbs M, Todd H, Barbeau O, Quevedo C, Botfield H, Young R, Ruddle R, Samuel L, Battersby A, Raynaud F, Allen N, Wilson S, Latinkic B, Workman P, McDonald E, Blagg J, Aherne W, Dale T. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010;70:5963–5973. doi: 10.1158/0008-5472.CAN-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]


