Abstract
MLN4924, an inhibitor of NEDD8 activating enzyme (NAE), has been reported to have activity against various malignancies. Here, we investigated the antitumor properties of MLN4924 and MLN4924 in combination with cisplatin on human cervical carcinoma (CC) in vitro and in vivo. Two human CC cell lines, ME-180 and HeLa, were used in this study. The cytotoxic effects of MLN4924 and/or cisplatin were measured by cell viability (MTT), proliferation (BrdU incorporation), apoptosis (flow cytometry with annexin V-FITC labeling), and the expression of cell apoptosis-related proteins (Western blotting). In vivo efficacy was determined in Nu/Nu nude mice with ME-180 and HeLa xenografts. The results showed that MLN4924 elicited viability inhibition, anti-proliferation and apoptosis in human CC cells, accompanied by activations of apoptosis-related molecules and Bid, Bcl-2 phosphorylation interruption, and interference with cell cycle regulators. Moreover, MLN4924 caused an endoplasmic reticulum stress response (caspase-4, ATF-4 and CHOP activations) and expression of other cellular stress molecules (JNK and c-Jun activations). Additionally, MLN4924 suppressed growth of CC xenografts in nude mice. Furthermore, we demonstrated that MLN4924 potentiated cisplatin-induced cytotoxicity in CC cells with activation of caspases. Consistently with this, MLN4924 significantly enhanced cisplatin-induced growth inhibition of CC xenografts. Together, these findings suggest that MLN4924 alone or in combination with cisplatin is of value in treating human CCs.
Keywords: MLN4924, cervical carcinoma, apoptosis, neddylation
Introduction
Cervical cancer (CC) is the second most common cancer in women worldwide [1]. Approximately 500,000 new cases and 275,000 deaths are reported annually in developing countries [15], and most cervical cancers are associated with high-risk human papilloma virus infection [3]. The treatment of the cervical cancer includes surgery, chemotherapy and radiotherapy [3,36]. but the prognosis for metastatic CC remains ominous due to drug resistance [49]. In addition, the toxicity and side effects of current chemotherapy are substantial. Therefore, the development of new drugs or combination treatments is important to improve patient survival and minimize the side effects of conventional chemotherapy.
A balance of protein degradation and formation is essential to maintain cellular homeostasis and is associated with transduction pathways related to cancer progression, metastasis, and drug resistance [30]. The ubiquitin-proteasome system, a non-lysosomal proteolysis system, responds to the accumulation of misfolded proteins by recognizing and rapidly degrading ubiquitin-modified proteins. This system is reportedly involved in the regulation of many cellular functions and cell survival. Specifically, the ubiquitin-proteosome system controls the degradation of ubiquitin-tagged proteins by two distinct steps: the covalent attachment of multiple ubiquitin molecules to the target proteins and the degradation of tagged proteins by the 26S proteasome [8,46]. First, ubiquitin is activated by ubiquitin-activating enzyme (E1) via ATP hydrolysis. The ubiquitin is then transferred to the ubiquitin-conjugating enzyme (E2) complex. Proteins tagged for degradation with ubiquitin are catalyzed by enzymes called ubiquitin ligases (E3) [46]. The tagging of a protein with a single ubiquitin molecule signals ligases to attach additional ubiquitin molecules. E3 ligases have an essential role by controlling the target substrate specificity for proteasome degradation. Three distinct classes of E3 ubiquitin ligases have been identified according to their core domains: HECT (homologous to E6-AP carboxy terminus), U-box domain and RING (Really Interesting New Gene) finger [11]. Neural-precursor-cell-expressed developmentally down-regulated protein 8 (NEDD8) is a ubiquitin-like molecule that can connect to the proteins of the cullin family [31]. NEDD8 is also required to regulate the activity of a subclass of ubiquitin E3 ligases, cullin-RING ligases (CRL) [13,29]. Specifically, NEDD8 first connects to the NEDD8-activating enzyme (NAE) in an ATP-dependent reaction and is subsequently activated. The NEDD8 conjugating enzyme (E2) then receives the activated NEDD8 and a protein substrate recognized by the CRL of an ubiquitin protein ligase (E3). Finally, E3 receives NEDD8 from the E2 and activates substrate ubiquitination [29]. Neddylation is the process by which NEDD8 is conjugated to its target proteins, and NEDD8-activating enzyme (NAE) is essential for Neddylation and CRL activity regulation.
MLN4924, a novel neddylation inhibitor, reportedly exhibits activity against hepatocellular carcinoma [25], ovarian cancer [28], acute myeloid leukemia [42], bladder cancer [19] and lymphoma [26] in vitro, in vivo and in the preclinical setting [41]. Specifically, the dysregulation of intracellular protein homeostasis after MLN4924 treatment is reportedly associated with cell cycle progression, autophagy, apoptosis, and other cellular responses [2,48]. Other studies also have demonstrated that MLN4924 can induce the production of reactive oxygen species, inhibit NFκB, and suppress tumor angiogenesis [26,42,48]. However, the antitumor effect of MLN4924 and the underlying mechanisms in human cervical cancer remain unclear.
In this study, we examined the cytotoxic effect of MLN4924 on human CC cells via apoptosis, proliferation inhibition, cell cycle interference and cellular stress induction assays. In addition, we investigated the ability of MLN4924 to enhance the antitumor effect of the conventional DNA-alkylating agent cisplatin in vitro and in vivo.
Materials and methods
Cell culture
Two human cervical cancer cell lines, ME-180 cell and HeLa, were used in this study. The ME-180 cell line was provided by Dr. Tai-Lung Cha (Division of Urology, Department of Surgery, National Defense Medical Center, Taipei, Taiwan) and has been authenticated by short tandem repeat (STR) DNA typing. The HeLa cell line was obtained from the Taiwan Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). The two cell lines were cultured in RPMI-1640 medium (for ME-180) or Dulbecco’s Modified Eagle’s Medium (for HeLa) containing 10% fetal bovine serum (HyClone, Logan, UT, USA), 1 mM sodium pyruvate (Invitrogen, Carlsbad, CA, USA) and penicillin (100 units/ml)/streptomycin (100 μg/ml) (Invitrogen) at 37°C in 5% CO2. All culture media were purchased from Corning (New York, NY, USA).
Reagents and antibodies
MLN4924 was purchased from Active Biochem (Maplewood, NJ, USA). Various concentrations of MLN4924 were prepared as suspensions in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) and then mixed with complete cell culture medium or saline. Cisplatin was obtained from clinical preparations of Abiplatin Solution (Pharmachemie BV, GA Haarlem, the Netherlands). The antibodies against various proteins used for the Western blot analysis (cleaved PARP, cleaved caspase-3, cleaved caspase-7, cleaved caspase-8, BH3 interacting-domain death agonist (Bid), phosphor-Bcl-2 (Ser70), phospho-c-Jun (Ser73), phospho-SAPK/JNK (Thr183/Tyr185), ATF-4, CHOP, phospho-Chk1 (Ser345), phospho-Chk2 (Thr68), phospho-p53 (Ser15), phospho-histone H2A.X (Ser139), p21 and p27) were purchased from Cell Signaling Technology (Danvers, MA, USA). The anti-caspase-4 antibody was obtained from MBL International Corporation (Woburn, MA, USA), and the anti--tubulin and anti-GAPDH antibodies were obtained from GeneTex (Irvine, CA, USA). The antibodies against Cullin-1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals were purchased from Sigma-Aldrich or Serva (Heidelberg, Germany).
Measurement of cell viability
Cell viability was determined by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT, Sigma-Aldrich). Briefly, cells were seeded with culture medium in 96-well microplates (4000 cells/well) and incubated at 37°C for 24 or 48 hours before drug treatments. At the end of the incubation, the cells were exposed to MTT (0.5 mg/ml) at 37°C for 4 hours. The reduced crystals were dissolved in DMSO (Sigma-Aldrich), and the absorbance was detected at 570 nm with a μQuant ELISA plate reader (Biotek, Winooski, VT, USA).
Western blot
To prepare cell or tissue samples for the Western blot analysis, cell pellets were lysed in lysis buffer (Cell Signaling Technology) and tissues were homogenized and lysed with T-PER tissue protein extraction reagent (Thermo Scientific Pierce, Rockford, IL, USA). After centrifugation at 13,000 rpm for 15 minutes at 4°C, the supernatants were collected, and the protein concentrations of supernatants were then measured using BCA protein assay reagent (Thermo Scientific Pierce, Rockford, IL, USA). Subsequently, equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto polyvinylidene fluoride (PVDF) membranes (GE Healthcare, Piscataway, NJ, USA), and then probed with various primary antibodies at 4°C overnight. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Genetex) at room temperature for 2 hours. Finally, the antibody-bound membranes were treated with enhanced chemiluminescent Western blot detection reagents (Millipore, Billerica, MA, USA) and visualized with an ImageQuant LAS 4000 system (GE Healthcare).
Analysis of apoptosis by fluorescence-activated cell sorting (FACS)
Apoptosis was determined by using flow cytometry with a commercial annexin V-FITC detection kit (Invitrogen). Briefly, after being treated at the different conditions, the cells were washed with ice-cold PBS and collected in trypsin-EDTA solution (Invitrogen). After centrifugation to remove the trypsin-EDTA, the cells were re-suspended in binding buffer containing annexin V-FITC and propidium iodide (PI) and then incubated for 15 minutes at room temperature in the dark prior to analysis on a Becton Dickinson LSR II flow cytometer (BD Bioscience, San Jose, CA, USA).
Cell proliferation assay
To evaluate the effect of MLN4924 on CC cell proliferation, we performed a 5-bromo-2’-deoxyuridine (BrdU) incorporation assays with a commercial kit (Millipore) as described previously [20]. The cells were cultured in 96-well microplates (4000 cells/well) and exposed to MLN4924 or DMSO (as the non-treated control) for 48 hours. After the designated hours of treatment, the cells were labeled with BrdU and tagged with the anti-BrdU antibody. Subsequently, the substrate was added, and the colored product was measured using a BioTek μQuant ELISA reader (Winooski) at dual wavelengths of 450-540 nm.
In vivo xenograft experiments
All animal care and experimental procedures were carried out in accordance with protocols approved by the National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee (IACUC). Moreover, all studies involving animals complied with the ARRIVE guidelines for reporting experiments involving animals. To develop xenograft tumors, ME-180 or HeLa cells in Matrigel (BD Biosciences) were injected subcutaneously into the dorsal flanks of 8-week-old Nu/Nu nude mice, which were obtained from the Taiwan National Laboratory Animal Center (Taipei, Taiwan). When the tumors reached approximately 150 mm3 in volume, the mice received respective treatments via intraperitoneal (i.p.) injection. The tumor volume was calculated as follows: volume = longest tumor diameter × (shortest tumor diameter)2/2. After 32 days of treatment, the mice were sacrificed, and the tumor tissues were collected for further analyses.
Statistical analysis
All data were further analyzed using GraphPad Prism® 5 software (La Jolla, CA, USA) and are presented as the means ± SDs or ± SEMs, unless otherwise indicated. Two sets of data were compared with a two-tailed Student’s t-test, whereas multiple groups were analyzed with a one-way ANOVA followed by Bonferroni’s post-hoc test. A P value < 0.05 was considered significant.
Results
MLN4924 inhibited cell viability and induced apoptosis in human CC cells
To determine the effect of MLN4924 on the viability of CC cells, ME-180 and HeLa cells were treated with various concentrations of MLN4924, and the cell viability was then measured with an MTT assay. First, MLN4924 decreased cullin neddylation, which is consistent with the inhibition of NEDD8 conjugation by MLN4924 (Figure 1A). In addition, MLN4924 significantly suppressed the viability of the two CC cell lines in a dose-dependent after 24 and 48 hours of treatment (Figure 1B). Furthermore, annexin V-FITC/PI labeling flow cytometry showed that 500 nM MLN4924 markedly induced apoptosis in the two CC cell lines after 48 hours of treatment (Figure 1C and 1D).
Figure 1.
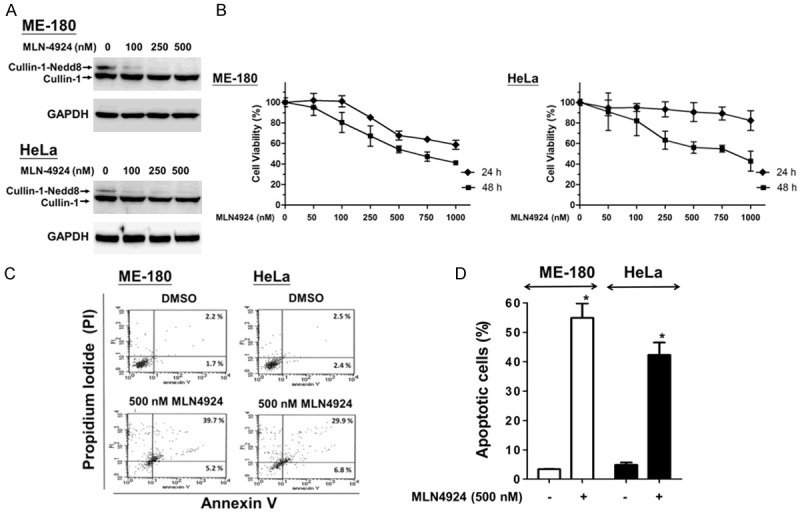
MLN4924 elicited inhibition of cell viability and apoptosis in human CC cells. A. Cullin-1 neddylation was completely inhibited by MLN4924. ME-180 and HeLa cells were exposed to different concentrations of MLN4924 or DMSO (as non-treated control) for 48 hours and then harvested to detect the expression of cullin-1 with a Western blot (GAPDH as control). B. The cells were treated with various concentrations of MLN4924 for 24 and 48 hours. Cell viability was assessed with an MTT assay. C. The cells were exposed to MLN4924 (500 nM) or DMSO (as the non-treated control) for 48 hours. Apoptotic cells were analyzed by FACS flow cytometry with annexin V-FITC/PI staining. The annexin V-positive cells (early apoptotic cells) are distributed in the lower-right panel; the late apoptotic cells, whose membranes are permeable to PI and annexin V staining, are distributed on the upper-right panel. D. Quantitative analyses of total apoptosis population following MLN4924 (500 nM) or DMSO treatments were performed. Data are presented as the means ± SD of at least three independent experiments. *P < 0.05 compared with the controls.
MLN4924 activated apoptosis-related molecules in human CC cells
We then measured the expression of apoptosis-related molecules in the total cell lysates of the two CC cell lines with a Western blot 48 hours after MLN4924 treatment (Figure 2A). Our results showed that MLN4924 activated caspase-3, caspase-7 and caspase-8, cleaved PARP, and phosphorylated histone H2A.X [40,44]. We also examined the effects of MLN4924 on Bid activation and Bcl-2 phosphorylation. Previous studies have indicated that B-cell lymphoma 2 (Bcl-2) plays an anti-apoptotic role, resulting in an overall pro-survival effect during cancer therapy. Specifically, the knockdown of Bcl-2 may potentiate chemotherapy efficacy [33], and the phosphorylation of Bcl-2 at Ser70 has been implicated in resistance to conventional therapies [10,33]. Moreover, Bid, a BH3 domain-containing pro-apoptotic molecule of the Bcl-2 family, can form a heterodimer with its antagonist, Bcl-2, to mediate mitochondrial damage induced by caspase-8 [6,23,24]. As shown in Figure 2B, MLN4924 resulted in Bid cleavage and concomitantly decreased Bcl-2 phosphorylation at Ser70 in human CC cells. These results consistently indicated that MLN4924 induces apoptosis in human CC cells.
Figure 2.
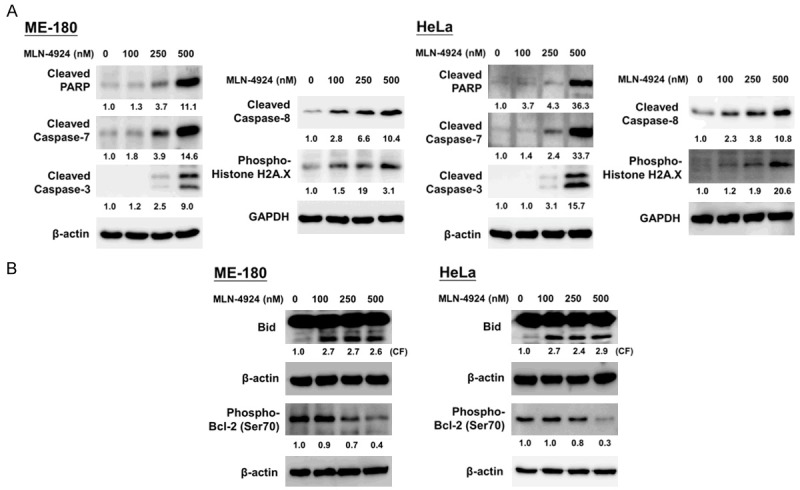
MLN4924 activated caspases, PARP, phospho-Histone H2A.X, and Bid and decreased Bcl-2 phosphorylation in human CC cells. Total cell lysates were harvested and analyzed by Western blotting with specific antibodies against (A) apoptosis-related molecules (PARP, cleaved caspase-3, -7 and -8), DNA damage response regulator (phospho-Histone H2A.X), (B) Bid, and phospho-Bcl-2 (Ser70). Results shown represent at least three independent experiments.
MLN4924 elicited cellular stress and the expression of ER stress-related signaling molecules in Human CC cells
MLN4924 has been reported to interfere the cellular proteome profile and homeostasis by interrupting protein degradation, which induced cellular stress and ER stress [9]. We demonstrated that MLN4924 activated the ER stress signaling pathway in a dose-dependent manner. As shown in Figure 3, MLN4924 treatment for 48 hours induced the expression of ER stress-related apoptotic proteins, such as ATF-4, CHOP, and cleaved caspase-4 [17,18,43]. Moreover, MLN4924 also activated the expression of cellular stress-related molecules, such as phospho-SAPK/JNK and its downstream mediator phospho-c-Jun (Figure 3) [4,12,22]. These findings indicated that MLN4924 treatment increased cellular stress and ER stress, thereby inducing apoptosis in human CC cells.
Figure 3.
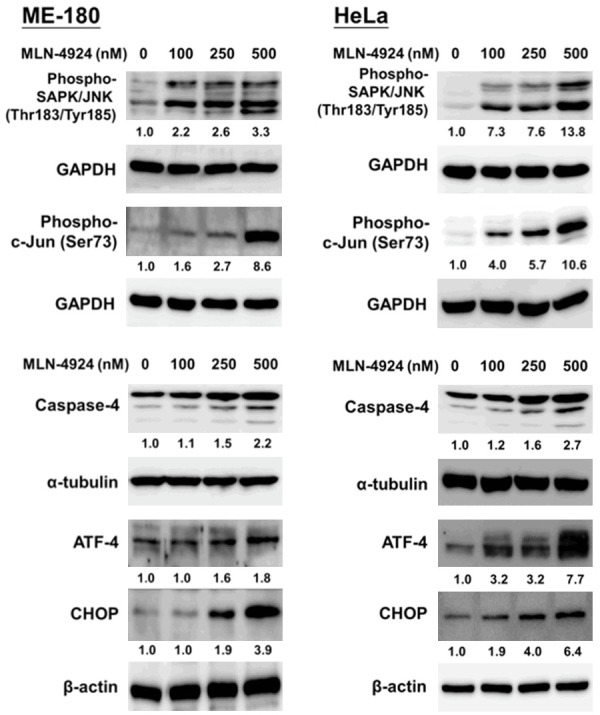
MLN4924 activated cellular stress and endoplasmic reticulum stress-related signaling in human CC cells. Cells were treated with several concentrations of MLN4924 or DMSO (as the non-treated control) for 48 hours. Cell lysates were harvested, and the expression levels of cell stress-related proteins (phospho-SAPK/JNK and phospho-c-Jun) and ER stress-related molecules (ATF-4, CHOP and caspase-4) were assessed via a Western blot. Results shown are representative of at least three independent experiments.
MLN4924 suppressed cell proliferation with cell cycle interference in human CC cells
We then determined the effect of MLN4924 on the proliferation of CC cells with a BrdU incorporation assay. Treatment with 500 nM MLN4924 for 48 hours significantly inhibited the proliferation of the human CC cells (Figure 4A). Previous studies have indicated that MLN4924 can interrupt the cell cycle [2,19]. Accordingly, we also observed that the expression of cyclin-dependent kinase inhibitors (p21 and p27) and checkpoint regulators (phospho-p53, phospho-Chk1 and phospho-Chk2) was up-regulated after MLN4924 treatment for 48 hours (Figure 4B). Together, these data indicate that MLN4924 interrupts cell cycle progression, thus inhibiting the proliferation of CC cells.
Figure 4.
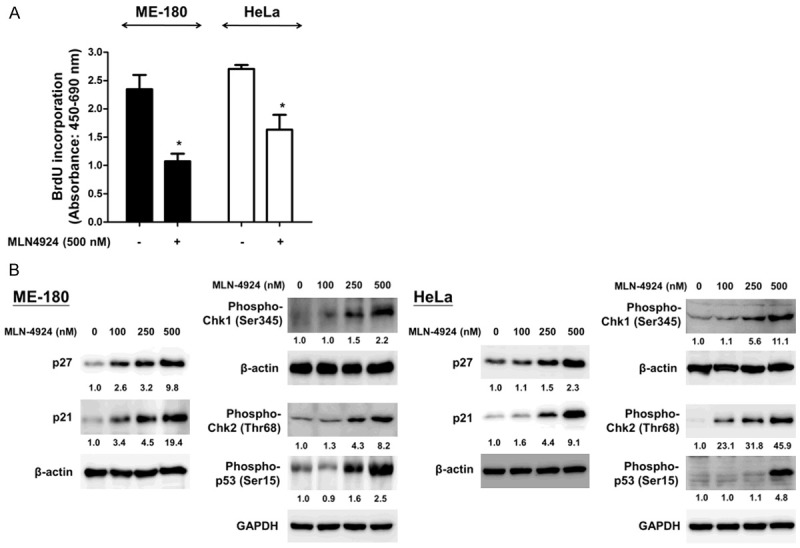
MLN4924 reduced cell proliferation and interfered cell cycle mediators in ME-180 and HeLa cell lines. A. Cells were exposed to MLN4924 (500 nM) or DMSO (as the non-treated control) for 48 hours. BrdU incorporation assay was adopted to determine the proliferation of human CC cells, and the cell proliferation of these two cell lines was decreased after MLN4924 treatments. B. After exposure to various concentrations of MLN4924 for 48 hours, the expression levels of cell cycle mediators, such as p21, p27, phospho-p53 (Ser15), phospho-Chk1 (Ser345) and phospho-Chk2 (Thr68), in ME-180 and HeLa cells were analyzed by Western blotting. Results represent at least three independent experiments.
MLN4924 significantly suppressed the growth of CC xenografts in Nu/Nu nude mice
To verify the in vitro effects of MLN4924 in vivo, we treated Nu/Nu nude mice with solid tumor xenografts generated by subcutaneous injections of 1 × 105 ME-180 or HeLa cells with MLN4924. After the tumors reached approximately 150 mm3 in volume, mice bearing ME-180 or HeLa tumor xenografts were intraperitoneally treated once per day with DMSO/normal saline (as the non-treated control) or MLN4924 (10 mg/kg) for 32 days.
At the end of the treatment, the tumors in the MLN4924-treated group were significantly smaller in size and weight than those in the control group (Figure 5A). Tumor growth was markedly inhibited in the MLN4924-treated group, whereas tumors in the control group continued to grow as large as 800 or 1100 mm3, with weights of 550 or 650 mg, respectively (Figure 5B). Notably, MLN4924 treatment did not affect the weight of the mice compared with the control group (data not shown), suggesting that MLN4924 was not toxic in vivo. These findings suggest that MLN4924 suppresses the growth of xenograft tumors in Nu/Nu nude mice without causing losses in body weight.
Figure 5.
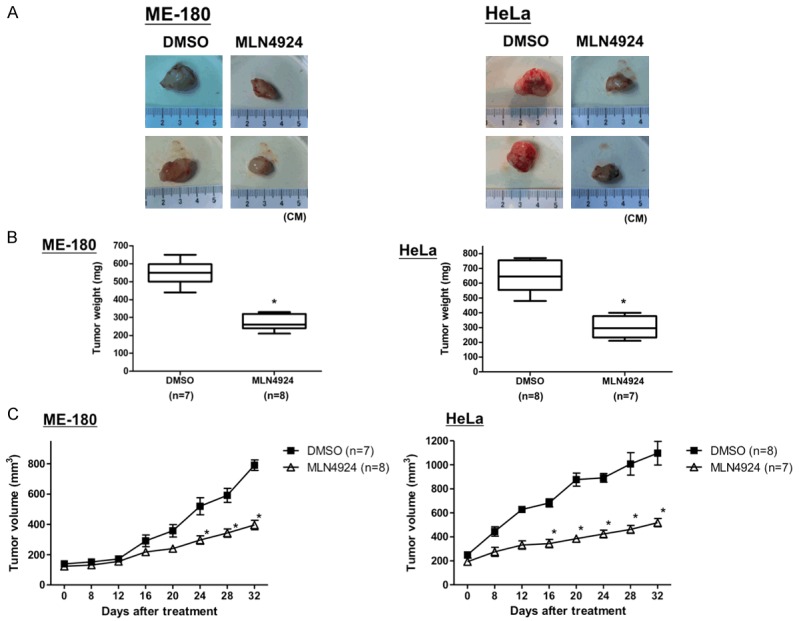
MLN4924 significantly abolished the growth of CC xenografts in vivo. Nu/Nu nude mice bearing ME-180 and HeLa CC tumor xenografts were injected intraperitoneally with DMSO (as the non-treated control; ME-180: n = 7, HeLa: n = 8) or MLN4924 (10 mg/kg; ME-180: n = 8, HeLa: n = 7) once per day for 32 days. A. The tumor images represent excised tumors from each group. B. The tumor weights were compared between the MLN4924-treated and control (DMSO) groups on the last day of treatment. Tumor weights are presented as the mean ± S.D.; *P < 0.05. C. The tumor volumes measured during the treatment are presented as the responses. Tumor volumes are presented as the mean ± SEM; *P < 0.05.
Cisplatin-induced cytotoxicity was enhanced by MLN4924 in human cervical cells
Furthermore, we studied the synergistic antitumor effects of MLN4924 in combination with cisplatin, doxorubicin or gemcitabine on CC cells. We found that MLN4924 did not increase the doxorubicin- or gemcitabine-induced cytotoxicity (data not shown). Surprisingly, MLN4924 significantly enhanced cisplatin-induced cytotoxicity. As shown in Figure 6A, 250 nM MLN4924 markedly potentiated the cytotoxicity of cisplatin in the two CC cell lines. Specifically, MLN4924 co-treatment further increased the cisplatin-induced activation of cleaved caspase-3 and caspase-8 (Figure 6B). These findings consistently indicated that MLN4924 enhances cisplatin-induced cytotoxicity and apoptosis in CC cells.
Figure 6.
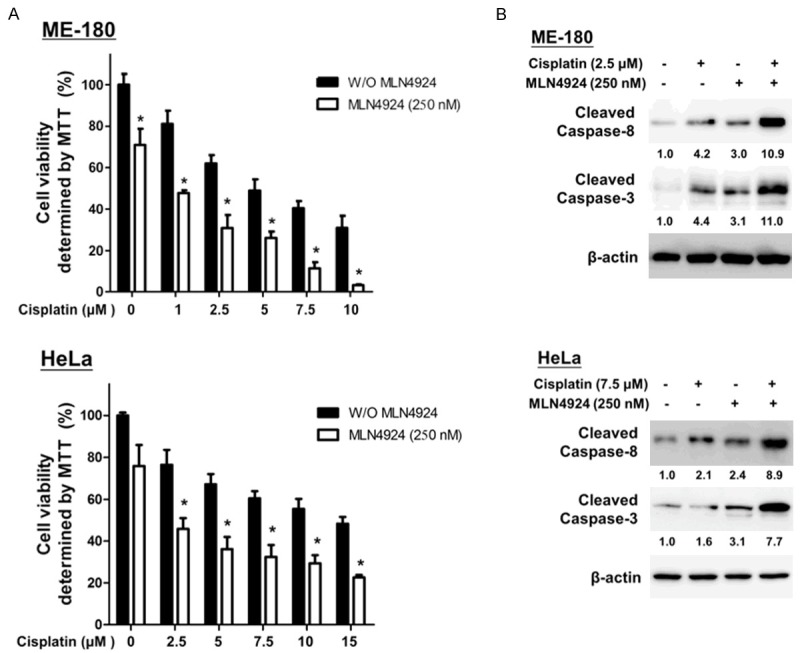
MLN4924 potentiated cisplatin-induced cytotoxicity in human CC cells. A. ME-180 and HeLa cells were incubated with MLN4924 (250 nM) and different concentrations of cisplatin alone or in combination for 48 hours. Cell viability was assessed with an MTT assay. Quantitative analyses of cell viability are presented as the means ± SD of five independents experiments. *P < 0.05 compared with cisplatin treatment alone. B. The total cell lysates were harvested and analyzed by Western blotting with specific antibodies against cleaved caspase-3 and caspase-8 after treatment with DMSO (as the non-treated control), cisplatin, MLN4924, and cisplatin/MLN4924 combination for 48 hours. Combination treatment with cisplatin/MLN4924 resulted in higher expression levels of apoptosis-associated proteins (cleaved caspase-3 and caspase-8) than the cisplatin or MLN4924 treatment alone. Results represent at least three independent experiments.
MLN4924 enhanced the antitumor effect of cisplatin in CC xenografts of Nu/Nu nude mice
Finally, we evaluated the antitumor effects of MLN4924 or cisplatin alone and in combination in a xenograft mouse model. ME-180 or HeLa cells were mixed with Matrigel and then injected subcutaneously into the flanks of Nu/Nu nude mice. The mice were divided into four groups and received intraperitoneal injections of one of the following: DMSO, cisplatin, MLN4924 and cisplatin/MLN4924 combination, as described in the Methods (n = 8/group in ME-180 xenograft; n = 7/group in HeLa xenograft). Consistently with the in vitro results, the combination of cisplatin and MLN4924 exerted the most significant antitumor effect, as evidenced by reductions in the tumor weights and volumes in both ME-180 and HeLa xenografts (Figure 7A-C) compared with either cisplatin or MLN4924 treatment alone or the control group.
Figure 7.
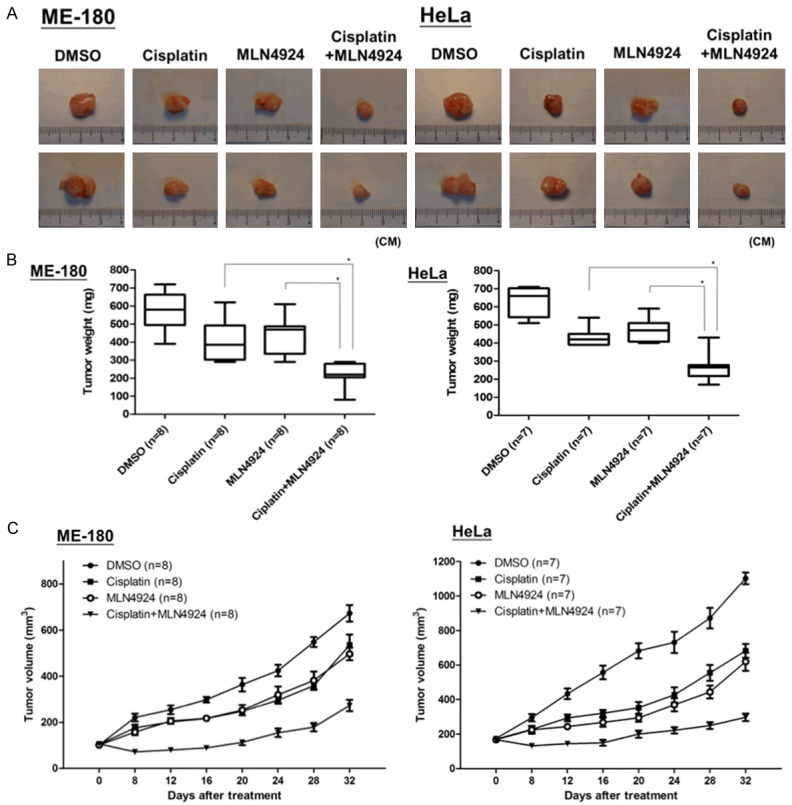
The anti-tumor efficacy of the combination of cisplatin and MLN4924 in a xenograft model of human cervical cancer. Nu/Nu nude mice bearing ME-180 or HeLa xenograft tumors were treated with DMSO (as non-treated control), cisplatin (1.2 mg/kg), MLN4924 (6 mg/kg) or the cisplatin/MLN4924 combination (1.2 mg/kg cisplatin plus 6 mg/kg MLN4924) by intraperitoneal injection once per day for 32 days. A. The tumor images represent excised tumors from each group. B. The tumor weights of each group (DMSO (as non-treated control), cisplatin, MLN4924 and combined treatment) measured on the last day of treatment were compared. C. The tumor volumes assessed during the different treatments are presented as the response. Quantitative analyses of tumor volumes are presented as the means ± SEM (n = 8 for each group of ME-180; n = 7 for each group of HeLa) and *P < 0.05 compared with cisplatin treatment alone or MLN4924 treatment alone.
Discussion
The selective NEDD8-activating enzyme inhibitor MLN4924, which disrupts neddylation, has recently been identified as a novel anticancer agent [41]. MLN4924 resembles the structure of AMP and forms an adduct with NEDD8, thus interfering with NEDD8 adenylation, which is involved in the first step of neddylation [5]. The disruption of this reaction suppresses neddylation on the major subset of the ubiquitin E3 ligase CRL and subsequent accumulation of numerous CRL substrates. Therefore, the MLN4924-mediated inhibition of neddylation interferes with the turnover of cellular proteins, which are manipulated by CRL via the ubiquitin-proteasome system [39]. These CRL substrates are reportedly associated with cell cycle arrest, cell death, and senescence in cancer cells as well as the suppression of tumor growth and invasion. Recent studies have also demonstrated that NEDD8 inhibition results in chemotherapeutic sensitization and radiosensitization [7,14,28,45]. Thus, new strategies targeting the NEDD8 pathway may be promising in cancer treatment.
In the present study, we demonstrated that MLN4924 significantly reduced cell survival and intensified the cisplatin-induced cytotoxicity of CC cells in vitro and in vivo. To our knowledge, this study is the first to demonstrate the anticancer effect of MLN4924 in CC as single agent or in combination with conventional chemotherapeutic agents. Furthermore, because it specifically targets CRL, MLN4924 is less toxic than bortezomib, an FDA-approved proteasome inhibitor for cancer treatment, which exhibits cytotoxicity by inhibiting the degradation of a broad range of cellular proteins [30]. Given the specificity and lower toxicity of MLN4924, it is currently being investigated in several clinical trials for cancer treatment [27].
The activity of CRL is associated with the turnover and homeostasis of proteins involved in DNA replication, cell cycle regulation, stress responses, and various signal transductions [34,35]. We verified that MLN4924 interferes with cell cycle regulation, activates the cell stress response, and induces apoptosis. We demonstrated that MLN4924 induces cell cycle arrest by up-regulating the expression of cyclin-dependent kinase inhibitors (p21 and p27) and the phosphorylation of checkpoint regulators (Chk1, Chk2 and p53).
Additionally, MLN4924 treatment results in the accumulation of CRL substrates and subsequent stress-related apoptosis. Cellular stresses, including radiation, drugs, cytokines or growth factors, induce the expression of SAPK/JNK and their activation by dimerization [10,21]. We also demonstrated that MLN4924 increases the phosphorylation of SAPK/JNK and its downstream transcription factor c-Jun, a member of the MAPK signaling pathway. Moreover, the intense accumulation of CRL substrate proteins may result in the aggregation of misfolded proteins in the cytosol or organelles, triggering endoplasmic reticulum stress [9] and subsequently triggering apoptosis [18,38,47] via the activation of caspase-4, ATF-4 and CHOP [16,17,43]. Accordingly, we demonstrated that MLN4924 induced ER stress and subsequent apoptosis via the activation of caspase-4, ATF-4 and CHOP. Together, the endoplasmic reticulum stress and unfolded protein response pathways play important roles in the MLN4924-induced apoptosis of CC cells.
Cisplatin, an alkylating agent, is a first-line chemotherapeutic agent for the treatment of solid tumors, including cervical cancer, and is often combined with other antitumor agents or radiotherapy [37]. However, the side effects and inevitable development of resistance to this drug compromise the efficacies and outcomes of treatments for advanced CC [32] and the prognosis of metastatic CC remains ominous. Thus, the development novel antitumor agents or new drug combination therapies against CC is imperative. Our study showed that MLN4924 significantly enhances the antitumor effect and apoptosis induced by cisplatin in human CC cells. This effect was further confirmed in the in vivo model, which demonstrated that this drug combination does not induce significant body weight loss in the study mice. Moreover, MLN4924 has been reported to radio sensitize some carcinoma cells [45], and radiotherapy is one of the major components of advanced CC treatment. Therefore, the efficacy of a combined MLN4924, cisplatin and radiotherapy regimen for the treatment of CC warrants further investigation.
In conclusion, we demonstrated that MLN4924 suppresses CC tumors, induces apoptosis, interferes with the cell cycle and induces endoplasmic reticulum stress. Moreover, MLN4924 enhances the cisplatin-induced antitumor effect on CC in vitro and in vivo. These findings provide a rationale for clinical trials of MLN4924 in CC. The combination of MLN4924 and cisplatin may serve as a promising therapeutic strategy in clinical practice.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of Taiwan (104-2314-B-002-164-MY3, 104-2320-B-002-014-MY3, 103-2314-B-002-161-MY3 and 102-2314-B-002-123-MY2) and the National Taiwan University Hospital (104-M2868). The authors thank Prof. Fang-Jen Lee for the kind provision of the HeLa cell line and Dr. Tai-Lung Cha for the kind provision of the ME-180 cell line. We also thank the personnel of the Second, Third and Sixth Core Laboratories of National Taiwan University Hospital.
Disclosure of conflict of interest
None.
Abbreviations
- NEDD8
neural-precursor-cell-expressed developmentally down-regulated protein 8
- SAPK
stress-activated protein kinases
- JNK
c-Jun N-terminal kinases
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
- BrdU
B5-bromo-2’-deoxyuridine
- FITC
fluorescein isothiocyanate
- ATF-4
activating transcription factor-4
References
- 1.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Blank JL, Liu XJ, Cosmopoulos K, Bouck DC, Garcia K, Bernard H, Tayber O, Hather G, Liu R, Narayanan U, Milhollen MA, Lightcap ES. Novel DNA damage checkpoints mediating cell death induced by the NEDD8-activating enzyme inhibitor MLN4924. Cancer Res. 2013;73:225–234. doi: 10.1158/0008-5472.CAN-12-1729. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, Soucy TA, Milhollen MA, Yang X, Burkhardt AL, Ma J, Loke HK, Lingaraj T, Wu D, Hamman KB, Spelman JJ, Cullis CA, Langston SP, Vyskocil S, Sells TB, Mallender WD, Visiers I, Li P, Claiborne CF, Rolfe M, Bolen JB, Dick LR. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. 2010;37:102–111. doi: 10.1016/j.molcel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chairatvit K, Ngamkitidechakul C. Control of cell proliferation via elevated NEDD8 conjugation in oral squamous cell carcinoma. Mol Cell Biochem. 2007;306:163–169. doi: 10.1007/s11010-007-9566-7. [DOI] [PubMed] [Google Scholar]
- 8.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cusimano A, Azzolina A, Iovanna JL, Bachvarov D, McCubrey JA, D’Alessandro N, Montalto G, Cervello M. Novel combination of celecoxib and proteasome inhibitor MG132 provides synergistic antiproliferative and proapoptotic effects in human liver tumor cells. Cell Cycle. 2010;9:1399–1410. doi: 10.4161/cc.9.7.11254. [DOI] [PubMed] [Google Scholar]
- 10.Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS Jr. Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- 11.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 12.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan M, Bigsby RM, Nephew KP. The NEDD8 pathway is required for proteasome-mediated degradation of human estrogen receptor (ER)-alpha and essential for the antiproliferative activity of ICI 182,780 in ERalpha-positive breast cancer cells. Mol Endocrinol. 2003;17:356–365. doi: 10.1210/me.2002-0323. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008, International journal of cancer. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abetainduced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang KH, Kuo KL, Chen SC, Weng TI, Chuang YT, Tsai YC, Pu YS, Chiang CK, Liu SH. Downregulation of glucose-regulated protein (GRP) 78 potentiates cytotoxic effect of celecoxib in human urothelial carcinoma cells. PLoS One. 2012;7:e33615. doi: 10.1371/journal.pone.0033615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo KL, Ho IL, Shi CS, Wu JT, Lin WC, Tsai YC, Chang HC, Chou CT, Hsu CH, Hsieh JT, Chang SC, Pu YS, Huang KH. MLN4924, a novel protein neddylation inhibitor, suppresses proliferation and migration of human urothelial carcinoma: In vitro and in vivo studies. Cancer Lett. 2015;363:127–136. doi: 10.1016/j.canlet.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Kuo KL, Lin WC, Ho IL, Chang HC, Lee PY, Chung YT, Hsieh JT, Pu YS, Shi CS, Huang KH. 2-methoxyestradiol induces mitotic arrest, apoptosis, and synergistic cytotoxicity with arsenic trioxide in human urothelial carcinoma cells. PLoS One. 2013;8:e68703. doi: 10.1371/journal.pone.0068703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 22.Leppä S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L, Chu Y, Yi J, Wang X, Sun Y, Jeong LS, Liu J, Jia L. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 26.Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, Dick LR, Garnsey JJ, Koenig E, Langston SP, Manfredi M, Narayanan U, Rolfe M, Staudt LM, Soucy TA, Yu J, Zhang J, Bolen JB, Smith PG. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 27.Nawrocki ST, Griffin P, Kelly KR, Carew JS. MLN4924: a novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs. 2012;21:1563–1573. doi: 10.1517/13543784.2012.707192. [DOI] [PubMed] [Google Scholar]
- 28.Nawrocki ST, Kelly KR, Smith PG, Espitia CM, Possemato A, Beausoleil SA, Milhollen M, Blakemore S, Thomas M, Berger A, Carew JS. Disrupting protein NEDDylation with MLN4924 is a novel strategy to target cisplatin resistance in ovarian cancer. Clin Cancer Res. 2013;19:3577–3590. doi: 10.1158/1078-0432.CCR-12-3212. [DOI] [PubMed] [Google Scholar]
- 29.Ohh M, Kim WY, Moslehi JJ, Chen Y, Chau V, Read MA, Kaelin WG Jr. An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep. 2002;3:177–182. doi: 10.1093/embo-reports/kvf028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 31.Pan ZQ, Kentsis A, Dias DC, Yamoah K, Wu K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene. 2004;23:1985–1997. doi: 10.1038/sj.onc.1207414. [DOI] [PubMed] [Google Scholar]
- 32.Peiretti M, Zapardiel I, Zanagnolo V, Landoni F, Morrow CP, Maggioni A. Management of recurrent cervical cancer: a review of the literature. Surg Oncol. 2012;21:e59–66. doi: 10.1016/j.suronc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb) 2011;3:279–296. doi: 10.1039/c0ib00144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podust VN, Brownell JE, Gladysheva TB, Luo RS, Wang C, Coggins MB, Pierce JW, Lightcap ES, Chau V. A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc Natl Acad Sci U S A. 2000;97:4579–4584. doi: 10.1073/pnas.090465597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, Pierce JW, Podust VN, Luo RS, Chau V, Palombella VJ. Palombella, Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol Cell Biol. 2000;20:2326–2333. doi: 10.1128/mcb.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose PG, Ali S, Watkins E, Thigpen JT, Deppe G, Clarke-Pearson DL, Insalaco S Gynecologic Oncology Group. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2007;25:2804–2810. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- 37.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 38.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Salon C, Brambilla E, Brambilla C, Lantuejoul S, Gazzeri S, Eymin B. Altered pattern of Cul-1 protein expression and neddylation in human lung tumours: relationships with CAND1 and cyclin E protein levels. J Pathol. 2007;213:303–310. doi: 10.1002/path.2223. [DOI] [PubMed] [Google Scholar]
- 40.Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 41.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 42.Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, Oberheu K, Padmanabhan S, O’Dwyer M, Nawrocki ST, Giles FJ, Carew JS. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- 43.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka T, Halicka D, Traganos F, Darzynkiewicz Z. Cytometric analysis of DNA damage: phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs) Methods Mol Biol. 2009;523:161–168. doi: 10.1007/978-1-59745-190-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei D, Morgan MA, Sun Y. Radiosensitization of Cancer Cells by Inactivation of Cullin-RING E3 Ubiquitin Ligases. Transl Oncol. 2012;5:305–312. doi: 10.1593/tlo.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 48.Yao WT, Wu JF, Yu GY, Wang R, Wang K, Li LH, Chen P, Jiang YN, Cheng H, Lee HW, Yu J, Qi H, Yu XJ, Wang P, Chu YW, Yang M, Hua ZC, Ying HQ, Hoffman RM, Jeong LS, Jia LJ. Suppression of tumor angiogenesis by targeting the protein neddylation pathway. Cell Death Dis. 2014;5:e1059. doi: 10.1038/cddis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yee GP, de Souza P, Khachigian LM. Current and potential treatments for cervical cancer. Curr Cancer Drug Targets. 2013;13:205–220. doi: 10.2174/1568009611313020009. [DOI] [PubMed] [Google Scholar]


