Abstract
Recently, an anti-angiogenic strategy to treat gastric cancer (GC) has been successful with the use of ramucirumab. The comprehensive network of VEGF, soluble VEGF receptor-2 (sVEGFR2) and cytokines and other angiogenic factors (CAF) in GC has not been reported. We aimed to reveal the CAF signature associated with VEGF and sVEGFR2, and to explore their prognostic implication in GC. We measured pretreatment serum levels of 52 CAFs, including VEGF and sVEGFR2, using multiplex bead immunoassays and ELISA, in 70 GC patients treated with palliative chemotherapy. Linear regression analysis for correlating CAFs with VEGF and sVEGFR2, and survival analysis were performed. Results from the current analysis showed the VEGF signature was shown to be associated with seven CAFs (IL-7, IL-12p70, IL-2Ra, IL-10, stem cell factor, FGF2b, IL-3). The sVEGFR2 signature was associated with IL-4 and PDGFb. VEGF and sVEGFR2 showed no association with each other. High VEGF was associated with worse OS (11.2 months, high-VEGF versus 16.7 months, low-VEGF; P = 0.061). However, among patients with high-sVEGFR2, OS was not different according to VEGF (12.1 months, high-VEGF versus 15.1 months, low-VEGF; P = 0.546). In patients with low-sVEGFR2, OS was significantly different according VEGF (10.9 months, high-VEGF versus 16.8 months, low-VEGF, P = 0.036). In multivariate analysis, a high VEGF/sVEGFR2 ratio was significantly correlated with worse OS (HR 1.78 [95% CI 1.08-2.94], P = 0.024). In conclusion, VEGF and sVEGFR2 had distinct CAF signatures in GC. Consideration of both VEGF and sVEGFR2 confers more accurate prognostic implication compared with VEGF alone in GC.
Keywords: VEGF, sVEGFR2, CAF, cytokine, gastric cancer
Introduction
Developing an effective strategy to treat unresectable or recurrent gastric cancer (GC) is of critical importance [1]. Tumor angiogenesis is one of the important components for tumorigenesis and progression [2,3]. Previous reports have shown that both tumor-expressed and secreted VEGF are a poor prognostic factor in major tumor types, including GC [4-7]. Targeting tumor angiogenesis is one of promising strategies in many solid tumor types [8-10].
So far, in GC, results of clinical trials regarding anti-angiogenic agents have been modest [11-14]. Adding bevacizumab (an antibody to VEGF [Vascular Endothelial Growth Factor]) to the chemotherapy regimen did not improve the overall survival (OS) of GC patients, compared with chemotherapy alone, even though there was an absolute gain in survival of 2 months with bevacizumab [11]. This anti-angiogenic strategy in GC was supported strongly by the recent success of ramucirumab, an antibody to the VEGF receptor 2 (VEGRF2). Ramucirumab improved OS of GC patients compared with the best supportive care in a second-line setting [12]. Furthermore, ramucirumab also improved the OS in combination with paclitaxel, compared with paclitaxel alone, in GC patients [13]. Targeting VEGFR2 was also successful with apatinib, a small-molecule VEGFR2 tyrosine kinase inhibitor, in GC patients [14].
The interaction of VEGF and VEGFR2 is an important key pathway for signaling angiogenesis. However, tumor angiogenesis is a very complex process that involves many factors, including various cytokines and angiogenic factors (CAFs), not limited to VEGF. The comprehensive network of VEGF and other CAFs has not yet been reported in the context of tumor angiogenesis. Recently, the role of VEGF in terms of immunosuppressive action has been highlighted. VEGF promotes an immunosuppressive microenvironment by inducing immature dendritic cells, myeloid-derived suppressor cells, and regulatory T cells, by means of immunosuppressive cytokines, such as interleukin (IL)-10 or transforming growth factor (TGF)-beta [15-17]. Therefore, the VEGF signature in the context of CAFs should be identified for accurate assessment of angiogenesis and the immunosuppressive status of the tumor microenvironment [2,3]. The biologic significance of the soluble form of VEGFR2 (sVEGFR2) has been suggested in several studies [18-21]. The sVEGFR2 could inhibit angiogenesis by binding to its ligand, VEGF, which blocks the binding of VEGF to VEGFR2 [18,19]. However, the clinical prognostic implication of sVEGFR2 has not been investigated in GC, especially when considering both VEGF and sVEGFR2.
In the current study, we hypothesized that VEGF and sVEGFR2 display distinct CAF-associated signatures, and considering both VEGF and sVEGFR2 could increase the prognostic implication in GC patients.
Materials and methods
Patients
This study was a retrospective analysis of de-identified patient-level data collected from medical charts. Patients diagnosed with GC at Seoul National University Hospital, Republic of Korea, from April 2005 to December 2011 were included in the analysis if they were older than 18 years of age and had histologically-confirmed recurrent or metastatic GC, an Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate organ function.
Sample preparation and CAF analysis
Patients provided written informed consent for the collection of blood samples for biomarker analysis. Specimens were obtained before initiation of palliative chemotherapy. A total of 52 CAFs were analyzed in the serum, according to the manufacturer’s instructions with multiplex bead suspension array kits using the Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, California, USA), including Human Group I and II cytokine panels, as described in previous reports [22,23]. Serum concentrations of soluble carbonic anhydrase IX (sCA9), soluble vascular endothelial growth factor receptor-2, placental growth factor, and osteopontin (OPN) were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota, USA). Each serum sample was analyzed in duplicate and mean CAF concentrations were reported in pg/ml. Analytes for which > 50% of patients had non-detectable levels or coefficients of variation > 20% were not included in the subsequent analyses. Analytes that had non-detectable levels were recorded as one-half of the lower threshold value.
Statistical analysis
The primary objective of this study was to determine the association of VEGF and sVEGFR2 with other CAFs, as well as clinical outcomes- including survival- of GC patients. The CAF concentrations analyzed in the study were log transformed, as concentrations were highly skewed in all samples. Linear regression analysis was performed for extracting any significant association of CAFs with VEGF or sVEGFR2. For unsupervised hierarchical clustering, the log-transformed concentration of each baseline CAF was standardized by subtracting the sample mean and dividing by the standard deviation. Hiera-rchical clustering and data presentation of the CAFs that were significantly correlated with VEGF or sVEGFR2, were performed with Cluster 3.0 and TreeView software (downloaded from http://www.eisenlab.org/) [24]. Overall survival (OS) and progression-free survival (PFS) were calculated from when palliative first-line chemotherapy was first administered up to the date of either death or the final follow-up visit, and to the date of disease progression (confirmed by imaging modality), respectively. All P values were two-sided and P < 0.05 was considered statistically significant. Additionally, for linear regression analysis to extract significant CAFs that were correlated with VEGF or sVEGFR2, a false discovery rate (FDR) and adjusted R-square value were applied to exclude false positive correlations. After filtering out using a FDR > 0.05, an adjusted R-square value of > 0.25 for VEGF-correlation and > 0.10 for sVEGFR2-correlation were considered as having a true significant correlation. Analyses were completed using STATA version 12 software (StataCorp LP, College Station, Texas, USA).
Ethics
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (H-1411-022-623). The study was conducted according to guidelines for biomedical research outlined in the Declaration of Helsinki.
Results
Patient and CAF characteristics
Characteristics of 70 patients included in the current study are summarized in Table 1. Eight patients (11.4%) were HER2-positive, 13 patients had poorly cohesive carcinoma. The median follow-up duration was 81.6 months (range 32.6-113 months) and the median OS and PFS of first-line palliative chemotherapy were 12.5 (95% CI 10.1-17) and 6 months (95% CI 4.3-6.9), respectively.
Table 1.
Patient characteristics
| Total N = 70 | ||
|---|---|---|
| Age | Median years (range) | 56 (26-77) |
| Sex | Male, n (%) | 44 (62.9) |
| Female, n (%) | 26 (37.1) | |
| ECOG | 0, N (%) | 7 (10) |
| 1, N (%) | 57 (81.4) | |
| 2, N (%) | 6 (8.6) | |
| Palliative setting | Metastatic, N (%) | 56 (80) |
| Recurrent, N (%) | 14 (20) | |
| HER2 | Negative, N (%) | 62 (88.6) |
| Positive, N (%) | 8 (11.4) | |
| Tumor location | Stomach, N (%) | 65 (92.9) |
| GEJ, N (%) | 5 (7.1) | |
| Pathology | Adenocarcinoma, N (%) | 56 (80.0) |
| (pure) PCC, N (%) | 13 (18.6) | |
| Others, N (%)* | 1 (1.4) | |
| SRC component | No, N (%) | 48 (68.6) |
| Yes, N (%) | 22 (31.4) | |
| Lauren | Intestinal, N (%) | 9 (12.9) |
| Diffuse, N (%) | 16 (22.9) | |
| Mixed, N (%) | 1 (1.4) | |
| Unknown, N (%)† | 44 (62.9) | |
| Chemotherapy regimen | FOLFOX, N (%) | 40 (57.1) |
| XP, N (%) | 28 (40.0) | |
| Others, N (%)‡ | 2 (2.9) | |
| Overall Survival | Median months (95% CI) | 12.5 (10.1-17) |
| Progression-free Survival | Median months (95% CI) | 6 (4.3-6.9) |
| Follow-up duration | Median months (range) | 81.6 (32.6-113) |
Adenosquamous carcinoma.
Lauren classification: not evaluable in 44 out of 70, due to small amount of tissue.
One patient in the HER2-positive group was treated with trastuzumab plus conventional chemotherapy, another patient was treated with irinotecan, 5-fluorouracil, and leucovorin.
Abbreviation: ECOG, Eastern Cooperative Group performance status; GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; PCC, poorly cohesive carcinoma; SRC, signet ring cell; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; XP, capecitabine and cisplatin.
A total of 52 CAFs were initially measured and analyzed, but 10 CAFs were excluded from the final analysis as more than half of the samples were outside of the detection range. The mean, standard error, median, and range of the 52 CAFs are listed in Table 2. The median concentrations of VEGF and sVEGFR2 were 207.0 pg/mL and 1324.6 pg/mL, respectively.
Table 2.
Cytokines and angiogenic factors profile
| CAF | N* | Mean (pg/ml) | Standard error* | Median (pg/ml) | Min (pg/ml) | Max (pg/ml) |
|---|---|---|---|---|---|---|
| CAFs Included in the final analysis (N = 42) | ||||||
| VEGF | 68 | 284.3 | 28.5 | 207.0 | 1.2 | 1146.8 |
| sVEGFR2 | 70 | 1314.8 | 34.0 | 1324.6 | 742.8 | 2172.8 |
| IL-2Ra | 70 | 163.1 | 15.5 | 126.7 | 12.7 | 726.8 |
| IL-3 | 55 | 201.7 | 33.7 | 123.3 | 12.5 | 1209.3 |
| IL-16 | 70 | 692.1 | 146.4 | 342.1 | 26.2 | 7298.3 |
| IL-18 | 70 | 123.7 | 13.9 | 82.4 | 23.2 | 564.0 |
| CTACK | 70 | 890.3 | 39.7 | 809.1 | 413.7 | 2175.5 |
| GRO-a | 69 | 257.8 | 43.2 | 176.7 | 5.7 | 2386.1 |
| HGF | 70 | 620.1 | 48.1 | 506.9 | 161.0 | 2428.2 |
| IFN-a2 | 58 | 26.4 | 3.2 | 20.8 | 0.2 | 125.9 |
| LIF | 37 | 27.5 | 3.1 | 25.3 | 1.4 | 88.9 |
| M-CSF | 46 | 31.9 | 4.2 | 22.2 | 1.1 | 156.9 |
| MIF | 70 | 4536.3 | 1346.0 | 850.0 | 104.4 | 77622.3 |
| MIG | 70 | 2312.1 | 495.9 | 1449.0 | 421.4 | 34713.6 |
| SCF | 70 | 138.3 | 8.0 | 132.5 | 42.8 | 399.1 |
| SCGF-b | 70 | 33494.8 | 4480.8 | 26173.7 | 4610.6 | 310063.7 |
| SDF-1a | 69 | 251.8 | 21.1 | 209.5 | 31.9 | 1335.6 |
| TRAIL | 47 | 46.9 | 6.2 | 32.6 | 1.5 | 275.7 |
| IL-1Ra | 70 | 305.9 | 130.2 | 106.4 | 30.1 | 9082.5 |
| IL-4 | 69 | 9.6 | 0.9 | 8.1 | 0.2 | 24.3 |
| IL-6 | 64 | 33.8 | 10.3 | 11.3 | 0.9 | 643.5 |
| IL-7 | 62 | 26.1 | 15.0 | 10.0 | 0.6 | 1060.0 |
| IL-8 | 65 | 88.5 | 51.9 | 19.1 | 1.3 | 3578.4 |
| IL-9 | 66 | 76.6 | 36.4 | 16.2 | 0.9 | 2171.1 |
| IL-10 | 49 | 108.2 | 80.4 | 14.5 | 1.3 | 5583.3 |
| IL-12p70 | 67 | 373.9 | 207.6 | 58.9 | 1.4 | 11784.3 |
| IL-13 | 70 | 22.9 | 11.2 | 8.6 | 1.7 | 788.3 |
| IL-17 | 61 | 59.2 | 5.6 | 49.5 | 0.9 | 191.3 |
| Eotaxin | 69 | 121.3 | 9.3 | 106.2 | 12.2 | 565.7 |
| FGF-basic | 68 | 40.7 | 4.0 | 32.7 | 1.2 | 185.8 |
| G-CSF | 70 | 569.8 | 481.3 | 76.3 | 17.6 | 33773.9 |
| IFN-g | 70 | 124.4 | 39.6 | 61.8 | 14.4 | 2734.7 |
| IP-10 | 69 | 2451.5 | 421.9 | 1686.1 | 299.2 | 27797.0 |
| MCP-1 | 70 | 106.2 | 13.3 | 74.3 | 8.6 | 717.8 |
| MIP-1a | 70 | 27.3 | 21.5 | 4.9 | 1.0 | 1511.7 |
| PDGF-bb | 70 | 7791.5 | 632.2 | 6650.9 | 152.3 | 24895.8 |
| MIP-1b | 70 | 277.2 | 107.4 | 154.4 | 50.4 | 7648.1 |
| RANTES | 70 | 28564.5 | 928.0 | 29143.9 | 2735.5 | 41739.8 |
| TNF-a | 46 | 36.2 | 11.7 | 22.0 | 2.6 | 806.2 |
| PIGF | 55 | 38.9 | 6.2 | 22.1 | 8.0 | 244.6 |
| sCA9 | 69 | 165.6 | 27.3 | 98.8 | 18.7 | 1374.5 |
| OPN | 70 | 5.7 | 0.5 | 4.4 | 1.3 | 22.8 |
| CAF excluded in the final analysis (N = 10) | ||||||
| IL-1a | 2 | 1.6 | 0.3 | 1.1 | 1.1 | 11.2 |
| IL-12p40 | 23 | 122.9 | 36.4 | 1.0 | 1.0 | 2105.1 |
| MCP-3 | 24 | 6.7 | 1.6 | 0.4 | 0.4 | 68.3 |
| BNGF | 16 | 4.2 | 1.3 | 0.6 | 0.6 | 72.5 |
| TNF-b | 16 | 4.7 | 0.9 | 1.0 | 1.0 | 32.6 |
| IL-1b | 20 | 3.3 | 1.4 | 0.3 | 0.3 | 84.0 |
| IL-2 | 16 | 6.9 | 4.0 | 0.7 | 0.7 | 276.4 |
| IL-5 | 6 | 2.0 | 0.6 | 0.7 | 0.7 | 37.1 |
| IL-15 | 8 | 2.3 | 0.6 | 0.9 | 0.9 | 26.9 |
| GM-CSF | 31 | 65.6 | 11.5 | 0.4 | 0.4 | 338.4 |
Number of analytes within detection range.
Abbreviation: CAF, cytokines and angiogenic factors; IL, interleukin; IL-2Ra, IL-2 receptor alpha; CTACK, cutaneous T-cell attracting chemokine; GROa, melanoma growth stimulating activity alpha; HGF, hepatocyte growth factor; IFN-a2, interferon alpha 2; LIF, leukemia inhibitory factor; M-CSF, macrophage colony-stimulating factor; MIF, macrophage migration inhibitory factor; MIG, monokine induced by interferon gamma; SCF, stem cell factor; SCGF-b, stem cell growth factor beta; SDF-1a, stromal cell-derived factor 1 alpha; IL-1Ra, IL-1 receptor alpha; TRAIL, Tumor Necrosis Factor Apoptosis-Inducing Ligand; FGF-basic, fibroblast growth factor 2 basic; GCSF, granulocyte colony stimulating factor; IFN-g, interferon gamma; IP-10, interferon gamma-induced protein 10; MCP-1, monocyte chemotactic protein 1; MIP-1A, macrophage inflammatory protein 1 alpha; PDGF-bb, platelet-derived growth factor beta polypeptide b; MIP-1B, macrophage inflammatory protein 1 beta; RANTES, regulated on activation; normal T cell expressed and secreted; TNF-a, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor A; PIGF, placenta growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; sCA9, soluble carbonic anhydrase 9; OPN, osteopontin; MCP-3, monocyte chemotactic protein 3; BNGF, beta-nerve growth factor; TNF-b, tumor necrosis factor beta; IL-1b, interferon-1 beta; GM-CSF, granulocyte macrophage colony-stimulating factor.
CAF signature associated with VEGF and sVEGFR2
VEGF and sVEGFR2 were not significantly associated with each other (R2 = 0.021 and P = 0.235, Figure 1A). A total of 40 CAFs were analyzed for linear regression with VEGF and sVEGFR2 (Table 3 and Figure 2). Seven CAFs including interleukin (IL)-7, IL-12p70, IL-2 Receptor alpha (IL-2Ra), IL-10, stem cell factor (SCF), FGF-basic (fibroblast growth factor 2 basic), and IL-3 were significantly associated with VEGF, with R2 > 0.250 and FDR of P value < 0.05. IL-4 and platelet-derived growth factor beta polypeptide b (PDGF-bb) were significantly associated with sVEGFR2, with R2 > 0.100 and FDR of P value < 0.05 (Table 4). Unsupervised hierarchical clustering analysis of VEGF and sVEGFR2, with their associated CAFs, clearly identified two groups of patients (Figure 1B, 1C). These distinct signatures of VEGF and sVEGFR2 revealed four groups of patients (Figure 3).
Figure 1.
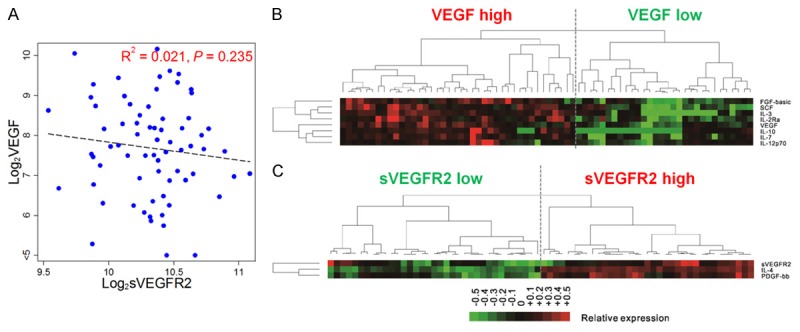
Cluster analysis of VEGF and sVEGFR2 with significantly correlated cytokines and angiogenic factors. Scatter plot of log 2 values of VEGF and sVEGFR2 (A). Unsupervised cluster analysis of cytokines and angiogenic factors (CAFs) that significantly correlated with VEGF (B), and sVEGFR2 (C) in patients with gastric cancer. The CAF concentration ratios are depicted by a log-transformed pseudo-color intensity scale. Abbreviations: VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; IL, interleukin; FDR, false discovery rate; SCF, stem cell factor; FGF-basic, fibroblast growth factor 2 basic; PDGF-bb, platelet-derived growth factor beta polypeptide b.
Table 3.
Lists of cytokines and angiogenic factors (CAFs) that correlated with VEGF and sVEGFR2 levels
| CAF | VEGF correlation | sVEGFR2 correlation | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| R2 | P value | FDR | R2 | P value | FDR | |
| VEGF | N/A | N/A | N/A | 0.021 | 0.235 | 0.338 |
| IL-7 | 0.409 | < 0.001 | < 0.001 | 0.006 | 0.538 | 0.630 |
| IL-12p70 | 0.397 | < 0.001 | < 0.001 | 0.003 | 0.668 | 0.721 |
| IL-2Ra | 0.287 | < 0.001 | < 0.001 | 0.025 | 0.193 | 0.331 |
| IL-10 | 0.281 | < 0.001 | < 0.001 | 0.016 | 0.295 | 0.391 |
| SCF | 0.256 | < 0.001 | < 0.001 | 0.052 | 0.057 | 0.233 |
| FGF-basic | 0.256 | < 0.001 | < 0.001 | 0.040 | 0.098 | 0.251 |
| IL-3 | 0.256 | < 0.001 | < 0.001 | 0.034 | 0.128 | 0.268 |
| M-CSF | 0.227 | < 0.001 | < 0.001 | 0.081 | 0.017 | 0.139 |
| IFN-a2 | 0.222 | < 0.001 | < 0.001 | 0.042 | 0.088 | 0.239 |
| TRAIL | 0.203 | < 0.001 | < 0.001 | 0.103 | 0.007 | 0.070 |
| LIF | 0.195 | < 0.001 | < 0.001 | 0.033 | 0.135 | 0.268 |
| IL-1Ra | 0.195 | < 0.001 | < 0.001 | 0.020 | 0.247 | 0.338 |
| CTACK | 0.188 | < 0.001 | 0.001 | 0.022 | 0.220 | 0.334 |
| MCP-1 | 0.167 | < 0.001 | 0.001 | 0.065 | 0.034 | 0.198 |
| IL-18 | 0.159 | < 0.001 | 0.002 | 0.035 | 0.121 | 0.268 |
| IL-16 | 0.156 | 0.001 | 0.002 | 0.006 | 0.511 | 0.616 |
| MIP-1b | 0.151 | 0.001 | 0.002 | 0.034 | 0.128 | 0.268 |
| HGF | 0.150 | 0.001 | 0.002 | 0.045 | 0.080 | 0.233 |
| SDF-1a | 0.131 | 0.002 | 0.005 | 0.011 | 0.390 | 0.495 |
| MIP-1a | 0.130 | 0.002 | 0.005 | 0.010 | 0.399 | 0.495 |
| IL-6 | 0.121 | 0.003 | 0.006 | 0.048 | 0.068 | 0.233 |
| SCGF-b | 0.106 | 0.006 | 0.011 | 0.020 | 0.246 | 0.338 |
| IL-13 | 0.098 | 0.008 | 0.015 | 0.000 | 0.999 | 0.999 |
| MIG | 0.094 | 0.010 | 0.016 | 0.000 | 0.955 | 0.979 |
| TNF-a | 0.093 | 0.010 | 0.016 | 0.047 | 0.071 | 0.233 |
| IL-9 | 0.093 | 0.010 | 0.016 | 0.003 | 0.655 | 0.721 |
| IP-10 | 0.088 | 0.013 | 0.020 | 0.004 | 0.609 | 0.694 |
| IFN-g | 0.071 | 0.026 | 0.038 | 0.045 | 0.078 | 0.233 |
| GRO-a | 0.070 | 0.027 | 0.038 | 0.028 | 0.163 | 0.305 |
| OPN | 0.037 | 0.108 | 0.148 | 0.046 | 0.075 | 0.233 |
| IL-8 | 0.037 | 0.112 | 0.148 | 0.024 | 0.203 | 0.333 |
| PDGF-bb | 0.036 | 0.116 | 0.148 | 0.132 | 0.002 | 0.041 |
| RANTES | 0.030 | 0.152 | 0.189 | 0.074 | 0.023 | 0.158 |
| sVEGFR2 | 0.021 | 0.235 | 0.276 | N/A | N/A | N/A |
| IL-17 | 0.021 | 0.226 | 0.273 | 0.103 | 0.007 | 0.070 |
| Eotaxin | 0.015 | 0.319 | 0.363 | 0.032 | 0.137 | 0.268 |
| sCA9 | 0.009 | 0.448 | 0.486 | 0.027 | 0.182 | 0.324 |
| MIF | 0.008 | 0.451 | 0.486 | 0.059 | 0.042 | 0.217 |
| IL-4 | 0.005 | 0.560 | 0.589 | 0.160 | 0.001 | 0.025 |
| PIGF | 0.001 | 0.781 | 0.800 | 0.029 | 0.216 | 0.334 |
| G-CSF | < 0.001 | 0.971 | 0.971 | < 0.001 | 0.939 | 0.979 |
Abbreviation: CAF, cytokines and angiogenic factors; other abbreviation of CAF, please see footnote of Table 2.
Figure 2.
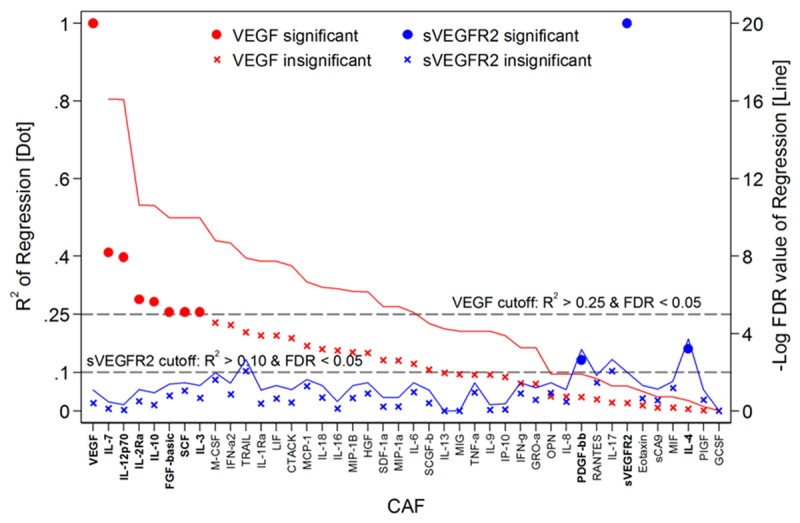
Correlation of VEGF and VEGFR2 with cytokines and angiogenic factors. R2 (line) and FDR value from linear regression of VEGF (red) or VEGFR2 (blue) with cytokines and angiogenic factors are plotted. Abbreviation: VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; CAF, cytokines and angiogenic factors; other abbreviation of CAF, please see footnote of Table 2.
Table 4.
List of cytokines and angiogenic factors (CAFs) that significantly correlated with VEGFA and sVEGFR2 levels
| CAF | VEGF correlation | ||
|
| |||
| R2 | P value | FDR | |
|
| |||
| IL-7 | 0.409 | < 0.001 | < 0.001 |
| IL-12p70 | 0.397 | < 0.001 | < 0.001 |
| IL-2Ra | 0.287 | < 0.001 | < 0.001 |
| IL-10 | 0.281 | < 0.001 | < 0.001 |
| SCF | 0.256 | < 0.001 | < 0.001 |
| FGF-basic | 0.256 | < 0.001 | < 0.001 |
| IL-3 | 0.256 | < 0.001 | < 0.001 |
|
| |||
| sVEGFR2 correlation | |||
|
|
|||
| R2 | P value | FDR | |
|
| |||
| IL-4 | 0.160 | 0.001 | 0.025 |
| PDGF-bb | 0.132 | 0.002 | 0.041 |
Abbreviation: CAF, cytokines and angiogenic factors; VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; IL, interleukin; FDR, false discovery rate; SCF, stem cell factor; FGF-basic, fibroblast growth factor 2 basic; PDGF-bb, platelet-derived growth factor beta polypeptide b.
Figure 3.
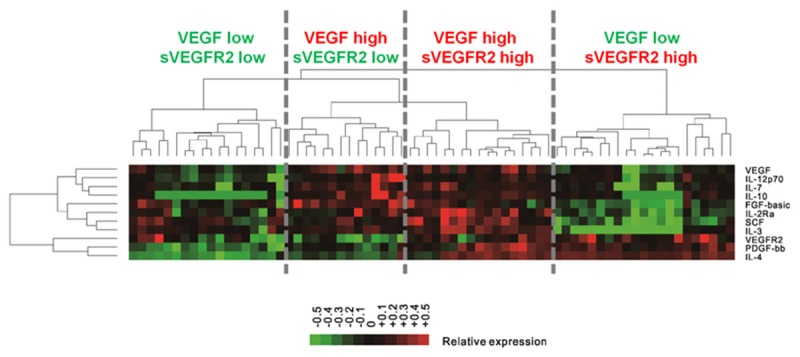
Cluster analysis of combinding VEGF and sVEGFR2 with significantly correlated cytokines and angiogenic factors. Unsupervised cluster analysis of cytokines and angiogenic factors (CAFs) with significantly correlated with VEGF and sVEGFR2 in patients with gastric cancer. The CAF concentration ratios are depicted by a log-transformed pseudocolor intensity scale. Abbreviation; VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; IL, interleukin; FDR, false discovery rate; SCF, stem cell factor; FGF-basic, fibroblast growth factor 2 basic; PDGF-bb, platelet-derived growth factor beta polypeptide b.
The prognostic implication of VEGF and sVEGFR2
Patients with high-VEGF (> median levels of VEGF) had worse OS than others (11.2 months in high-VEGF versus 16.7 months in low-VEGF, P = 0.061, Figure 4A). However, sVEGFR2 itself did not confer any significant prognostic impact (P value of OS and PFS = 0.423 and 0.272, respectively, Figure 5). Interestingly, prognostic implication of VEGF differed according to the sVEGFR2 level. Among patients with high-sVEGFR2 (> median levels of sVEGFR2), the prognostic impact of VEGF was not observed (12.1 months in high-VEGF versus 15.1 months in low-VEGF, P = 0.546, Figure 4B). However, in patients with low-sVEGFR2, OS was significantly different according VEGF levels (10.9 months in high-VEGF versus 16.8 months in low-VEGF, P = 0.036).
Figure 4.
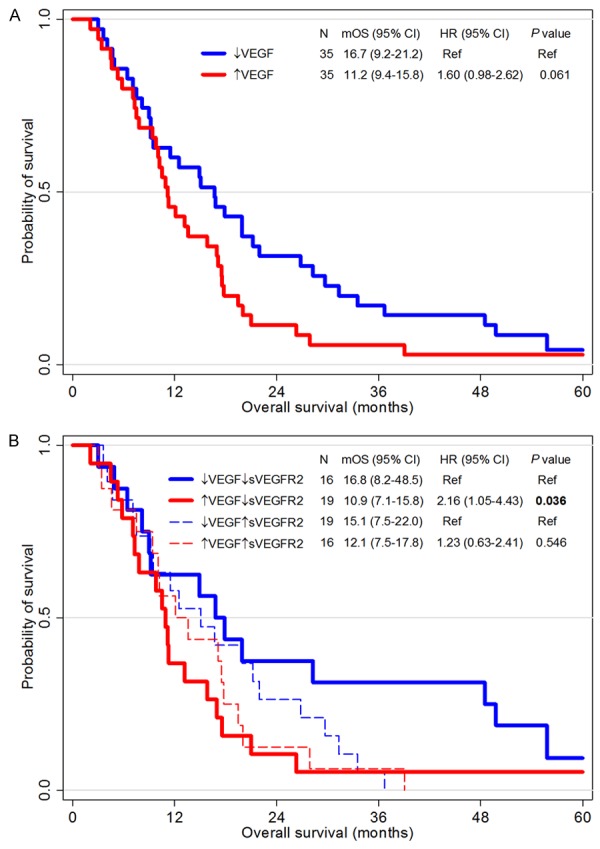
Survival analysis according to VEGF and sVEGFR2 levels. Kaplan-Meier curves for overall survival of two groups divided by the VEGF level higher or lower than its median value (A), and of four groups divided by the VEGF level and the sVEGFR level according to their median levels (B). Abbreviations: CI, confidential interval; HR, hazard ratio; mOS, median overall survival; VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2.
Figure 5.
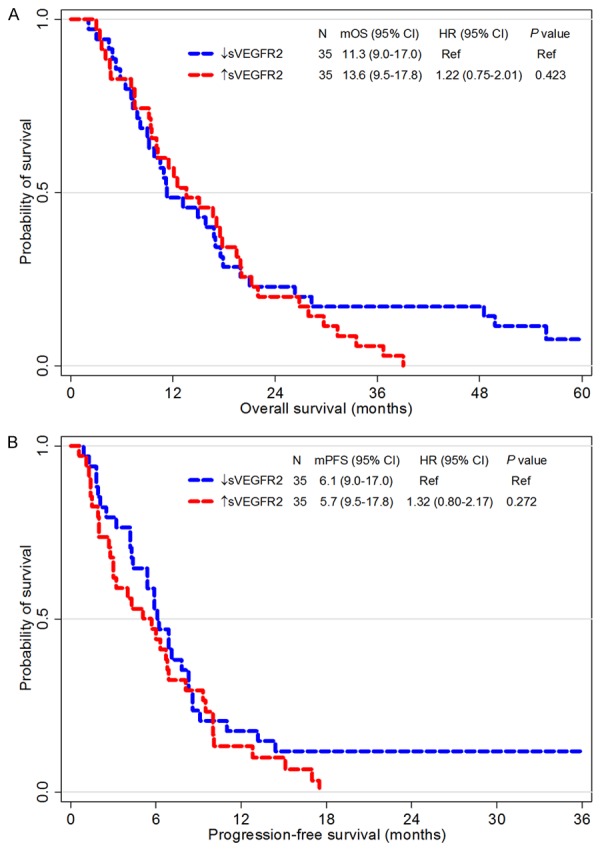
Survival analysis according to sVEGFR2. Kaplan-Meier curves for overall survival (A) and progression-free survival (B) of two groups divided by the sVEGFR2 level higher or lower than its median value. Abbreviation: Abbreviations; CI, confidential interval; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; sVEGFR2, soluble vascular endothelial growth factor receptor 2.
To consider both VEGF and sVEGFR2, we calculated the VEGF/sVEGFR2 ratio (median 0.75, range 0.02-1.03). OS was significantly worse in the high VEGF/sVEGFR2 ratio group than in the low VEGF/sVEGFR2 ratio group (11.2 months versus 16.7 months, P = 0.042, Figure 6A). In multivariate analysis of survival, the VEGF/sVEGFR2 ratio was a significant poor prognostic factor, along with poor performance status and signet ring component (P = 0.024, Table 5 and Figure 6B). Clinico-pathological characteristics were not significantly different according to VEGF/sVEGFR2 ratio (Table 6).
Figure 6.
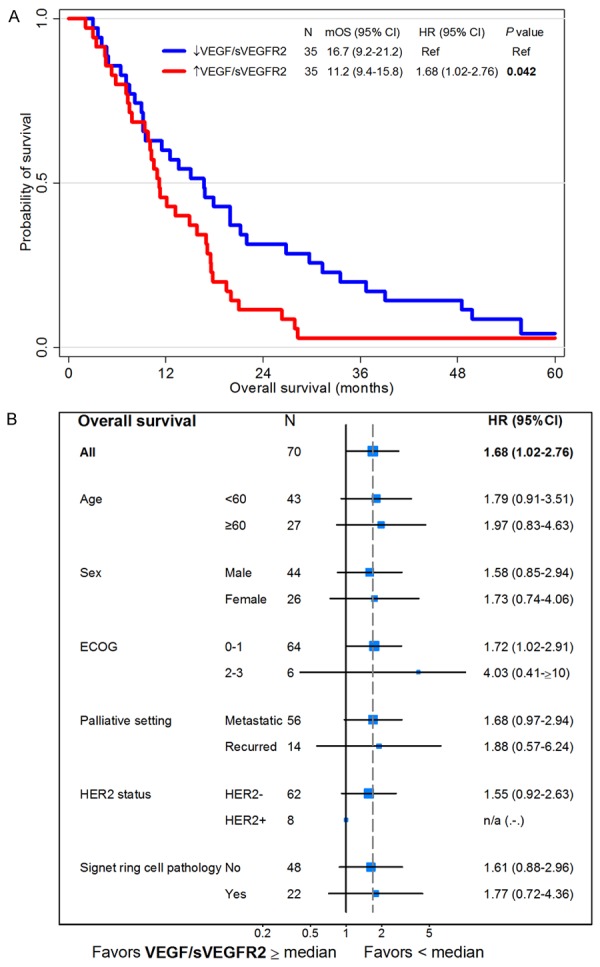
Survival analysis according to the VEGF/sVEGFR2 ratio. Kaplan-Meier curves for overall survival of two groups divided by the VEGF/sVEGFR2 ratio higher or lower than its median value (A). Forest plot of hazard ratios (HR) and 95% confidence intervals (CI) for overall survival assessed by subgroup factors (B). Abbreviation: ECOG, Eastern Cooperative. Group performance status; HER2, human epidermal growth factor receptor 2; mOS, median overall survival; VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; n/a, not applicable.
Table 5.
Univariate and multivariate Cox survival analysis of OS according to circulating VEGF and sVEGFR2 levels
| Overall survival | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age ≥ 60 (vs. < 60) | 0.92 | 0.56-1.51 | 0.732 | |||
| Sex: female (vs. male) | 1.28 | 0.79-2.10 | 0.320 | |||
| ECOG ≥ 2 (vs. 0-1) | 4.17 | 1.72-10.1 | 0.002 | 5.33 | 2.14-13.3 | < 0.001 |
| Recurrent (vs. metastatic) | 0.82 | 0.44-1.50 | 0.512 | |||
| HER2+ (vs. HER2-) | 1.01 | 0.48-2.14 | 0.971 | |||
| SRC component | 1.87 | 1.09-3.18 | 0.022 | 2.00 | 1.17-3.43 | 0.012 |
| VEGF/sVEGFR2* | 1.67 | 1.02-2.76 | 0.042 | 1.78 | 1.08-2.94 | 0.024 |
VEGF/sVEGFR2 ≥ median (vs. < median).
Abbreviation: VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; ECOG, Eastern Cooperative Group performance status; HR, hazard ratio; CI, confidence interval; HER2, human epidermal growth factor receptor 2; SRC, signet ring cell.
Table 6.
Patient characteristics according to VEGF/ sVEGFR2 ratio
| VEGF/sVEGFR2↓ N = 35 | VEGF/sVEGFR↑ N = 35 | P value | ||
|---|---|---|---|---|
| Age | Median years (range) | 54 (29-74) | 60 (26-77) | 0.094 |
| Sex | Male, N (%) | 21 (60.0) | 23 (65.7) | |
| Female, N (%) | 14 (40.0) | 12 (34.3) | 0.621 | |
| ECOG | 0, N (%) | 4 (11.4) | 3 (8.6) | |
| 1, N (%) | 28 (80.0) | 29 (82.8) | ||
| 2, N (%) | 3 (8.6) | 3 (8.6) | 0.923 | |
| Palliative | Metastatic, N (%) | 27 (77.1) | 29 (82.9) | |
| Recurrent, N (%) | 8 (22.9) | 6 (17.1) | 0.550 | |
| HER2 | Negative, N (%) | 33 (94.3) | 29 (82.9) | |
| Positive, N (%)* | 2 (5.7) | 6 (17.1) | 0.133 | |
| Tumor location | Stomach, N (%) | 32 (91.4) | 33 (94.3) | |
| GEJ, N (%) | 3 (8.6) | 2 (5.7) | 0.643 | |
| Pathology | ADC, N (%) | 30 (85.7) | 26 (74.3) | |
| PCC, N (%) | 4 (11.4) | 9 (25.7) | ||
| Others, N (%) | 1 (2.9) | 0 (0) | 0.201 | |
| SRC component | No, N (%) | 25 (71.4) | 23 (65.7) | |
| Yes, N (%) | 10 (28.6) | 12 (34.3) | 0.607 | |
| Lauren | Intestinal, N (%) | 6 (17.1) | 3 (8.6) | |
| Diffuse, N (%) | 9 (25.7) | 7 (20.0) | ||
| Mixed, N (%) | 0 (0) | 1 (2.8) | ||
| Unknown, N (%) | 20 (57.1) | 24 (68.6) | 0.455 | |
| Follow-up | Median months (range) | 76.6 (32.6-106) | 85.9 (62-113) | 0.350 |
One patient in the VEGF/sVEGFR2↓ group was treated with irinotecan, 5-fluorouracil, and leucovorin, and another patient in the VEGF/sVEGFR2↑ was treated with trastuzumab plus conventional chemotherapy.
Abbreviation: VEGF, vascular endothelial growth factor; sVEGFR2, soluble vascular endothelial growth factor receptor 2; ECOG, Eastern Cooperative Group performance status; GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; ADC, adenocarcinoma; PCC, poorly cohesive carcinoma; SRC, signet ring cell; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; XP, capecitabine and cisplatin.
Discussion
In this study, we analyzed pretreatment serum levels of 52 CAFs including VEGF and sVEGFR2 in patients with advanced GC. Clustering analysis of the CAF signature in association with VEGF and sVEGFR2 independently showed two distinct groups. High-VEGF levels were associated with a poor prognosis, and this was only significant in patients with low-sVEGFR2. Taken together, a high-VEGF/sVEGFR2 ratio showed a statistically significant poor prognosis.
Previous research has shown that tumor angiogenesis is promoted by a complex network of immune cells and their related circulating factors [2,3]. However, comprehensive analysis of multiple array-based cytokines and angiogenic factors (CAF) that are associated with VEGF, has not yet been reported. In the current study, using a strict statistical filtering criteria, we found that VEGF is significantly associated with seven CAFs, namely IL-7, IL-12p70, IL-2Ra, IL-10, stem cell factor (SCF), fibroblast growth factor-basic (FGF-basic), and IL-3. As a response to hypoxia, hypoxia-inducible factor 1 activates micro-vessel formation by producing pro-angiogenic cytokines such as VEGF and FGF-basic [25,26]. VEGF and SCF produced by hypoxic cells bind to bone marrow-derived angiogenic cells, recruiting them to the tumor, and stimulating vascularization [26,27]. Moreover, IL-7 is able to mediate VEGF-induced tumor stromal activation, inducing lymphangiogenesis in the surrounding tumor [28,29]. As well as activating the angiogenic pathway, VEGF modulates immunosuppressive features, by activating Th2-related cytokines, such as IL-10 and TGF-beta [2,3,25]. The results of the current study elucidate the associations between VEGF and other CAFs in the patients of GC.
The membrane-bound form of VEGFR2 is up-regulated along with VEGF by the signaling pathway response to hypoxia, and can bind to VEGF-A, C, D, and E, thus promoting growth and development of new vessels [2,3]. Since proteolytic hydrolysis of the membrane form of VEGFR2 is a regulatory mechanism, over-expression of the membrane form of VEGFR2 would increase the soluble form of VEGFR2 [19,30]. However, comprehensive association studies of CAFs which correlate with sVEGFR2 have rarely been described. In the current study, IL-4 and platelet derived growth factor beta polypeptide b (PDGF-bb) were significantly correlated with sVEGFR2. Although IL-4 has been established to play a primary role in the Th2-response along with IL-10 [31], this result implies that IL-4 might play a distinct biological role in tumor angiogenesis, independently from IL-10. Previous results have shown that PDGF-bb is involved in angiogenesis [26,32,33]. In our study, PDGF-bb significantly correlated with sVEGFR2, along with IL-4.
As described above, VEGF not only directly promotes tumor angiogenesis but also promotes an immunosuppressive network [15-17]. Previous reports consistently showed the poor prognostic impact of VEGF in GC patients treated with conventional chemotherapy, although statistical significances varied in these studies [7,34]. In the current study, a high-VEGF level showed the trend toward poor survival (P = 0.061). However, sVEGFR2 level was not correlated with prognosis, in accordance with previous reports in various clinical settings [35,36]. Previous studies have reported that sVEGFR2 traps VEGF in its circulating form, inhibiting its ordinary biological role of angiogenesis and immunosuppression [18-20]. Intriguingly, in the current study, the poor prognostic impact of VEGF is observed only in patients with low-sVEGFR2, not in patients with high-sVEGFR2. A high VEGF/sVEGFR2 ratio, which represents the un-trapped form of VEGF, was significantly correlated with poor prognosis. To the best of our knowledge, this is the first report regarding the clinical impact of VEGF combined with sVEGFR2 in GC.
The translational importance of this study is that consideration of both VEGF and sVEGFR2 confers more accurate prognostic implication compared with VEGF alone in GC.
Although the clinical trial using bevacizumab in GC was unsuccessful [11], recent trials of ramucirumab and apatinib were successful in previously-treated GC [12-14]. Moreover, as a previous biomarker study of bevacizumab clearly showed that high-VEGF patients might benefit from bevacizumab [37], the precise patient selection based on relevant biomarkers is crucially important. The result of the current study implies that sVEGFR2 could be investigated where the clinical implication of VEGF is concerned.
Although the current study presents a new scope of prognostic impact and associated CAFs of VEGF and sVEGFR2, there are several limitations, such as retrospective analysis, with a relatively small sample size. The findings should be validated in a separate cohort or independent studies.
In conclusion, VEGF and sVEGFR2 have distinct CAF signatures. Consideration of both VEGF and sVEGFR2 confers more accurate prognostic implication compared with VEGF alone in GC.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant no. 2013R1A1A2008705) and supported by a grant from Seoul National University College of Medicine (800-20140609). We would like to thank the patients who participated in this study.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 5.Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, Muraoka R, Iki M. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res. 1996;56:2671–2676. [PubMed] [Google Scholar]
- 6.Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Graves CA, Camphausen K, Kim HJ. Prognostic significance of serum levels of vascular endothelial growth factor and insulin-like growth factor-1 in advanced gastric cancer patients treated with FOLFOX chemotherapy. Chemotherapy. 2012;58:426–434. doi: 10.1159/000345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 13.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastrooesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 15.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 16.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 17.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 18.Kou B, Li Y, Zhang L, Zhu G, Wang X, Li Y, Xia J, Shi Y. In vivo inhibition of tumor angiogenesis by a soluble VEGFR-2 fragment. Exp Mol Pathol. 2004;76:129–137. doi: 10.1016/j.yexmp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Collet G, Lamerant-Fayel N, Tertil M, El Hafny-Rahbi B, Stepniewski J, Guichard A, Foucault-Collet A, Klimkiewicz K, Petoud S, Matejuk A, Grillon C, Jozkowicz A, Dulak J, Kieda C. Hypoxia-regulated overexpression of soluble VEGFR2 controls angiogenesis and inhibits tumor growth. Mol Cancer Ther. 2014;13:165–178. doi: 10.1158/1535-7163.MCT-13-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riely GJ, Miller VA. Vascular endothelial growth factor trap in non small cell lung cancer. Clin Cancer Res. 2007;13:s4623–4627. doi: 10.1158/1078-0432.CCR-07-0544. [DOI] [PubMed] [Google Scholar]
- 21.Becker J, Pavlakovic H, Ludewig F, Wilting F, Weich HA, Albuquerque R, Ambati J, Wilting J. Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitor sVEGFR-2. Clin Cancer Res. 2010;16:1431–1441. doi: 10.1158/1078-0432.CCR-09-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montero AJ, Diaz-Montero CM, Millikan RE, Liu J, Do KA, Hodges S, Jonasch E, McIntyre BW, Hwu P, Tannir N. Cytokines and angiogenic factors in patients with metastatic renal cell carcinoma treated with interferon-alpha: association of pretreatment serum levels with survival. Ann Oncol. 2009;20:1682–1687. doi: 10.1093/annonc/mdp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zurita AJ, Jonasch E, Wang X, Khajavi M, Yan S, Du DZ, Xu L, Herynk MH, McKee KS, Tran HT, Logothetis CJ, Tannir NM, Heymach JV. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:46–52. doi: 10.1093/annonc/mdr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32:4057–4063. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidenaar AC, ter Elst A, Kampen KR, Meeuwsen-de Boer TG, de Jonge HJ, Scherpen FJ, den Dunnen WF, Kamps WA, de Bont ES. Stromal interaction essential for vascular endothelial growth factor A-induced tumour growth via transforming growth factor-beta signalling. Br J Cancer. 2011;105:1856–1863. doi: 10.1038/bjc.2011.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming J, Zhang Q, Qiu X, Wang E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jundependent vascular endothelial growth factor-D up-regulation: a mechanism of lymphangiogenesis in lung cancer. Eur J Cancer. 2009;45:866–873. doi: 10.1016/j.ejca.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004;2:315–326. [PubMed] [Google Scholar]
- 31.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 33.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 34.Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, Kim YH, Kim JS. Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol. 2010;40:1147–1153. doi: 10.1093/jjco/hyq111. [DOI] [PubMed] [Google Scholar]
- 35.Kontovinis LF, Papazisis KT, Touplikioti P, Andreadis C, Mouratidou D, Kortsaris AH. Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer. 2009;9:82. doi: 10.1186/1471-2407-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Q, Dey AL, Yang Y, Shen Y, Jilani IB, Estey EH, Kantarjian HM, Giles FJ, Albitar M. Soluble vascular endothelial growth factor receptor 1, and not receptor 2, is an independent prognostic factor in acute myeloid leukemia and myelodysplastic syndromes. Cancer. 2004;100:1884–1891. doi: 10.1002/cncr.20187. [DOI] [PubMed] [Google Scholar]
- 37.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, Shah MA. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 2012;30:2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]


