Abstract
Breast cancer is the most common cancer in women worldwide. Triple-negative breast cancer patients have higher metastatic rate than patients with other breast cancer subtypes. Distant metastasis is one of the causes leading to the high mortality rates. Cyclooxygenase-2 (COX2) is associated with breast cancer metastasis and the downstream prostaglandin E2 (PGE2) exerted its effect through EP receptors (EP1-EP4). However, the exact molecular events of EP receptors in breast cancer metastasis remain undefined. Expressions of EP receptors were determined during cancer development in NOD-SCID mice inoculated with MB-231 and MB-231-EP2 clone. EP2 overexpressing stable clone was constructed to investigate the proliferation and invasion potentials in vivo and in vitro. Drug transporter array was used to identify EP2 receptor-associated drug transported genes in breast cancer metastasis. Localization of EP2 receptor in primary tissues and xenografts were examined by immunostaining. Stable EP2-expression cells formed larger tumors than parental cells in mice model and was highly expressed in both primary and metastatic tissues. Silencing of EP2 receptor by siRNA and antagonist (AH 6809) significantly decreased cell proliferation and invasion, concomitant with reduced MMP-2 and MMP-9 expressions. Results from array data showed that expression of SLC19A3 was markedly increased in EP2 siRNA transfected cells. Ectopic expression of SLC19A3 retarded cell proliferation, invasion and MMPs expressions. Notably, SLC19A3 had a lower expression in primary tissues and was negatively correlated with EP2 receptor expression. Our novel finding revealed that EP2 receptor regulated metastasis through downregulation of SLC19A3. Thus, targeting EP2-SLC19A3 signaling is a potential therapeutic therapy for treating metastatic breast cancer.
Keywords: EP2 receptor, SLC19A3, MMP-2, MMP-9, breast cancer metastasis
Introduction
Breast cancer is the leading cancer and second most common cause of death in women. According to the GLOBOCAN statistics from the World Health Organization, approximately 1.67 million women were diagnosed with breast cancer in 2012 (Globocan 2012, http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx). About 5-10% of breast cancers are metastatic at diagnosis and up to 70% of breast cancer involving lymph nodes will relapse [1]. Surgery is the mainstay treatment of breast cancer while hormonal therapy, chemotherapy, targeted therapy and/or radiation therapy are given based on the tumor grade and molecular subtypes. Triple-negative breast cancer (TNBC) is the most aggressive molecular subtype that associates with poor prognosis and high recurrence rate. The recurrence rate of TNBC patients is approximately 34%, which is 10% higher than other molecular subtypes [2]. The survival rate after first diagnosis of distant metastasis is also shorter when compared with other subtypes [3]. Due to the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), standard neoadjuvant and prophylactic treatments of advanced TNBC are limited to chemotherapy only. In addition, TNBC is associated with germline mutations in BRCA1 and BRCA2 and tends to arise in young women [4]. Therefore, understanding the molecular basis of metastatic breast cancer, better therapeutic strategies and novel prognostic markers can be developed to improve the clinical outcome.
Epithelial-mesenchymal transition (EMT) is a key process during embryonic development, as well as pathological conditions such as cancer development and cancer metastasis. Epithelial cells increase motility and reduce intercellular adhesion during morphological transition to mesenchymal cells [5]. Similarly, invasive and metastatic behaviours of tumor cells are enhanced by EMT pathways and are associated with poor prognosis [5]. Eicosanoid metabolism and signaling have been extensively studied in the past decade and the roles of cyclooxygenase-2 (COX-2) during tumorigenesis and metastasis have been identified [6,7]. COX-2 inhibitors have shown promising anti-tumor effect, however, the unwanted side effects could not be ignored. Hence, it is important to look for alternative therapeutic agents for breast cancers. Prostaglandin E2 (PGE2) is one of the downstream products of COX-2 and exerted its effect through EP receptors [6]. Under normal condition, PGE2 regulates angiogenesis and other signaling pathways by binding to one of the EP receptors, namely, EP1- EP4, and each receptor has distinct biochemical functions [8,9]. PGE2 promotes angiogenesis and cell proliferation during tumorigenesis by inhibiting apoptosis [10]. Elevated level of PGE2 has been reported in metastatic breast cancer patients [11]. Expressions of EP2 and EP4 receptors were induced in proliferating mammary gland during pregnancy and lactation [12]. Silence of EP2 and EP4 receptors suppressed CCR7 expression and lymph node metastasis [13]. Both EP2 and EP4 receptors coupled to the activation of adenylate cyclase and the stimulation of cAMP formation, the amino acid sequences of these two receptors share less than 40% identity [9,14]. Low expression of EP1 receptor has been shown to correlate with breast cancer metastasis and poor overall survival [15]. Recently, COX-2 induced EP4 receptor regulated stem-like cell properties in breast carcinogenesis [16,17]. These studies suggest EP receptors play important roles in tumorigenesis and metastasis [12].
Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade extracellular matrix [18]. Overexpression of MMP-2 and MMP-9 have been reported in breast and prostate cancer and are positively correlated with COX-2 signaling pathway [19,20]. Human ATP-binding cassette (ABC) transporter family and solute-carrier (SLC) gene superfamily are critical transporters during chemotherapeutic treatments [21,22]. Chemoresistance in tumor cells are associated with decreased intracellular drug permeability in ABC proteins, including breast cancer resistance protein (BCRP), multidrug resistance proteins (MRPs) and P-glycoprotein (P-gp) [23]. Extensive research has shown the importance of ABC proteins during tumorigenesis [19], however, the role of SLC transporters is not well characterized. SLC19A3 belongs to solute carrier family 19 that codes for thiamine transporter-2, and downregulation of SLC19A3 expression was seen in primary breast cancer tissues [24]. Our group was the first to delineate the low expression of SLC19A3 in was due to promoter methylation, which could be detected in plasma of breast cancer patients. To this end, the connection between EP2 receptor and SLC19A3 in breast cancer metastasis is largely unknown.
The aims of this study were to identify the role of EP receptors during breast cancer metastasis and the regulation of EMT genes in vivo and in vitro. Also, the effect of SLC19A3 on EMT modulation and the clinical association with the patients’ outcome will be assessed.
Materials and methods
Patient recruitment
This study was approved by Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Breast cancer patients with no previous treatment history of any cancer were eligible to join this study. Patients with breast cancer were recruited from the Department of Surgery, Queen Mary Hospital and Tung Wah Hospital from 2008 to 2013. Study-related information was explained to each subject and signed consents were obtained from all recruited subjects before joining the study. Tumor samples were extracted during surgical operation.
Cell lines
Human metastatic TNBC cell line (MDA-MB-231, American Type Culture Collection, Rocheville, MD, USA) was used as parental cell line in this study. We constructed an EP2-overexpressing cells, MB-231-EP2 clone. Breast cancer cells were cultured in RPMI 1640 medium (Gibco, Life technologies, Carlsbad, CA, USA) supplemented with 10% heat inactivated fetal bovine serum and 1% antibiotic-antimycotic (Gibco) at 37°C in a humidified atmosphere containing 5% carbon dioxide. Cells were transfected using Qiagen HiPerFect Transfection Reagent (Qiagen, Hilden, Germany) according to manufacturer’s recommendation. Expression of EP receptors was assessed by real-time PCR followed by agarose gel electrophoresis.
Tumorigenicity
Human xenograft breast cancer models were used to study the expression of EP receptors, tumor proliferation and tumor invasion. This study was approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR) in the University of Hong Kong. Cells (MB-231 and MB-231-EP2 clone) were trypsinized and the final cell concentration was adjusted to 1 × 106 cells/ml. One hundred microliters of cell suspension were injected into the mammary fat pad of nude mice. Xenografted tumor size was assessed by external caliper weekly and was calculated by using formula V = L × W2/2 where V = tumor volume, L = tumor length and W = tumor width [25]. Mice were sacrificed after 1 week, 2 weeks, 3 weeks, or 3 months and inoculated tumors were extracted for histological examination.
Tumor metastatic animal model
NOD/SCID mice, 4-6 weeks old, were implanted with MB-231 cells stably express luciferase gene in a human breast cancer metastasis model. Briefly, 100 µl of cell suspension (2 × 106 cells) was injected into the mammary fat pad of mice. These were divided into two groups of eight each: (i) MB-231 and (ii) MB-231-EP2 clone. Tumor growth and metastasis were monitored at regular intervals using in vivo fluorescence imaging system (Xenogen100). After 3 months, the tumors were harvested and collected for further analysis.
Immunohistochemistry
Expressions of EP receptors in primary and metastatic tumors were assessed by immunostaining using established protocol with modification. Briefly, xenografted tissue was fixed embedded in paraffin. Tumor was sectioned at 6 μm and was dewaxed and rehydrated by serially immersed in ethanol. Sections were quenched with 3% hydrogen peroxide and treated with 10 mM sodium citrate. Blocking solution was applied for one hour to block the non-specific binding sites. Sections were incubated with monoclonal primary antibody targeting EP1-4 receptors (1:1000 dilutions, Cayman, Michigan, USA) overnight at 4°C. After washing, sections were incubated with secondary antibody for 30 minutes. Sections were visualized using Nikon Eclipse 80i (Nikon, Tokyo, Japan) using iView DAB detection kit (Ventana, Arizona, USA) and photographs were taken with Spot Advanced software.
Cell proliferation
Cell proliferation was studied using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium reduction assay. Cells were seeded at 96-well plate and were treated with 20 nM and 40 nM of EP2-siRNA and control siRNA (Qiagen) for 3 days. Culture medium was removed and 3% MTT in serum free medium was added to each well and further incubated for 2 hours. Medium was discarded and dimethyl sulfoxide (DMSO) was added and incubated at room temperature for 30 minutes. Absorbance at 570 nm was measured using a Multiskan FC Microplate Photometer (Thermo Scientific, Florida, USA) and average absorbance was calculated for statistical analysis.
Cell invasion assay
Invasion potential was assessed using Bio Coat Matrigel Invasion Chamber (BD Biosciences) according to manufacturer’s recommendation. After transfection, cells were trypsinized and resuspended in serum free medium at a concentration of 1 × 104 cells/ml. Resuspended cells were placed in the upper chamber and complete medium was added to the bottom chamber. Cells were incubated and quantified after 24 hours. Non-invasive cells were removed and invasive cells were quantified under microscope at 100 × magnification after stained with crystal violet.
Drug transporter array and real-time RT-PCR
Gene expression profiling of MB-231 transfected with EP2-siRNA and control-siRNA was performed using Drug Transporters RT2 Profiler PCR Array (Qiagen) to identify genes involved in the cancer development and metastasis. Expressions of EP2 receptor and genes related to EMT pathway were confirmed by real-time PCR. RNA from primary tissues and cell lines were extracted using RNeasy Mini kit (Qiagen) and was reversed-transcribed into cDNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). All experiments were carried out using Light Cycler 480 Real-time PCR system (Roche, Basel, Switzerland). The expression levels were calculated by using comparative threshold cycle (Ct) method with formula 2-ΔΔCt. Beta-actin (β-actin) or Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as control to calculate fold changes.
Statistical analysis
Statistical analysis was performed using ANOVA or two-tailed student’s t test for comparing the means between two groups by statistical package SPSS, release 20. All P-values are two-sided and less than 0.05 was considered statistically significant.
Results
EP2 receptor promotes cell proliferation and invasion
To investigate the expression levels of EP receptors during cancer development, MB-231 cells were injected into mammary fat pad of nude mice and examine the expressions of EP1, EP2, EP3, and EP4 receptor at different time points by real-time RT-PCR. Expressions of EP2 and EP4 receptors were significantly induced during cancer development when compared with EP1 and EP3 receptors at 2 weeks and 3 weeks post-injection (Figure 1A). Higher expression of EP2 receptor was observed in xenografts 3 weeks after cell inoculation, this suggests that EP2 receptor contributed in the progression of breast cancer.
Figure 1.
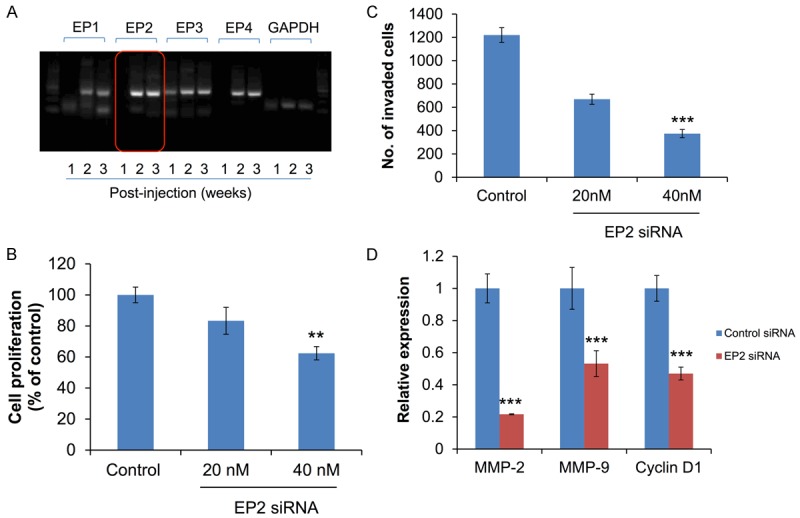
Effect of EP2 receptors inhibition on cell proliferation and invasion in MB-231 cells. A. Expression levels of EP1, EP2, EP3 and EP4 receptors were measured and quantified using RT-PCR after 1 week, 2 weeks, and 3 weeks post-injection of MB-231 cells. EP2 receptors were highest among the four receptors. B. Cell proliferation was determined by MTT assay. All experiments were performed in triplicate. C. Cell invasion potential was measured using Matrigel Invasion Chamber. D. Expression of MMP-2, MMP-9, and Cyclin D1 were quantified by real-time RT-PCR. **P<0.01 and ***P<0.001 are considered as statistically significance.
To validate the functional role of EP2 in metastasis, cell proliferation and invasion were examined in cells transfected with EP2 siRNA. Silence of EP2 receptor significantly reduced cell proliferation and invasion dose-dependently (Figure 1B and 1C). Expressions of MMP-2, MMP-9, and cyclin D1 were downregulated in EP2-siRNA transfection (Figure 1D).
EP2 promoted EMT and metastasis
A stable EP2-expression cell line (MB-231-EP2) was used to study tumorigenesis and distant metastasis in metastatic breast cancer mice model. Cells from MB-231 and MB-231-EP2 clone were injected into the mammary fat pad of NOD-SCID mice. Primary and metastatic tumors were visualized under in vivo imaging system (Xenogen100). Distant metastases, including pancreas, lung, and liver, were found in mice with MB-231 and MB-231-EP2 cells. As shown in Table 1, a higher tumor frequency was found in mice bearing MB-231-EP2 cells. Larger tumor volume was found in MB-231-EP2 clone than MB-231 (Figure 2A). EP2 receptors were predominantly expressed in metastatic tumor than in primary tumor (Figure 2B).
Table 1.
Characteristics of MB-231 and MB-231-EP2 clone in metastatic breast cancer mice model
| Cell line | MB-231 (n = 20) | MB-231-EP2 clone (n = 20) |
|---|---|---|
| Number of mice with tumor | 10 | 13 |
| Number of mice had metastatic tumor | 6 | 4 |
Figure 2.
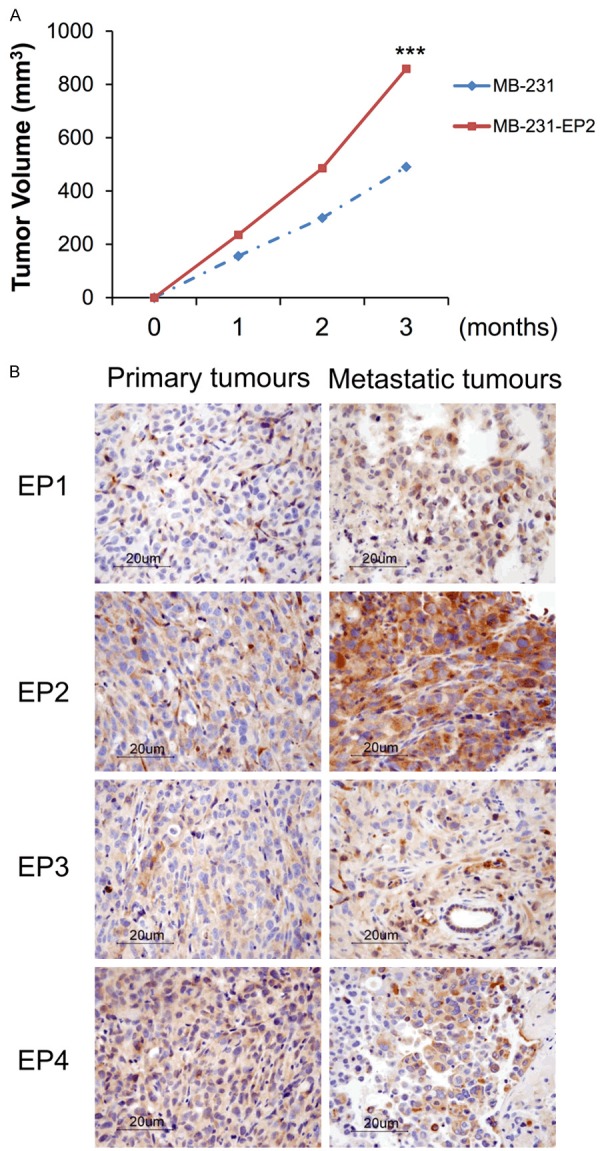
Involvement of EP receptors in breast cancer metastasis. A. Mice were injected with MB-231 and MB-231-EP2 clone in NOD-SCID mice. Tumor volume was measured every 7 days, and metastatic tumors were monitored using in vivo imaging system. B. Immunostaining of EP receptors were performed in primary tumor and metastatic tumor tissues. ***P<0.001 is considered as statistically significance.
To further identify EMT genes involved in metastasis induced by EP2 receptor, we compared the gene expression of drug transporter array in EP2-siRNA and control-siRNA. Among 84 genes, there were 17 differentially expressed genes in EP2-siRNA transfected cells (Figure 3) with a cutoff value of 2-fold difference. As shown in Table 2, there were 7 upregulated (ABCC10, ABCC2, SLC19A3, SLC22A9, SLC28A1, SLC28A2, and SLC7A8) and 10 downregulated (ABCC5, ABCG2, SLC16A3, SLC29A1, SLC31A1, SLC3A1, SLC7A11, SLCO1B1, SLCO2A1 and SLCO4A1) genes in EP2-siRNA transfected cells. Significant increase in SLC19A3 expression was seen in EP2-siRNA when compared with control siRNA, which was comparable with the array data (Figure 5A). This result pointed to the importance of EP2 receptor in the regulation of SLC19A3.
Figure 3.
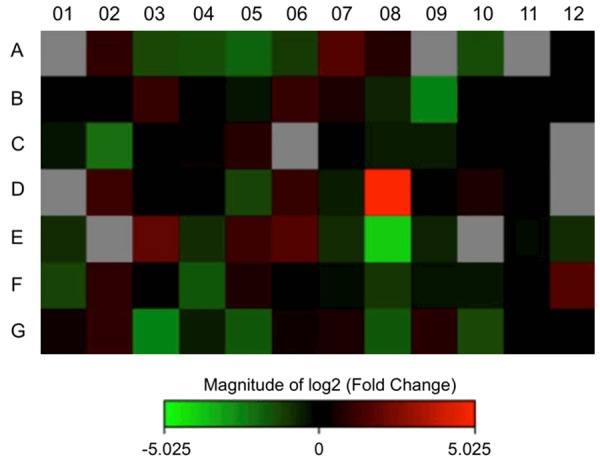
Drug transporter gene expression profiling of control-siRNA and EP2-siRNA treated MB-231 cells.
Table 2.
Differential expression of drug transporter genes in EP2 siRNA cells
| Symbol | Fold change | Full name |
|---|---|---|
| ABCC10 | 2.0491 | ATP-binding cassette, sub-family C (CFTR/MRP), member 10 |
| ABCC2 | 2.1214 | ATP-binding cassette, sub-family C (CFTR/MRP), member 2 |
| SLC19A3 | 32.5594 | Solute carrier family 19 (thiamine transporter), member 3 |
| SLC22A9 | 3.7581 | Solute carrier family 22 (organic anion transporter), member 9 |
| SLC28A1 | 2.2191 | Solute carrier family 28 (concentrative nucleoside transporter), member 1 |
| SLC28A2 | 3.1167 | Solute carrier family 28 (concentrative nucleoside transporter), member 2 |
| SLC7A8 | 3.1932 | Solute carrier family 7 (amino acid transporter light chain, L system), member 8 |
| ABCC5 | -6.0419 | ATP-binding cassette, sub-family C (CFTR/MRP), member 5 |
| ABCG2 | -4.5473 | ATP-binding cassette, sub-family G (WHITE), member 2 (Junior blood group) |
| SLC16A3 | -2.4538 | Solute carrier family 16 (monocarboxylate transporter), member 3 |
| SLC29A1 | -16.1113 | Solute carrier family 29 (equilibrative nucleoside transporter), member 1 |
| SLC31A1 | -2.4623 | Solute carrier family 31 (copper transporter), member 1 |
| SLC3A1 | -3.2154 | Solute carrier family 3 (amino acid transporter heavy chain), member 1 |
| SLC7A11 | -2.0705 | Solute carrier family 7 (anionic amino acid transporter light chain, xc-system), member 11 |
| SLCO1B1 | -6.0629 | Solute carrier organic anion transporter family, member 1B1 |
| SLCO2A1 | -3.3519 | Solute carrier organic anion transporter family, member 2A1 |
| SLCO4A1 | -3.3288 | Solute carrier organic anion transporter family, member 4A1 |
RNA was extracted from control-siRNA and EP2-siRNA treated MB-231 cells. Expression levels of 84 genes were analyzed using drug transporter array. Only genes that were upregulated or downregulated by at least 2-fold are listed.
Figure 5.
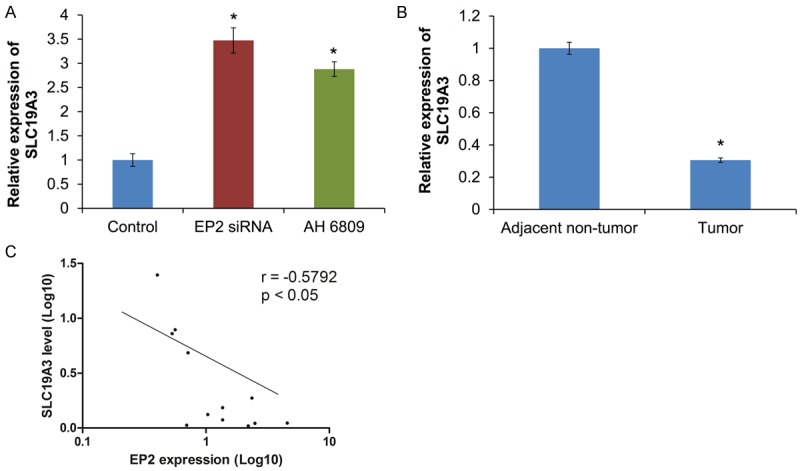
A. Effect of EP2 siRNA or antagonist in EMT-related genes. B. Expression of SLC19A3 in human primary tissues. C. A negative correlation between EP2 receptor and SLC19A3 expression in paired primary tissues (n = 12). *P<0.05 is considered statistically significance.
Tumor suppressive role of SLC19A3 in cell proliferation and cell invasion
To examine the role of SLC19A3 during tumorigenesis and metastasis, cells were transfected with pcDNA3.1(+) or pcDNA3.1(+) with SLC19A3 insert to test the effect on cell proliferation assay and invasion. Ectopic expression of SLC19A3 remarkably suppressed cell proliferation relative to cells without SLC19A3 insert (Figure 4A). Also, cell invasion assay showed that the number of invaded cells was reduced in SLC19A3-overexpressing cells than empty vector (Figure 4B). Importantly, EMT-related genes, MMP-2 and MMP-9, were suppressed by SLC19A3 overexpression (Figure 4C).
Figure 4.
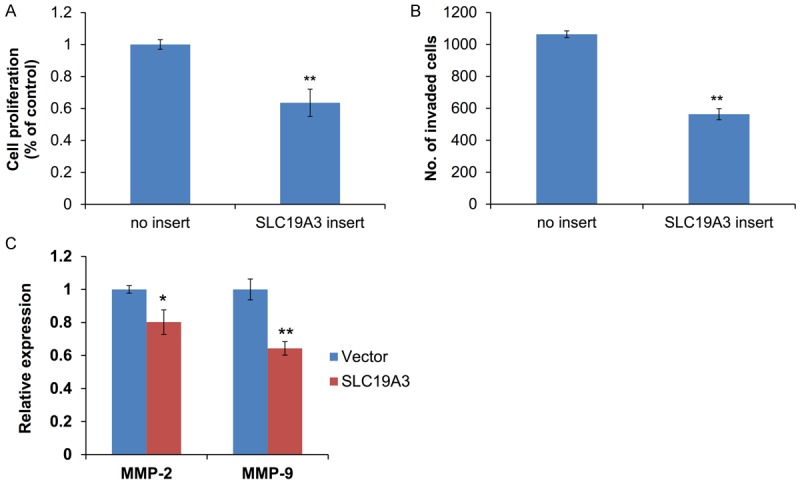
Effect of SLC19A3 on cell proliferation and invasion in MB-231 cells. A. Cell proliferation was measured by MTT assay. B. Cell invasion potential was measured by MTT. C. Expressions of MMP-2 and MMP-9 were measured by real-time RT-PCR. *P<0.05 and **P<0.01 are considered statistically significance.
Expression of SLC19A3 is inversely correlated with EP2 receptor
To further investigate the correlation between SLC19A3 and EP2 receptor, expression of SLC19A3 will be evaluated in cells transfected with EP2-siRNA and EP2 antagonist (AH6809). Silencing of EP2 receptor significantly restored the expression of SLC19A3 (Figure 5A). The expression of SLC19A3 was lowered in primary tumor when compared with adjacent non-tumor tissues (Figure 5B). Our results indicated that expression of EP2 receptor was inversely correlated to the level of SLC19A3 in tumor tissues (Figure 5C).
Discussion
Metastasis is one of the major factors that lead to poor prognosis in breast cancer patients, especially in patients with TNBC subtype. TNBC is associated with poor prognosis with limited therapeutic options, resulting in high recurrence rate and metastatic rate than other breast cancer subtypes [26]. Previous studies have shown that TNBC patients with residual diseases after treated with neoadjuvant chemotherapy showed poor 3-year overall survival rate when compared with non-TNBC patients [27]. To this end, understanding the molecular mechanism of metastasis may help to design better drugs to prevent or treat metastasis.
EP receptors have been studied extensively due to its association with cancer development and metastasis in cancers of colon, lung, prostate and skin [20,28]. While there are four EP coupling receptors mediating PGE2 signaling, our data suggested that overexpression of EP2 receptor increased cell proliferation and invasion during tumorigenesis and EP2 receptor were also responsible for developing distant metastasis for TNBC in animal models. Previous studies have shown that EP2 and EP4 receptors are predominantly expressed in mammary tumors [12]. Several lines of evidence reported that EP2 and EP4 receptors had a positive correlation with increased breast cancer metastasis [12,13,17,29-32]. EP2 receptor was found to be strongly induced in mammary adenoma cells [12]. Moreover, breast cancer metastasis was inhibited by antagonizing EP4 receptor [29]. Cancer stem cells have been demonstrated with their ability to promote cancer metastasis [33], and expression of EP4 receptor has been associated with inhibition of breast cancer stem-cell properties [17]. EP2 receptor increased tumor proliferation and tumor invasiveness in the present study. The effect of EP2 receptor on cell proliferation and invasiveness was validated and confirmed by EP2-siRNA knockdown in vitro. This reduction in cell proliferation and invasion suggested that EP2 receptor play an important role during cancer progression, which was in line with previous study [12]. Our data indicated that EP2 receptor had the strongest expression among other EP receptors during cancer development. In addition, EP2 receptor was not only predominantly expressed in mammary tumor, but also expressed in metastatic tumors in this study. Moreover, average tumor volume was larger with a higher percentage of mice implanted with EP2 clone developed tumor compared to MB-231, suggesting the invasive nature of EP2 receptor during cancer development. Although there were fewer mice with distant metastasis in mice-bearing EP2 clone, these mice had a poor survival rate than mice with MB-231 within the experimental period, which result in lower metastasis frequency in mice with EP2-clone.
The association between COX-2 and MMPs has been demonstrated in many cancers [19,20]. High expression level of EP2 receptor in metastatic tissues with increased cell proliferation and invasion capacities implicated a possible correlation between EP2 receptor and MMP-2 and MMP-9. MMPs are classified into collagenases, gelatinases, stromelysin, matrilysin and the membrane-type MMP [34]. The increased MMPs induced by EP2 receptor during tumorigenesis would facilitate the process of angiogenesis and cell migration. Degradation of basement membrane by MMP-2 and MMP-9 enhanced the migration potential of cancer cells leading to tumor spread [34]. High expression of MMP-9 was associated with TNBC and higher chance of nodal and distant metastasis [18]. On the other hand, cyclin D1 is one of the common genes that overexpressed in breast tumors which involved in cell cycle progression [35].
Chemosensitivity and resistance in tumor cells are contributed by the variation of membrane transporters’ activities [36]. Low expression of SLC19A3 in primary breast tumor tissue was seen, which was consistent with our earlier finding in the plasma of breast cancer patients [20]. Expression of SLC19A3 was restored by suppressing EP2 receptor through siRNA and EP2 antagonist, suggested that expression of SLC19A3 was regulated by EP2 receptor. Downregulation of SLC19A3 in breast, lung and gastric cancers has been reported [37-39]. SLC19A3 was downregulated in breast cancer by hypermethylation of CpG region in the promoter [24]. Similar findings on SLC19A3 downregulation by DNA methylation and histone deacetylation in colon cancer cell lines have also been reported [40]. In contrast, upregulation of SLC19A3 in breast cancer cell lines was found under hypoxic condition [23]. The discrepancy may due to variations in the use of different types of breast cancer cell lines and experimental methods. Since breast cancer is a heterogeneous disease that may explain the variations in expression levels in different breast cancer subtypes. Further studies are warranted to investigate the change of SLC19A3 expression under different conditions in various breast cancer subtypes.
This is the first study to demonstrate a negative correlation between EP2 receptor and SLC19A3 in breast cancer progression and metastasis. The current study proposed a pathway that illustrates the regulatory mechanism of EP2 receptor through downregulation of SLC19A3 on MMPs (Figure 6). However, further study is needed to validate our results in TNBC patients by increasing sample size and compared with patients with metastasis. Moreover, COX-2 inhibitors have been widely used as therapeutic targets for treating cancers due to its anti-tumorigenic effect. The use of COX-2 inhibitors alone or combined with hormonal therapy in treating metastatic breast cancer has achieved promising results [41,42]. However, the cardiovascular safety is the major drawback in using COX-2 inhibitor as the standard therapeutic treatments [43]. Results from this study provide the basis for the development of blocking EP2/SLC19A3 signaling pathway as alternative treatment for breast cancer metastasis.
Figure 6.
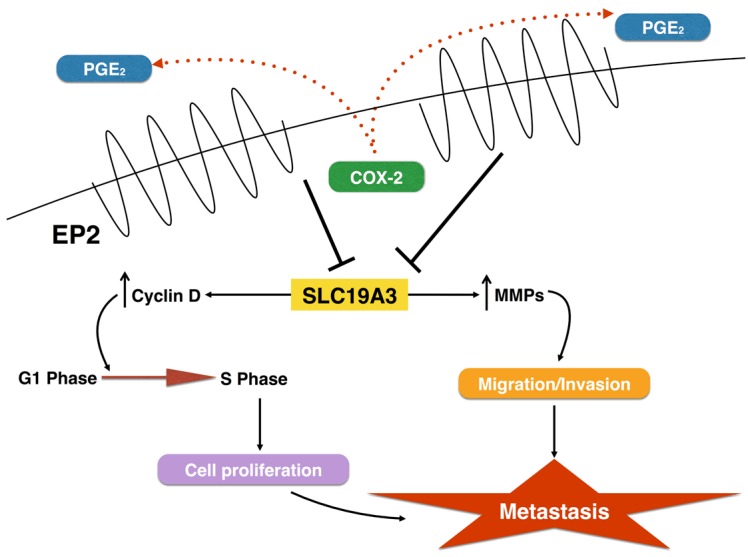
A schematic diagram showing the proposed mechanism of breast cancer metastasis induced by EP2 receptor. EP2 receptor regulates SLC19A3 expression, thereby activates MMPs and leads to the development of metastasis.
Acknowledgements
This study was supported by Dr Ellen Li Charitable Foundation, Kerry Kuok Foundation, Hong Kong Hereditary Breast Cancer Family Registry and the Seed Funding from Committee on Research and Conference Grants, The University of Hong Kong (201402159002, 201411159091). We thank Mr. Chung Wing Bun for assisting in immunostaining.
Disclosure of conflict of interest
None.
References
- 1.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11–19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 2.Schmadeka R, Harmon BE, Singh M. Triplenegative breast carcinoma: current and emerging concepts. Am J Clin Pathol. 2014;141:462–477. doi: 10.1309/AJCPQN8GZ8SILKGN. [DOI] [PubMed] [Google Scholar]
- 3.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 4.Evans DG, Howell A, Ward D, Lalloo F, Jones JL, Eccles DM. Prevalence of BRCA1 and BRCA2 mutations in triple negative breast cancer. J Med Genet. 2011;48:520–522. doi: 10.1136/jmedgenet-2011-100006. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zhou BP. Epithelial-mesenchymal Transition---A Hallmark of Breast Cancer Metastasis. Cancer Hallm. 2013;1:38–49. doi: 10.1166/ch.2013.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranger GS, Thomas V, Jewell A, Mokbel K. Elevated cyclooxygenase-2 expression correlates with distant metastases in breast cancer. Anticancer Res. 2004;24:2349–2351. [PubMed] [Google Scholar]
- 8.Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 10.Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary tumorigenesis. FASEB J. 2010;24:1105–1116. doi: 10.1096/fj.09-141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011;30:449–463. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan MR, Hou MF, Chang HC, Hung WC. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J Biol Chem. 2008;283:11155–11163. doi: 10.1074/jbc.M710038200. [DOI] [PubMed] [Google Scholar]
- 14.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Kundu N, Ioffe OB, Goloubeva O, Konger R, Baquet C, Gimotty P, Reader J, Fulton AM. Prostaglandin E receptor EP1 suppresses breast cancer metastasis and is linked to survival differences and cancer disparities. Mol Cancer Res. 2010;8:1310–1318. doi: 10.1158/1541-7786.MCR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder M, Landman E, Liu L, Hess D, Lala PK. COX-2 Elevates Oncogenic miR-526b in Breast Cancer by EP4 Activation. Mol Cancer Res. 2015;13:1022–33. doi: 10.1158/1541-7786.MCR-14-0543. [DOI] [PubMed] [Google Scholar]
- 17.Kundu N, Ma X, Kochel T, Goloubeva O, Staats P, Thompson K, Martin S, Reader J, Take Y, Collin P, Fulton A. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res Treat. 2014;143:19–31. doi: 10.1007/s10549-013-2779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousef EM, Tahir MR, St-Pierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer. 2014;14:609. doi: 10.1186/1471-2407-14-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang HF, Shu P, Murphy TF, Aisner S, Fitzhugh VA, Jordan ML. Significance of divergent expression of prostaglandin EP4 and EP3 receptors in human prostate cancer. Mol Cancer Res. 2013;11:427–439. doi: 10.1158/1541-7786.MCR-12-0464. [DOI] [PubMed] [Google Scholar]
- 21.He L, Vasiliou K, Nebert DW. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics. 2009;3:195–206. doi: 10.1186/1479-7364-3-2-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweet R, Paul A, Zastre J. Hypoxia induced upregulation and function of the thiamine transporter, SLC19A3 in a breast cancer cell line. Cancer Biol Ther. 2010;10:1101–1111. doi: 10.4161/cbt.10.11.13444. [DOI] [PubMed] [Google Scholar]
- 24.Ng EK, Leung CP, Shin VY, Wong CL, Ma ES, Jin HC, Chu KM, Kwong A. Quantitative analysis and diagnostic significance of methylated SLC19A3 DNA in the plasma of breast and gastric cancer patients. PLoS One. 2011;6:e22233. doi: 10.1371/journal.pone.0022233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG, Colaco B, Pires MJ, Colaco J, Ferreira R, Ginja M. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim (NY) 2013;42:217–224. doi: 10.1038/laban.254. [DOI] [PubMed] [Google Scholar]
- 26.Cleator S, Heller W, Coombes RC. Triplenegative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 27.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 28.Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res. 2006;66:9794–9797. doi: 10.1158/0008-5472.CAN-06-2067. [DOI] [PubMed] [Google Scholar]
- 29.Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 30.Robertson FM, Simeone AM, Mazumdar A, Shah AH, McMurray JS, Ghosh S, Cristofanilli M. Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J Exp Ther Oncol. 2008;7:299–312. [PubMed] [Google Scholar]
- 31.Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, Fulton AM. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2:e22647. doi: 10.4161/onci.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C. Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp Cell Res. 2003;289:265–274. doi: 10.1016/s0014-4827(03)00269-6. [DOI] [PubMed] [Google Scholar]
- 33.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–U554. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 34.Somiari SB, Somiari RI, Heckman CM, Olsen CH, Jordan RM, Russell SJ, Shriver CD. Circulating MMP2 and MMP9 in breast cancer--potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. Int J Cancer. 2006;119:1403–1411. doi: 10.1002/ijc.21989. [DOI] [PubMed] [Google Scholar]
- 35.Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–727. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Sadee W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239:168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Lam EK, Wang X, Zhang J, Cheng YY, Lam YW, Ng EK, Yu J, Chan FK, Jin H, Sung JJ. Promoter hypermethylation mediates downregulation of thiamine receptor SLC19A3 in gastric cancer. Tumour Biol. 2009;30:242–248. doi: 10.1159/000243767. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Huang H, Lu X, Golinski M, Comesse S, Watt D, Grossman RB, Moscow JA. Downregulation of thiamine transporter THTR2 gene expression in breast cancer and its association with resistance to apoptosis. Mol Cancer Res. 2003;1:665–673. [PubMed] [Google Scholar]
- 39.Liu S, Stromberg A, Tai HH, Moscow JA. Thiamine transporter gene expression and exogenous thiamine modulate the expression of genes involved in drug and prostaglandin metabolism in breast cancer cells. Mol Cancer Res. 2004;2:477–487. [PubMed] [Google Scholar]
- 40.Ikehata M, Ueda K, Iwakawa S. Different involvement of DNA methylation and histone deacetylation in the expression of solute-carrier transporters in 4 colon cancer cell lines. Biol Pharm Bull. 2012;35:301–307. doi: 10.1248/bpb.35.301. [DOI] [PubMed] [Google Scholar]
- 41.Falandry C, Canney PA, Freyer G, Dirix LY. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20:615–620. doi: 10.1093/annonc/mdn693. [DOI] [PubMed] [Google Scholar]
- 42.Brandao RD, Veeck J, Van de Vijver KK, Lindsey P, de Vries B, van Elssen CH, Blok MJ, Keymeulen K, Ayoubi T, Smeets HJ, Tjan-Heijnen VC, Hupperets PS. A randomised controlled phase II trial of pre-operative celecoxib treatment reveals anti-tumour transcriptional response in primary breast cancer. Breast Cancer Res. 2013;15:R29. doi: 10.1186/bcr3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazhar D, Ang R, Waxman J. COX inhibitors and breast cancer. Br J Cancer. 2006;94:346–350. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]


