Abstract
Soft-tissue sarcomas (STS) are a heterogeneous group of mesenchymal tumors whose classification and treatment is complicated by molecular heterogeneity within the histological subtypes and by the lack of prognostic/therapeutic biomarkers. This study analyses expression of target proteins involved in insulin-like growth factor pathway (IGF1Rβ, IRS1 S612 and IGFBP7) in high-grade STS to stratify patients with the worst prognosis. Tissue microarray analysis performed on 145 high-grade STS samples revealed a uniform expression of IGF1Rβ and IRS1 S612, while IGFBP7 was more strongly expressed in metastatic than in metastasis-free patients. This was confirmed by multivariate regression analysis that demonstrated the independent poor prognostic role of IGFBP7 overexpression with a significant increase of risk of metastasis (HR = 6.358, 95% CI = 2.946-13.721; P < 0.0005). Given the evidence that circulating protein may generate from tissue tumor cells, in 59/145 patients who had available serum we measured IGFBP7 concentration. The ELISA assay revealed significantly higher levels in tumor patients than in the control with a possible threshold value of 25 ng/ml. Differentiating sera according to primary tumor histotype, significantly higher IGFBP7 concentration was found in synovial sarcoma and liposarcoma than in other STS histotypes. This study revealed that tissue expression of IGFBP7, considered a tumor stroma marker in mesenchymal derived cells, was highly prognostic in poor metastasis-free survival. In parallel, the determination of serum protein levels might contribute to STS diagnosis. Subsequent analyses will be crucial to understand the clinical relevance of IGFBP7 protein in STS.
Keywords: Soft tissue sarcoma, tissue microarray, circulating biomarkers, insulin-like growth factor pathway, prognosis
Introduction
Soft-tissue sarcomas (STS) are a heterogeneous group of mesenchymal tumors [1] that comprise 1% of adult cancers including approximately 50 subtypes [2,3].
Complete surgical removal in association with radiation and chemotherapy increased 5-year disease-free survival in localized high-grade STS patients, while clinical outcome of patients with advanced/metastatic tumors at diagnosis, or following adjuvant therapy, remains strongly unfavourable. Since the majority of sarcomas present multiple genomic diversity [4] even among tumors with the same diagnosis, STS clinical management requires a more profound knowledge about the molecules dictating tumor cell metastatic potential.
In previous studies on high-grade STS, bone metastasis [5,6] and bone tumors [7] we identified proteins highly associated with metastatic events. Moreover, several clinical parameters such as tumor size, depth, histological tumor grade and age have been defined as predictive factors for STS patient survival [8-10]. In particular, 50% of patients with high-grade tumors die of disease [10]. Therefore, there is a clear need to establish easily determinable biomarkers that can be used for a better patient stratification and new therapeutic strategies.
The insulin-like growth factor (IGF) system is one of the most extensively studied target systems in sarcomas [11]. IGF-I receptor (IGF1R) and its substrate, insulin receptor substrate 1 (IRS1) are kinase-activated proteins in IGF axis that play a role in cell proliferation and drug resistance [12]. Previously found highly expressed in STS bone metastases [5] they are thus potential targets for sarcoma treatment [13,14]. IGF1R correlates with poor prognosis in malignant peripheral nerve sheath tumor [14] promoting cell survival [15] and acting as biomarker in human sarcomas [16,17]. In human rhabdomyosarcomas cell lines IGF-II overexpression mediates AKT phosphorylation [18].
IGF signaling is modulated by IGF binding proteins (IGFBPs) that act as tumor suppressor genes or oncogenes depending on the context [19-21]. In particular, IGFBP7 binds insulin with high affinity and may be considered a tumor stroma marker in malignant epithelial and mesenchymal derived cells [22].
In the current study we focused on tissue and circulating levels of IGFBP7 in high-grade STS patients and demonstrated that a high tissue expression had a significant poor prognostic value in terms of metastasis-free survival. Moreover, circulating IGFBP7 levels might be useful in discriminating tumor from non tumor patients and in contributing to STS diagnosis.
Materials and methods
Patients and tumor samples
145 patients (82 males and 63 females) diagnosed at Rizzoli Orthopedic Institute (IOR) from October 1991 to April 2011 with high-grade primary STS according to the Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors were included in the study. The primary tumors, deeply localized and with a diameter > 5 cm, arose from soft tissues of the extremities and chest wall. Selection criteria excluded patients previously treated with radio/chemotherapy and with local relapses at presentation.
The diagnosis based on histological, cytogenetic and immunohistochemical criteria, according to the World Health Organization International Histological Classification of Tumours [2], was confirmed by independent pathologists (Table 1). Follow-up time was considered from the date of diagnosis to the first event (metastasis) or to the last follow-up (minimum follow-up 3 years for metastasis-free patients). Patients underwent wide local excision of the primary tumor. 98 patients received adjuvant treatment within 3 months after tumor excision (Table 1).
Table 1.
Clinical characteristics of STS patients in discovery (IHC) and validation (IHC and ELISA) sets
| IHC (n = 86) | IHC, ELISA (n = 59) | |
|---|---|---|
| Gender | No. | No. |
| Male | 50 | 32 |
| Female | 36 | 27 |
| Median age - yr (range) | 62 (19-92) | 53 (13-83) |
| Site | No. | No. |
| Upper Limbs | 15 | 7 |
| Lower Limbs | 65 | 48 |
| Axial Skeleton | 6 | 4 |
| Total median follow up - months | 42 (3-204) | 52 (5-239) |
| Metastasis median time - months | 4 (0-98) | 17 (0-187) |
| Outcome | No. | No. |
| Alive with Disease | 39 | 39 |
| Dead of Disease | 47 | 20 |
| Clinical Course | No. | No. |
| Non Metastatic STS | 34 | 32 |
| Metastatic STS | 52 | 27 |
| Histotype | No. | No. |
| LMS | 32 | 8 |
| UPS | 27 | 4 |
| SS | 10 | 16 |
| LS | 10 | 25 |
| FS | 7 | 6 |
| Adjuvant Treatments | No. | No. |
| Chemotherapy | 29 | 13 |
| Radiotherapy | 37 | 19 |
Abbreviations: ELISA, Enzyme-linked Immunosorbent Assay; IHC, Immunohistochemistry; LMS, Leiomyosarcoma; UPS, Undifferentiated Pleomorphic Sarcoma; SS, Synovial Sarcoma; FS, Fibrosarcoma; LS, Liposarcoma.
First, IGF pathway protein expression was analyzed by immunohistochemistry on tissue macroarray (TMA) sections of archived STS paraffin-embedded samples from 86 patients (Table 1). The most significant protein, IGFBP7, was then validated by TMA in another 59 high-grade STS with paired serum available (Table 1) and its circulating levels were determined on serum by ELISA assay.
The research protocol was approved by the Rizzoli Institute Ethic Committee (n = 865; 1-14-2009) and all patients provided appropriate informed consent. The work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Tissue microarray analysis (TMA)
Hematoxylin-eosin sections from paraffin-embedded tumor samples were reviewed by pathologists and the most representative area was chosen for TMA construction using TMA Master System (Euroclone SpA, Milano, Italy).
Protein expression was evaluated by immunohistochemistry (IHC).
After antigen retrieval and blocking with ready-to-use Universal Block (catalog no. 71-00-61; KPL, Gaithersburg, MD, USA) for 20 minutes at room temperature, sections were incubated with rabbit polyclonal anti-IGF1Rβ (Cat. #sc-713, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-phospho-IRS1 (S612) (Cat. #PAB12628, 1:1000; Abnova, Walnut, CA, USA) and goat polyclonal anti-IGFBP7 (Cat. #AF1334, 5 µg/ml; R&D Systems, Abingdon, UK) at 4°C overnight. Negative controls were included by omitting antibodies during primary incubation. IHC staining was performed using Dako LSAB + System-HRP followed by treatment with 3,3-diaminobenzidine (DAB) solution as chromogen (Dako, Milan, Italy) and sections were counterstained with Meyer’s hematoxylin.
According to the percentage of positive tumor cells, samples were scored as 0 (≤ 10% positive cells); 1 (11%-25%); 2 (26%-49%); 3 (≥ 50%). Staining intensity was scored as 0, no visual staining; 1, weak; 2, moderate; 3, strong. Cut-off levels for the sum of scores were applied as 0-1 for negative cases, 2-4 for weak positivity or moderate positivity in less than 50% of tumor cells and 5-6 for strong and moderate expression in more than 50% of tumor cells. This was considered protein overexpression.
Enzyme-linked immunosorbent assay (ELISA)
The levels of circulating IGFBP7 protein were measured by ELISA in 59 sera from patients with high-grade STS and 10 healthy controls. Briefly, blood samples were centrifuged at 3000 g for 20 min (4°C), and the supernatants were collected, made to aliquots, and stored in -80°C until assayed. Time interval between processing and freezing was less than 2 h per sample. None of the samples were thawed more than twice before analysis. Individual serum samples (50 µl) were mixed with 450 µl dilution buffer and the concentration of IGFBP7 (ELISA kit from Boster Biological Technology Co., Fremont, CA, USA) was determined according to manufacturer’s instructions. The plate was read at 450 nm in a microtiter plate reader (Promega, Milan, Italy). The average percentage recovery was approximately 100%. Sensitivity of the assay was < 20 pg/ml.
Statistical analysis
Kaplan-Meier survival analysis was used to assess the influence of nominal variables on the development of metastasis. Cox Regression univariate analysis was used to assess the influence of continuous variables on the development of metastasis.
Cox Regression multivariate analysis was used to select variables independently predicting development of metastasis.
Mann-Whitney and Kruskal-Wallis followed by post hoc Dunnett test for multiple comparisons were used for data with non-homogeneous variance test.
For all tests P < 0.05 was considered significant.
All statistical analyses were performed using SPSS v.19.0 (IBM Corp., Armonk, NY, USA).
Results
Protein expression in high-grade STS surgical specimens
Population study
34 of 86 patients with primary high-grade STS included in the discovery set for IHC analysis were metastasis-free. 52 patients had metastasis during follow-up and 28/52 presented metastasis at diagnosis or within the first 4 months from diagnosis (Table 1). Average metastasis-free survival (MFS) was 80.8 months (95% CI = 59.9-101.7) with a median of 19 months (95% CI = 0-47.7) (Figure 1A).
Figure 1.
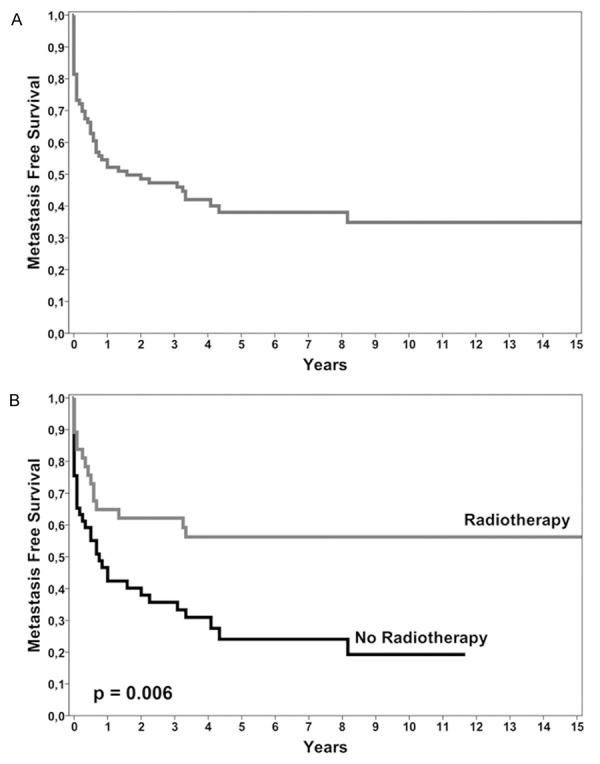
Survival curves. A. Metastasis-free survival of 86 high-grade STS patients analyzed in discovery set. B. Kaplan-Meier survival curves show that the radiotherapy treatment is associated with a better prognosis.
Age, gender, tumor size and site, histotype and chemotherapy did not significantly influence the risk of metastatic progression, while Kaplan-Meier analysis showed a statistically significant difference between MFS curves based on radiotherapy treatment (log-rank = 7.619; P = 0.006) (Figure 1B), with a higher probability of MFS for patients who were given radiotherapy.
Immunohistochemical analysis
In order to verify the role of proteins controlling IGF pathway signaling on malignant progression, thus providing prognostic information and new strategies for high-grade STS management, we performed TMA analysis of IGF1Rβ, IRS1 S612 and IGFBP7 proteins.
In detail, 81% (42/52) of metastatic STS presented a moderate to strong IGFBP7 staining in more than 25% of tumor cells, with both cytoplasmatic and nuclear intracellular distribution more intense and uniform in 24/29 patients with metastasis at presentation or within the first 4 months (Figure 2A).
Figure 2.
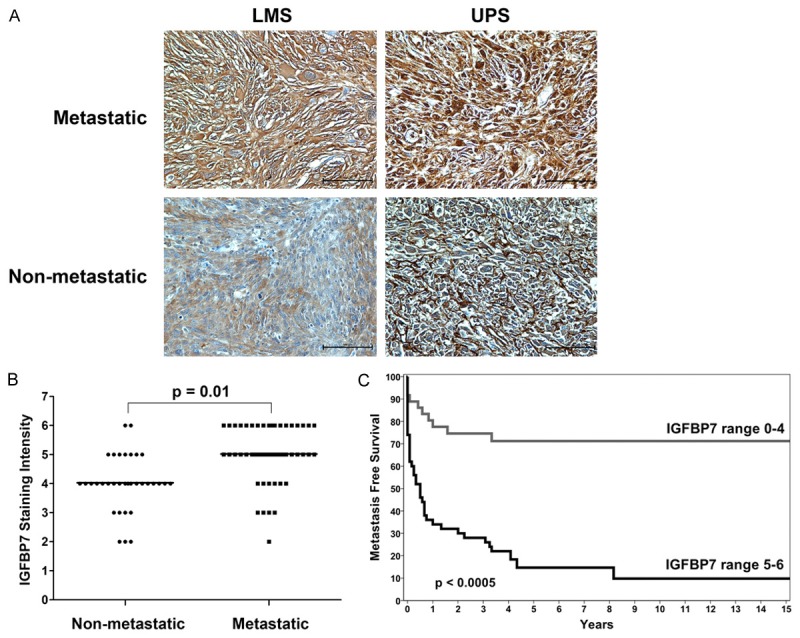
IGFBP7 protein expression. A. Representative immunostaining of IGFBP7 in leiomyosarcoma (LMS) and undifferentiated pleomorphic sarcoma (UPS). A stronger expression is evident in metastatic patients, with predominant cytoplasmatic distribution (IHC 20 ×). B. Mann-Whitney analysis reveals statistically significant higher IGFBP7 staining levels in metastatic compared to non metastatic samples. C. Kaplan-Meier survival curves show the higher probability of the metastatic event in patients with high IGFBP7 expression (score 5-6).
In 76% of disease-free samples staining intensity ranged from negative to moderate in less than 25% of tumor cells with cytoplasmatic and/or nuclear intracellular distribution (Figure 2A). Based on cytoplasmatic IGFBP7 staining intensity score (range 1-6), univariate Cox analysis demonstrated that the risk of metastasis increased of 78.4% (95% CI = 1.32-2.41; P < 0.0005) for each increasing score. Accordingly, Mann-Whitney analysis revealed statistically significant higher IGFBP7 staining levels in metastatic compared to non metastatic samples (P = 0.01) (Figure 2B).
No significantly different expression intensity was seen according to histological subtypes.
When the clinical course of patients with IGFBP7 overexpression (score 5-6) was matched with that of other patients (score ≤ 4), we found that MFS probability was 16% versus 72% respectively and the difference of Kaplan-Meier curves was statistically significant (log-rank = 25.811; P < 0.0005) (Figure 2C). No significant differences were seen for overall survival. IGF1Rβ and IRS1 S612 expression was uniformly distributed across STS samples. IGF1Rβ was moderately to strongly positive in the majority of STS samples with a predominant cytoplasmatic immunostaining in more than 50% of tumor cells (Figure 3A), while IRS1 S612 presented also nuclear immunoreactivity in 35 of 86 (41%) tumor tissues (Figure 3B). No significant differences were observed between metastatic and non metastatic subsets in terms of IGF1Rβ and IRS1 S612 expression. Univariate analysis for MFS and overall survival based on IGF1Rβ and IRS1 S612 expression was not statistically significant.
Figure 3.
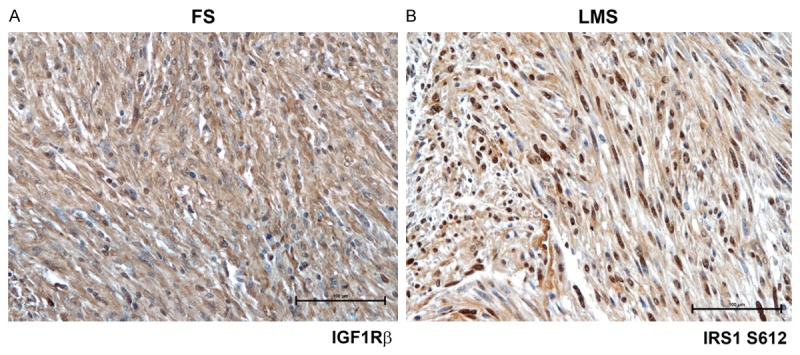
Representative immunostaining of IGF1Rβ and IRS1 S612 in fibrosarcoma (FS) and leiomyosarcoma (LMS). A, B. IGF1Rβ and IRS1 S612 were uniformly distributed with predominant cytoplasmatic and nuclear staining respectively (IHC 20 ×).
Multivariate analysis
Cox regression model including patient variables, IGFBP7 staining intensity (score 1-6), IGFBP7 overexpression (score 5-6), histotype, and treatment, showed that IGFBP7 cytoplasmatic overexpression had the strongest poor predictive value (HR = 6.358, 95% CI = 2.946-13.721; P < 0.0005) while radiotherapy (HR = 0.564, 95% CI = 0.310-1.027; P = 0.061) was protective for metastatic event.
IGFBP7 validation and serum levels
Population study
IGFBP7 expression was validated in another 59 high-grade STS tissue samples with paired serum available (Table 1).
32 out of 59 patients were metastasis-free, 27 developed metastases. Four had metastasis at presentation or within the first 4 months (Table 1).
MFS rate was 80.6% ± 5.3% at 1 year, 77% ± 5.6% at 2 years, 59% ± 6.9% at 5 years and 44.4% ± 8.3% at 10 years (Figure 4A). Average MFS was 103 months (95% CI = 79-126) with a median of 69 months (95% CI = 35-102).
Figure 4.
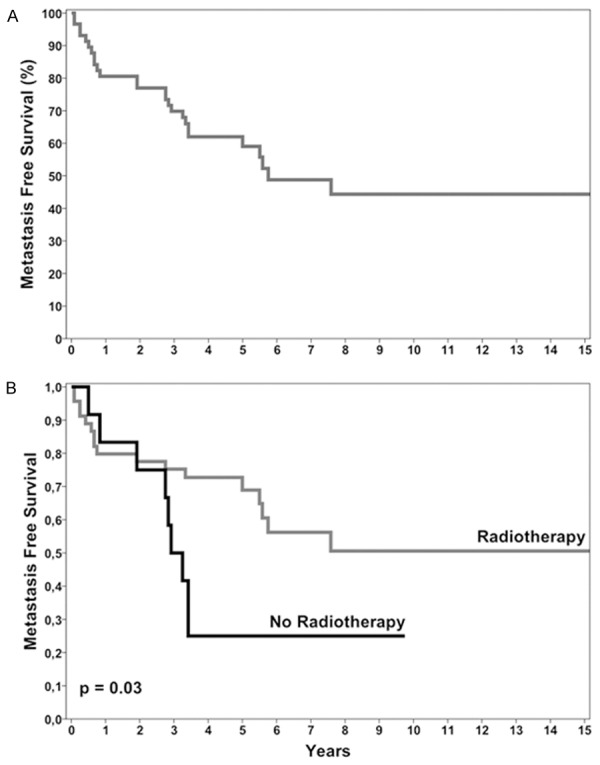
Survival curves. A. Metastasis-free survival of 59 STS patients with available serum. B. Kaplan-Meier survival curves show that radiotherapy treatment is associated with a better prognosis.
Long-term good response to radiotherapy treatment (Figure 4B) was confirmed by Kaplan-Meier analysis (log rank = 4.893; P = 0.03), while age, gender, chemotherapy, and primary tumor site did not significantly affect the risk of metastasis (P ≥ 0.05).
IHC validation and ELISA analysis
IHC analysis performed on additional 59 STS confirmed a higher IGFBP7 expression in metastatic when compared with non metastatic patients (P < 0.01) with an increased risk of metastasis for patients with IGFBP7 overexpression (score 5-6) (HR = 7.032, 95% CI = 3.272-15.115; P < 0.0005).
When the levels of circulating protein were determined by ELISA test, a significant difference was seen between serum IGFBP7 median values in STS (37.5 ng/ml; 14.58-114.44) and control (18.83 ng/ml; 4.69-32.14) (Mann-Whitney test P < 0.0005) (Figure 5A).
Figure 5.
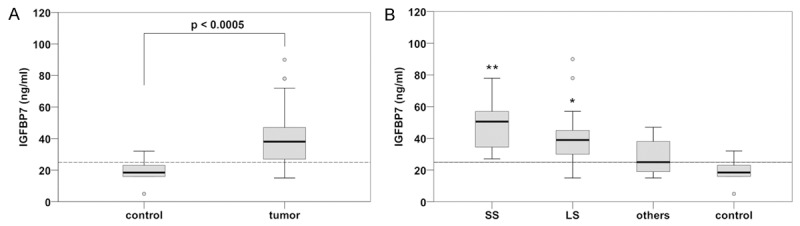
IGFBP7 serum level in STS patients. A. Mann-Whitney test shows that serum levels of STS patients were significantly higher than the control, revealing a threshold level of 25 ng/ml. B. Kruskal-Wallis analysis followed by Dunnett test demonstrated that synovial sarcoma (SS) and liposarcoma (LS) had significantly higher IGFBP7 levels when compared to both the control and the other tumors (**P < 0.0005 and *P = 0.001 respectively).
Examining IGFBP7 distribution across the samples we found that 75% of non-tumor patients had IGFBP7 serum levels ≤ 23 ng/ml, while 75% of STS patients had IGFBP7 serum levels ≥ 27 ng/ml (Figure 5A), thus providing a possible threshold of 25 ng/ml to distinguish tumor from non-tumor patients. The reliability of the test was assessed by a sensitivity and specificity of 79.7% and 80% respectively. Positive and negative predictive value analyses were 95.9% and 40% respectively with Cohen’s kappa coefficient of 0.422.
Then, the sera were differentiated according to primary tumor histotype (Figure 5B). Kruskal-Wallis analysis followed by Dunnett test demonstrated that IGFBP7 protein had significantly higher levels in SS (48.69 ± 15.37 ng/ml) and LS (42.48 ± 22.6 ng/ml) than in other STS including LMS, UPS and FS that together presented a median value of 27.8 ± 10.2 ng/ml (P = 0.001 for SS and P = 0.04 for LS respectively). No significant differences were found between metastatic and non metastatic subgroups.
Discussion
Besides conventional combined treatment high-grade STS require new therapeutic approaches to target aberrant key end-points in signalling pathways. IGF signaling was found frequently altered in sarcomas so to be considered a potential therapeutic target in sarcoma [5,13,14,23,24].
Inhibition of IGF1R led to attenuation of mitogen-activated protein kinase pathways [14,18]. Recently, we demonstrated that nuclear IGF1R expression was significantly related to poor survival in synovial sarcoma patients who did not receive adjuvant chemotherapy, differentiating a subgroup of synovial sarcoma patient’s candidate to the treatment [25].
In this study, IGF1Rβ and its substrate IRS1 phosphorylated at S612 were uniformly expressed across high-grade STS samples with moderate to strong immunostaining and variable intracellular localization independently from patient clinical course.
In contrast, staining intensity and distribution of IGFBP7 protein was significantly higher and more homogeneous in metastatic than in non metastatic tumors. Regression analysis showed that metastatic risk significantly increased by increasing staining intensity score and indicated tissue IGFBP7 overexpression as an independent poor prognostic biomarker, also confirming the role of radiotherapy treatment in preventing metastatic progression [25].
IGFBP7, a cell adhesive glycoprotein of about 30kDa also known as IGFBP7-related Protein 1 (IGFBP-rP1) or tumor-derived adhesion factor/angiomodulin, is a member of the IGFBP superfamily that modulating cell functions through dependent- and independent-IGF mechanism may be a useful prognostic biomarker [19,26].
Both increased and decreased expression of IGFBP7 has been reported in different tumors, suggesting a complex role in tumor cells [27-29]. Recent data recognized IGFBP7 as a tumor antigen in mesenchymal-derived tumors acting in TGFβ signaling pathway [30]. In addition, IGFBP7 interacts in vitro with various extracellular matrix proteins to stimulate adhesion and migration/invasion [31,32].
Although IGFBP7 was also detectable in human body fluids, very few studies examined the relationship between circulating IGFBP levels and tumor stage [21]. Given the evidence that circulating protein may generate from tissue tumor cells, we measured circulating IGFBP7 in high-grade STS sera with an easily quantifiable assay. Significantly higher mean levels were found in STS when compared with controls, providing a threshold value of 25 ng/ml to distinguish tumor from non tumor patients. However, further studies on a larger series of STS are necessary to confirm a potential clinical validity.
Differentiating sera according to primary tumor histotype, IGFBP7 circulating protein had a significantly higher concentration in synovial sarcoma and liposarcoma than in other STS. Unlike STS tissue data, no significant differences between metastatic and non metastatic serum were found.
Conclusion
In accordance with previous studies that found IGFBP7 associated with poor prognosis [33], tissue IGFBP7 expression appears to have a predominant prognostic role in high-grade STS independently from histological subtype. In parallel, the concentration of serum IGFBP7 might be useful in providing a threshold value of 25 ng/ml to discriminate tumor from non tumor patients. Subsequent experiments will be crucial to understand the clinical relevance of IGFBP7 and its role in providing information about the presence and progression of disease.
Acknowledgements
We thank Dr. Elettra Pignotti for statistical analysis, Dr. Alba Balladelli for editing the paper, Ms. Cristina Ghinelli for the graphics, and the Rizzoli Institute surgical pathology technicians. This work was supported by: EU Project “Eurosarc” and 5‰ donation (Italy). Amalia Conti was supported by Post-Doctoral Fellowship 2014 “Fondazione Umberto Veronesi”.
Disclosure of conflict of interest
None.
Abbreviations
- STS
Soft-tissue sarcomas
- IGF
insulin-like growth factor
- IGF1R
IGF-I receptor
- IRS1
insulin receptor substrate 1
- IGFBPs
IGF binding proteins
- UICC
Union for International Cancer Control
- TMA
tissue macroarray
- IHC
immunohistochemistry
- DAB
3,3-diaminobenzidine
- ELISA
Enzyme-linked Immunosorbent Assay
- MFS
metastasis-free survival
- IGFBP-rP1
IGFBP7-related Protein 1
References
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CDM, Unni KK, Mertens F. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2013. WHO classification of tumours of soft tissue and bone. [Google Scholar]
- 3.Cormier JN, Pollock RE. Soft Tissue Sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 4.Coindre JM. Molecular biology of soft-tissue sarcomas. Bull Cancer. 2010;97:1337–1345. doi: 10.1684/bdc.2010.1213. [DOI] [PubMed] [Google Scholar]
- 5.Conti A, Espina V, Chiechi A, Magagnoli G, Novello C, Pazzaglia L, Quattrini I, Picci P, Liotta LA, Benassi MS. Mapping protein signal pathway interaction in sarcoma bone metastasis: linkage between rank, metalloproteinases turnover and growth factor signaling pathways. Clin Exp Metastasis. 2014;31:15–24. doi: 10.1007/s10585-013-9605-6. [DOI] [PubMed] [Google Scholar]
- 6.Chiechi A, Novello C, Magagnoli G, Petricoin EF 3rd, Deng J, Benassi MS, Picci P, Vaisman I, Espina V, Liotta LA. Elevated TNFR1 and serotonin in bone metastasis are correlated with poor survival following bone metastasis diagnosis for both carcinoma and sarcoma primary tumors. Clin Cancer Res. 2013;19:2473–2485. doi: 10.1158/1078-0432.CCR-12-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti A, Rodriguez GC, Chiechi A, Blazquez RM, Barbado V, Krènacs T, Novello C, Pazzaglia L, Quattrini I, Zanella L, Picci P, De Alava E, Benassi MS. Identification of potential biomarkers for giant cell tumor of bone using comparative proteomics analysis. Am J Pathol. 2011;178:88–97. doi: 10.1016/j.ajpath.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2084 localized primary adult soft tissue sarcoma. Ann Surg. 2002;235:424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefannovski PD, Bido E, Paoli AD, Buonadonna A, Boz G, Libra M, Morassut S, Rossi C, Carbone A, Frustaci S. Prognostic factors in soft tissue sarcomas: a study of 395 patients. Eur J Surg Oncol. 2002;28:153–164. doi: 10.1053/ejso.2001.1242. [DOI] [PubMed] [Google Scholar]
- 10.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J. Clin. Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 11.Rikhof B, de JS, Suurmeijer AJ, Meijer C, van der Graaf WT. The insulin-like growth factor system and sarcomas. J Pathol. 2009;217:469–482. doi: 10.1002/path.2499. [DOI] [PubMed] [Google Scholar]
- 12.Reuveni H, Flashner-Abramson E, Steiner L, Makedonski K, Song R, Shir A, Herlyn M, Bar-Eli M, Levitzki A. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013;73:4383–4394. doi: 10.1158/0008-5472.CAN-12-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong H, Fazenbaker C, Breen S, Chen C, Huang J, Morehouse C, Yao Y, Hollingsworth RE. MEDI-573, alone or in combination with mammalian target of rapamycin inhibitors, targets the insulin-like growth factor pathway in sarcomas. Mol Cancer Ther. 2014;13:2662–2673. doi: 10.1158/1535-7163.MCT-14-0144. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Ylipää A, Sun Y, Chen C, Huang J, Morehouse C, Yao Y, Hollingsworth RE. Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin Cancer Res. 2011;17:7563–7573. doi: 10.1158/1078-0432.CCR-11-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baserga R. The contradictions of the insulinlike growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- 16.Lin F, Shen Z, Xu X, Hu BB, Meerani S, Tang LN, Zheng SE, Sun YJ, Min DL, Yao Y. Evaluation of the expression and role of IGF pathway biomarkers in human sarcomas. Int J Immunopathol Pharmacol. 2013;26:169–177. doi: 10.1177/039463201302600116. [DOI] [PubMed] [Google Scholar]
- 17.Friedrichs N, Küchler J, Endl E, Koch A, Czerwitzki J, Wurst P, Metzger D, Schulte JH, Holst MI, Heukamp LC, Larsson O, Tanaka S, Kawai A, Wardelmann E, Buettner R, Pietsch T, Hartmann W. Insulin-like growth factor-1 receptor acts as a growth regulator in synovial sarcoma. J Pathol. 2008;216:428–439. doi: 10.1002/path.2438. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGFII-overexpressing rhabdomyosarcomas cells. Oncogene. 2003;22:8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- 19.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insight. Nat Rev Cancer. 2014;14:329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 20.Ruan W, Xu E, Xu F, Ma Y, Deng H, Huang Q, Lv B, Hu H, Lin J, Cui J, Di M, Dong J, Lai M. IGFBP7 plays a potential tumor suppressor role in colorectal carcinogenesis. Cancer Biol Ther. 2007;6:354–359. doi: 10.4161/cbt.6.3.3702. [DOI] [PubMed] [Google Scholar]
- 21.Sepiashvili L, Hui A, Ignatchenko V, Shi W, Su S, Xu W, Huang SH, O’Sullivan B, Waldron J, Irish JC, Perez-Ordonez B, Liu FF, Kislinger T. Potentially novel candidate biomarkers for head and neck squamous cell carcinoma identified using an integrated cell line-based discovery strategy. MCP. 2012;11:1404–1415. doi: 10.1074/mcp.M112.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rupp C, Scherzer M, Rudisch A, Unger C, Haslinger C, Schweifer N, Artaker M, Nivarthi H, Moriggl R, Hengstschläger M, Kerjaschki D, Sommergruber W, Dolznig H, Garin-Chesa P. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene. 2015;34:815–825. doi: 10.1038/onc.2014.18. [DOI] [PubMed] [Google Scholar]
- 23.Janeway KA, Maki RG. New strategies in sarcoma therapy: linking biology and novel agents. Clin Cancer Res. 2012;18:5837–5844. doi: 10.1158/1078-0432.CCR-12-0875. [DOI] [PubMed] [Google Scholar]
- 24.Kolb EA, Gorlick R. Development of IGF-IR inhibitors in pediatric sarcomas. Curr Oncol Rep. 2009;11:307–313. doi: 10.1007/s11912-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 25.Palmerini E, Benassi MS, Quattrini I, Pazzaglia L, Donati D, Benini S, Gamberi G, Gambarotti M, Picci P, Ferrari S. Prognostic and predictive role of CXCR4, IGF-1R and Ezrin expression in localized synovial sarcoma: is chemotaxis important to tumor? Orphanet J Rare Dis. 2015;10:6. doi: 10.1186/s13023-014-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komiya E, Sato H, Watanabe N, Ise M, Higashi S, Miyagi Y, Miyazaki K. Angiomodulin, a marker of cancer vasculature, is upregulated by vascular endothelial growth factor and increases vascular permeability as a ligand of integrin αvβ3. Cancer Med. 2014;3:537–549. doi: 10.1002/cam4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005;41:1515–1527. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Degeorges A, Wang F, Frierson HF Jr, Seth A, Chung LW, Sikes RA. Human prostate cancer expresses the low affinity insulin-like growth factor binding protein IGFBP-rP1. Cancer Res. 1999;59:2787–2790. [PubMed] [Google Scholar]
- 29.Wang Z, Wang Z, Liang Z, Liu J, Shi W, Bai P, Lin X, Magaye R, Zhao J. Expression and clinical significance of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues from patients with non-small cell lung cancer. Onco Targets Ther. 2013;6:1437–1444. doi: 10.2147/OTT.S51997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato J, Hasegawa S, Akaogi K, Yasumitsu H, Yamada S, Sugahara K, Miyazaki K. Identification of cell-binding site of angiomodulin (AGM/TAF/Mac25) that interacts with heparan sulfates on cell surface. J Cell Biochem. 1999;75:187–195. [PubMed] [Google Scholar]
- 31.Kishibe J, Yamada S, Okada Y, Sato J, Ito A, Miyazaki K, Sugahara K. Structural requirements of heparan sulfate for the binding to the tumor-derived adhesion factor/angiomodulin that induces cord-like structures to ECV-304 human carcinoma cells. J Biol Chem. 2000;275:15321–15329. doi: 10.1074/jbc.275.20.15321. [DOI] [PubMed] [Google Scholar]
- 32.Pen A, Moreno MJ, Durocher Y, Deb-Rinker P, Stanimirovic DB. Glioblastoma secreted factors induce IGFBP-7 and angiogenesis by modulating Smad-2-dependent TGF-b signaling. Oncogene. 2008;27:6834–6844. doi: 10.1038/onc.2008.287. [DOI] [PubMed] [Google Scholar]
- 33.Adachi Y, Itoh F, Yamamoto H, Arimura Y, Kikkawa-Okabe Y, Miyazaki K, Carbone DP, Imai K. Expression of angiomodulin (tumor-derived adhesion factor/mac25) in invading tumor cells correlates with poor prognosis in human colorectal cancer. Int J Cancer. 2001;95:216–222. doi: 10.1002/1097-0215(20010720)95:4<216::aid-ijc1037>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]


