Abstract
This study is to investigate the effects of human umbilical cord-mesenchymal stem cells (HUC-MSCs) transplantation combined with minimally invasive hematoma aspiration on neural functional recovery and p53 gene expression in rats with intracerebral hemorrhage (ICH). Collagenase type-IV was injected to the caudate nucleus of the rats to make ICH models. One hundred and twenty Sprague-Dawley rats with successful modeling were randomly divided into 4 groups, including the ICH group, hematoma aspiration group, HUC-MSCs transplantation group and HUC-MSCs transplantation combined with hematoma aspiration group (combination group). Neural functional status of the rats was assessed by modified neurological severity score (mNSS). Expression of p53 in the cerebral tissues surrounding ICH was detected by immunohistochemical assays. The scores of mNSS and the expression of p53 gene in the hematoma aspiration group, the HUC-MSCs transplantation group and the combination group were significantly lower than those in the ICH group at each indicated time point (p < 0.05). Intriguingly, mNSS scores and p53 expression in the combination group were significantly lower than those in the hematoma aspiration group on day 7, 14 and 30 (p < 0.05), and significantly lower than those in the HUC-MSCs transplantation group on day 14 and 30 (p < 0.05). HUC-MSCs transplantation combined with minimally invasive hematoma aspiration is more effective than either therapy alone in rats with ICH and could distinctly reduce the damage of nerve cells.
Keywords: Human umbilical cord-mesenchymal stem cell transplantation, minimally invasive hematoma aspiration, intracerebral hemorrhage, rats, p53
Introduction
Intracerebral hemorrhage (ICH) is a common vascular disease of the nervous system and accounts for 10% of all cases of cerebrovascular disease with the annual incidence rate of 60~80 per 100,000. Twenty to thirty percent of the strokes are caused by ICH in China and 30%~40% patients die in the acute phase of ICH. Secondary lesions could be the main cause of brain injury after ICH [1]. The hematoma after ICH engenders space-occupying effect and direct damage to surrounding tissues. Other lesions include hypoperfusion surrounding the hematoma [2,3], secondary inflammatory reaction [4,5], a chain reaction caused by the generation of prothrombin coagulation, toxicity effect induced by dissolving red blood cells [6,7], cerebral edema [7]. Reactive Oxygen Species (ROS) reaction [8], the complementary cascade and neuron apoptosis.
Reducing the intracranial pressure by dehydrating agents is widely used in the treatment for ICH. However, the prognosis of the patients is poor. Many patients suffer from sequelae of ICH such as neurological dysfunction. Therefore, it is important to investigate effective treatment for ICH. Nowadays, minimally invasive hematoma aspiration has aroused much attention. It has been reported that the optimal time window for minimally invasive hematoma aspiration is within 6 hr after ICH [9]. Aspiration treatment at the early stage of ICH could reduce the expansion of the hematoma, the space-occupying effects, the production of inflammatory mediators and the direct chemical damage to the brain tissue, thus preventing the secondary lesions of ICH [10]. However, neurons are non-renewable and minimally invasive hematoma aspiration alone could not effectively promote the recovery of neurological functions.
In recent years, stem cell therapy has become a hot topic [11]. Neural stem cells are stem cells in the nervous system. They can self- renew and give rise to offspring cells to generate lineages of neurons glia, and oligodendrocytes. However, allogeneic transplantation of neural stem cells could cause host rejection. Human umbilical cord mesenchymal stem cells (HUC-MSCs) have multiple differentiation potentials and can differentiate into nerve cells, adipocytes, liver cells, cardiac cells, etc. HUC-MSCs are derived from the umbilical cord tissue. They are easily obtained, available in abundant supply and do not involve ethical and moral issues. The therapeutic benefits of HUC-MSCs transplantation for ischemic stroke in rats have been reported, which was likely due to the ability of the cells to produce growth-promoting factors [12]. HUC-MSC transplantation could accelerate functional neurological recovery of rats after stroke, which may be mediated by their ability to promote angiogenesis [13]. HUC-MSC treatment is capable of alleviating the motor impairments, cerebellar atrophy and decreasing the number of apoptotic cells in the ataxic mouse model, probably via promoting particular neurotrophic factors [14]. HUC-MSCs have the potential for treatment of Parkinson’s disease [15]. Transplantation of HUC-MSCs is also beneficial to wound healing after spinal cord injury in rats [16].
There is no report on the effects of combined treatment of HUC-MSCs transplantation and minimally invasive hematoma aspiration on the therapy of ICH. In this study, the effects of HUC-MSCs transplantation, minimally invasive hematoma aspiration and HUC-MSCs transplantation combined with minimally invasive hematoma aspiration on neural functional recovery and p53 expression in rats with ICH were investigated.
Materials and methods
Reagents
HUC-MSCs basal medium, fetal bovine serum, penicillin, streptomycin and glutamine were purchased from Cyagen Biotech Co., Ltd. (Guangzhou, China). Rabbit anti-p53 polyclonal antibody and rabbit anti-human CD29, CD34, CD44, CD90 as well as CD105 polyclonal antibodies were purchased from Wuhan Boster Biological Engineering Co., Ltd. (Wuhan, China). The Streptavidin-Peroxidase (SP) kit, DAB color kit and mouse anti-BrdU monoclonal antibody were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Intracerebral hemorrhage
The study was approved by the Shandong University Institutional Animal Care and Use Committee. One hundred and forty-three healthy male Sprague-Dawley (SD) rats weighing 250 to 280 g were provided by Laboratory Animal Center of Shandong University of Traditional Chinese Medicine and kept under clean conditions. For induction of hemorrhage, each rat was anesthetized with pentobarbital (0.3%, intraperitoneal injection) and placed in a stereotactic frame (Rui Wode Life Science and Technology Co., Ltd, Shenzhen, China). Collagenase-induced ICH in rats was produced according to the reports with minor modification [17]. Through a hole drilled in the skull, a microinjector (Rui Wode Life Science and Technology Co., Ltd, Shenzhen, China) was introduced into the caudate nucleus (0.1 mm anterior to the anterior fontanelle, 3.0 mm lateral right to the sagittal suture, depth 5.5 mm below the surface of the skull), and 2 μl of saline containing 0.4 IU collagenase type- IV (Soledad Biological Technology Co., Ltd, Beijing, China) was infused over 5 minutes. After the infusion, the needle was left in the place for 10 minutes and then removed. The bone hole was sealed with bone wax, the scalp wound was sutured.
Six hours after the surgery, the Bederson score was used to monitor the neurologic functions of the rats: score of 0, no observable neurologic deficit; score of 1, rats with forelimb flexion when their tails were raised; score of 2, decreased resistance to lateral push (and forelimb flexion) without circling; score of 3, rats showed the same behavior as in score of 2, with circling. Sixteen rats died after the surgery. Seven rats with a Bederson score of < 2 were excluded.
One hundred and twenty rats with a Bederson score of ≥ 2 were equally randomized into 4 groups, ICH group without treatment, hematoma aspiration group with minimally invasive hematoma aspiration, HUC-MSCs transplantation group and HUC-MSCs transplantation combined with hematoma aspiration group (combination group). In each group, 6 rats were sacrificed after assessed by modified neurological severity score (mNSS) on day 1, 3, 7, 14 and 30 after ICH respectively.
Minimally invasive hematoma aspiration
Six hours after ICH, the rats were anesthetized again and placed in the stereotactic frame. With the same stereotactic coordinates, urokinase (5 μl, 200 IU/μl, Livzon Pharmaceutical Factory, Zhuhai, China) was injected by microinjector into the hematoma over 5 minutes. The microinjector was left in the place for 30 minutes and then removed. Then aspiration was performed slowly using our homemade device. The bone hole was sealed with bone wax, the scalp wound was sutured, and the rats were placed back in the feeding room.
mNSS test
The mNSS was used to grade neurologic deficits including motor, ground walking, sensory, coordination of movement, reflex, abnormal movements, etc. Neurological function was graded on a scale of 0 to 18 (normal score, 0; maximal deficit score, 18). The testing was performed on day 1, 3, 7, 14 and 30 after ICH by the observer who did not know the treatment of each group. Each rat was tested twice and the average score was calculated.
Preparation of HUC-MSCs
Human umbilical cords were collected from the healthy puerperas with full-term pregnancy and cesarean section in the department of obstetrics of Shandong Provincial Hospital with written informed consent from these puerperas. Umbilical artery, vein and the surrounding tissues were stripped from the human umbilical cords. The umbilical cords were shredded and cultured in HUC-MSCs complete medium with 10% fetal bovine serum, 1% penicillin/streptomycin and 1% glutamine in an atmosphere of 95% air/5% CO2 at 37°C for 4~5 days. Then the media was removed completely and replaced with fresh media and the non-adherent cells were removed. When HUC-MSCs reached 80% confluency, the cells were digested with 0.25% trypsin (Hyclone, USA) and passaged [18]. Forty-eight to seventy-two hours before transplantation, the HUC-MSCs were labeled with 10 μM BrdU (Sigma, USA) [19].
Transplantation of HUC-MSCs
Six hours after ICH, the rats were anesthetized and placed in the stereotactic frame. Under sterile conditions, 2 μl of HUC-MSCs suspension (1 × 105/rat) was injected by microinjector into the side of the brain damage at the speed of 1 μl/min. The microinjector was left in the place for 30 minutes and then removed. The bone hole was sealed with bone wax, the scalp wound was sutured, and the rats were placed back in the feeding room.
Cardiac perfusion fixation
The rats were anesthetized. Normal saline and 4% formaldehyde solution were administered to perform cardiac perfusion fixation. Then the rats were decapitated. The tissues around the hematoma with the size of 2 mm × 2 mm × 2 mm were removed and fixed in 4% paraformaldehyde solution for at least 24 hours followed by dehydration with gradient ethanol and embedding with paraffin. The sections of the brain tissues were obtained for immunohistochemical assays.
Immunocytochemistry/immunohistochemistry
The expression of CD29, CD34, CD44, CD90 and CD105 in HUC-MSCs were detected using immunocytochemistry. The cells were seeded on the slides placed in the 6-well plates 2~3 days before the detection. The samples were fixed with 4% paraformaldehyde solution for 15 minutes and incubated with 0.5% TritonX-100 for 20 minutes, then 3% H2O2 for 15 minutes. After blocking with goat serum for 20 minutes, the slides were incubated with rabbit anti-human CD29, CD34, CD44, CD90 and CD105 polyclonal antibodies at 4°C overnight. Secondary antibodies were added and incubated at 37°C for 30 minutes. Then the slides were developed with DAB chromogenic reagent for 10 minutes. Finally, sections were counterstained with haematoxylin for 10 minutes, mounted with neutral gum and observed under microscopy (CSW-DZ01 Christie Granville Optical Instrument Co., Ltd, Shenzhen, China).
Expression of p53 in the brain tissues of the rats was detected using immunohistochemistry. The sections were dewaxed, rehydrated in graded alcohols and processed to antigen retrieval. After incubated with 3% H2O2 at 37°C for 15 minutes and blocked with goat serum, the sections were incubated with rabbit anti-p53 polyclonal antibody (1:50) at 4°C overnight, biotinylated secondary antibody at 37°C for 30 minutes and avidin-peroxidase at 37°C for 30 minutes. The sections were developed with DAB chromogenic reagent for about 90 seconds. Finally, sections were counterstained with haematoxylin for 2 minutes. After 1% hydrochloric acid differentiation and dehydration, sections were mounted with neutral gum and observed under microscopy. For each section, five fields were randomly taken under a magnification of 400 ×. The positive cells and negative cells were counted in each field. The positive rate of each field was the percentage of the ratio of the number of positive cells to the number of total cells (the sum of positive and negative cells). The positive rate of each tissue section was expressed as the mean of the positive rate of the five fields.
Expression levels of BrdU in HUC-MSCs in the brain sections of the rats were detected using immunohistochemistry. The sections were dewaxed, incubated with 3% H2O2 for 15 minutes, penetrated with 0.1% TritonX-100/0.1% sodium citrate at 37°C for 3 hours and digested with 0.125% trypsin at room temperature for 10 minutes. Then the sections were treated with 0.1 M hydrochloric acid at 4°C for 10 minutes, 2 M hydrochloric acid at 37°C for 30 minutes and 0.1 M Na2B4O7 at room temperature for 10 minutes. After that, the sections were incubated with mouse anti-BrdU monoclonal antibody (1:50) at 37°C for 2 hours and biotinylated secondary antibody at 37°C for 30 minutes. The sections were developed with DAB chromogenic reagent and counterstained with haematoxylin. After dehydration and dimethylbenzene transparency, the sections were mounted with neutral gum.
Statistical analysis
All data were processed using SPSS17.0 statistical package. The data were presented as means ± standard deviation. The one-way ANOVA was performed to determine statistical significance of the differences. LSD method was further used to determine the pairwise differences. p-values of less than 0.05 were considered statistically significant.
Results
Successful modeling of ICH in rats
In this study, collagenase-induced ICH rats were produced and the Bederson score was used to monitor the neurologic functions of the rats. Sixteen rats died after the surgery. Seven rats with a Bederson score of < 2 were excluded. One hundred and twenty rats with a Bederson score of ≥ 2 were enrolled in the following experiments.
ICH usually occurs at the caudate nucleus which is the largest gray matter nuclei in the brain. Furthermore, the location and observation of caudate nucleus are convenient. We injected collagenase type- IV into the caudate nucleus to make ICH models. As shown in Figure 1, the brain samples of the rat in the ICH group, hematoma aspiration group, HUC-MSCs transplantation group and combination group subjected to cardiac perfusion fixation 14 days after ICH confirmed that the caudate nucleus was indeed the site of ICH.
Figure 1.
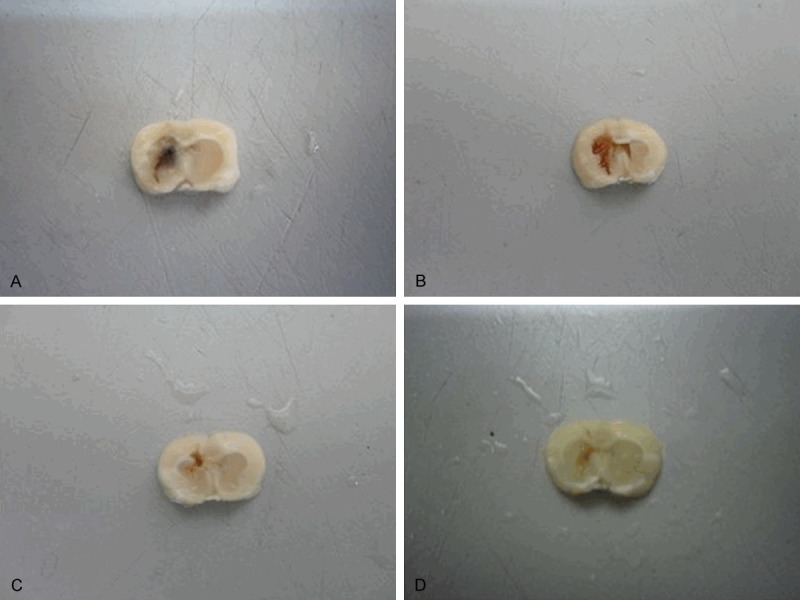
Brain tissue specimens of the rat subjected to cardiac perfusion fixation. Collagenase type- IV was injected to the caudate nucleus of the rats to make ICH models. The representative pictures of the brain samples of the rats in the in the ICH group (A), hematoma aspiration group (B), HUC-MSCs transplantation group (C) and combination group (D) subjected to cardiac perfusion fixation 14 days after ICH were shown. These results confirmed that the caudate nucleus was indeed the site of ICH. ICH, intracerebral hemorrhage, HUC-MSCs, human umbilical cord-mesenchymal stem cells.
Characterization of HUC-MSCs
The cultured HUC-MSCs showed a fibroblast-like morphology. To further confirm the mesenchymal stem cell characteristics of these cells, we detected the expression of the cell markers of HUC-MSCs using immunocytochemistry. As shown in Figure 2, the cultured cells expressed CD29 (with brown staining in the cytoplasm), CD44 (with brown staining on the cell membrane), CD90 (with brown staining in the cytoplasm) CD105 (with brown staining in the cytoplasm), but not CD34 (with blue staining in the cytoplasm). These results indicate that these cells have the characteristics of mesenchymal stem cells, and not the characteristics of haematopoietic cells. The cultured cells are HUC-MSCs.
Figure 2.
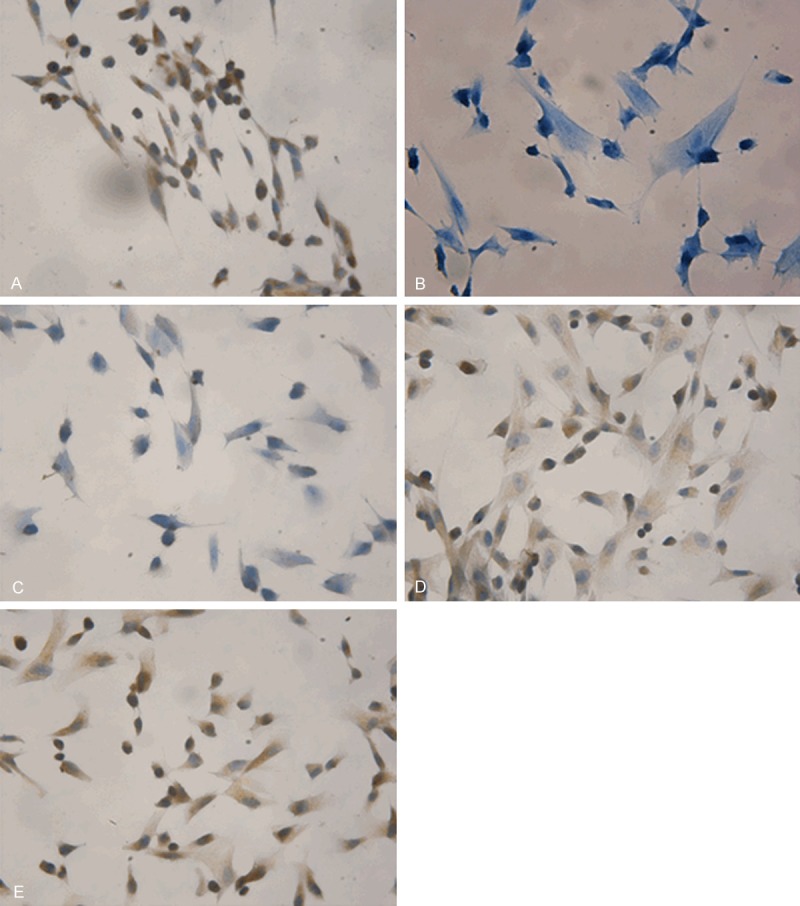
Cell markers expression in HUC-MSCs using immunocytochemistry (magnification × 400). HUC-MSCs were seeded on the slides placed in the 6-well plates 2~3 days before the detection. The expression of CD29, CD34, CD44, CD90 and CD105 in HUC-MSCs were detected using immunocytochemistry. Positive cells were stained brown. Representative immunocytochemical staining results were shown. The cells expressed CD29 (A), CD44 (C), CD90 (D), CD105 (E), but not CD34 (B). HUC-MSCs, human umbilical cord-mesenchymal stem cells.
Distribution of HUC-MSCs in the brain
In the HUC-MSCs transplantation group and the combination group, HUC-MSCs labeled with BrdU were transplanted into the side of the brain damage. To investigate the distribution of HUC-MSCs in the brain, BrdU expression was detected using immunohistochemistry. As expected, there was no BrdU expression in the brain tissues of the rats in the ICH group and hematoma aspiration group which did not receive HUC-MSCs transplantation (data not shown). BrdU expression could be detected in the brain tissues of the rats in the HUC-MSCs transplantation group and the combination group on day 1, 3, 7, 14 and 30 after ICH. The HUC-MSCs with BrdU staining mainly located at the site with hemorrhage, hippocampus, cerebral cortex, etc (Figure 3). It seems that after transplantation, the HUC-MSCs could migrate to the site of hemorrhage.
Figure 3.

BrdU expression in the brain of the rats injected with HUC-MSCs using immunohistochemistry (magnification × 400). HUC-MSCs were labeled with BrdU to trace the distribution of these cells. BrdU expression was detected using immunohistochemistry. Positive cells were stained brown. Representative immunohistochemical staining results were shown. BrdU expressions could be detected in the brain of the rats in the HUC-MSCs transplantation group (A) and the HUC-MSCs transplantation combined with hematoma aspiration group (B). HUC-MSCs, human umbilical cord-mesenchymal stem cells.
Lower mNSS scores in the groups with treatment
Then the effects of minimally invasive hematoma aspiration, HUC-MSCs transplantation and HUC-MSCs transplantation combined with minimally invasive hematoma aspiration on the neural functional recovery in rats with ICH were investigated by mNSS tests. As shown in Table 1, the scores of mNSS in the hematoma aspiration group, the HUC-MSCs transplantation group and the combination group were significantly lower than those in the ICH group at each indicated time point (p < 0.05). The scores of mNSS in the combination group were significantly lower than those in the hematoma aspiration group on day 7, 14 and 30 (p < 0.05), and significantly lower than those in the HUC-MSCs transplantation group on day 14 and 30 (p < 0.05). The scores of mNSS in the HUC-MSCs transplantation group were significantly lower than those in the hematoma aspiration group on day 7 and 14 (p < 0.05), while not on day 30. These results suggest that minimally invasive hematoma aspiration, HUC-MSCs transplantation and HUC-MSCs transplantation combined with minimally invasive hematoma aspiration all could promote the neural functional recovery in rats with ICH. And HUC-MSCs transplantation combined with minimally invasive hematoma aspiration may be more effective than either therapy alone.
Table 1.
mNSS testing in rats with ICH
| ICH | Hematoma aspiration | HUC-MSCs transplantation | Combination | |
|---|---|---|---|---|
| 1 d | 10.00 ± 1.26 | 7.50 ± 0.55* | 8.00 ± 1.01* | 8.17 ± 1.28* |
| 3 d | 10.83 ± 0.40 | 8.33 ± 1.50* | 8.67 ± 0.82* | 8.83 ± 1.17* |
| 7 d | 8.17 ± 0.75 | 7.33 ± 0.82*,# | 5.83 ± 0.75*,Δ | 5.33 ± 0.82*,Δ |
| 14 d | 4.67 ± 0.82Δ,# | 3.67 ± 0.52*,# | 3.00 ± 0.00*,Δ | 2.33 ± 0.52*,Δ,# |
| 30 d | 3.50 ± 0.55Δ,# | 2.83 ± 0.41* | 2.50 ± 0.55* | 1.83 ± 0.41*,Δ,# |
Note: ICH, intracerebral hemorrhage group without treatment; HUC-MSCs, human umbilical cordmesenchymal stem cells; Combination, HUC-MSCs transplantation combined with hematomaaspiration group. The data represents means ± SD. One-way ANOVA and LSD;
p < 0.05 vs. ICH group;
p < 0.05 vs. hematoma aspiration group;
p < 0.05 vs. HUC-MSCs transplantation group.
Reduced p53 expression in the groups with treatment
The effects of minimally invasive hematoma aspiration, HUC-MSCs transplantation and HUC-MSCs transplantation combined with minimally invasive hematoma aspiration on the expression of p53 around hematoma were studied using immunohistochemistry. As shown in Figure 4, p53 expression could be detected in the cell nucleus of the tissues at the side of the brain damage. The cells with p53 staining mainly located at the striatal region around hematoma, cerebral cortex, subcortical region, hippocampus, etc. The positive rates of p53 expression were shown in Table 2. p53 expression in the hematoma aspiration group, the HUC-MSCs transplantation group and the combination group were significantly lower than those in the ICH group at each indicated time point (p < 0.05). p53 expression in the combination group was significantly lower than that in the hematoma aspiration group on day 7, 14 and 30 (p < 0.05), and significantly lower than those in the HUC-MSCs transplantation group on day 14 and 30 (p < 0.05). The expressions of p53 in the HUC-MSCs transplantation group were significantly lower than those in the hematoma aspiration group on day 7 and 14 (p < 0.05), while not on day 30. These results were consistent with what we observed in mNSS tests. It seems that minimally invasive hematoma aspiration, HUC-MSCs transplantation and HUC-MSCs transplantation combined with minimally invasive hematoma aspiration all could significantly reduce p53 expression around hematoma. And HUC-MSCs transplantation combined with minimally invasive hematoma aspiration may be more effective than either therapy alone.
Figure 4.

p53 expression around hematoma using immunohistochemistry (magnification × 400). Expression of p53 in the brain tissues of the rats were detected using immunohistochemistry. Positive cells were stained brown. Representative immunohistochemical staining results of p53 expression in the brain tissues of the rats in the ICH group without treatment (A), hematoma aspiration group (B), HUC-MSCs transplantation group (C) and HUC-MSCs transplantation combined with hematoma aspiration group (D) 3 days after ICH were shown. HUC-MSCs, human umbilical cord-mesenchymal stem cells; ICH, intracerebral hemorrhage.
Table 2.
The positive rates of p53 expression in brain tissue
| ICH | Hematoma aspiration | HUC-MSCs transplantation | Combination | |
|---|---|---|---|---|
| 1 d | 0.78 ± 0.08 | 0.67 ± 0.02* | 0.67 ± 0.03* | 0.66 ± 0.07* |
| 3 d | 0.92 ± 0.06 | 0.86 ± 0.02* | 0.85 ± 0.01* | 0.84 ± 0.01* |
| 7 d | 0.57 ± 0.05 | 0.47 ± 0.03*,# | 0.41 ± 0.01*,Δ | 0.39 ± 0.08*,Δ |
| 14 d | 0.47 ± 0.22Δ,# | 0.38 ± 0.02*,# | 0.35 ± 0.02*,Δ | 0.31 ± 0.03*,Δ,# |
| 30 d | 0.41 ± 0.02Δ,# | 0.31 ± 0.01* | 0.30 ± 0.02* | 0.22 ± 0.03*,Δ,# |
Note: ICH, intracerebral hemorrhage group without treatment; HUC-MSCs, human umbilical cord mesenchymal stem cells; Combination, HUC-MSCs transplantation combined with hematoma aspiration group. The data represents means ± SD. One-way ANOVA and LSD;
p < 0.05 vs. ICH group;
p < 0.05 vs. hematoma aspiration group;
p < 0.05 vs. HUC-MSCs transplantation group.
Discussion
ICH is a common vascular disease with high incidence rates and high mortality rates in the acute phase. In this study, we produced collagenase-induced ICH in rats and investigated the effects of HUC-MSCs transplantation, minimally invasive hematoma aspiration and HUC-MSCs transplantation combined with minimally invasive hematoma aspiration on neural functional recovery and p53 expression.
Rats are inexpensive to house and can be easily handled and observed. They have similar anatomical structures to humans in the brain [20] and are most commonly used in the research of ICH. Thus in this study, we produced ICH models using SD rats. ICH could be induced via infusion of autologous whole blood [21], mechanical damage [22], using the stroke-prone strains and injection of collagenase [17]. Collagenase can digest extracellular matrix (ECM) components and the collagen of vascular basement membrane, which allows the blood to penetrate into the surrounding tissues. This process is different from the spontaneous ICH in patients, and with more severe secondary inflammatory responses. However, collagenase-induced ICH is reproducible and easy for operation, with similar pathophysiological and biochemical processes to human ICH. Thus in this study, we produced collagenase-induced ICH in rats.
It has been reported that aspiration of the hematoma after collagenase-induced hemorrhage improved acute functional outcome and reduced neuronal loss from the striatum [23]. Minimally invasive hematoma aspiration could release hematoma-induced space-occupying effects and chemical damage to surrounding tissues and has been used in clinical treatment of ICH [10,24]. In this study, minimally invasive hematoma aspiration could promote the neural functional recovery in rats with ICH, which was consistent with the reports mentioned above.
Nowadays, HUC-MSCs transplantation for treatment of nervous system diseases has aroused much attention. HUC-MSCs could be injected into the brain using the stereotactic frame or injected intravascularly [25,26]. In this study, we directly injected the HUC-MSCs into the brain surrounding the damage. This process could cause lesions in the brain, while it ensured the biological effects of transplanted HUC-MSCs.
HUC-MSCs expressed CD10, CD13, CD29, CD44, CD73, CD90, CD105, CD166, etc, but not CD19 (B cell antigen), CD31 (endothelial cell-specific antigen), CD34 (hematopoietic stem cell antigen), CD45 (leukocyte common antigen), or HLA-DR (MHC II) [27,28]. Consistent with this, our cultured HUC-MSCs had the characteristics of mesenchymal stem cells with the expression of CD29, CD44, CD90, CD105, but not CD34. We labeled HUC-MSCs with BrdU to trace the distribution of these cells. In the HUC-MSCs transplantation group and the combination group, the HUC-MSCs with BrdU staining mainly located at the site with hemorrhage, cerebral cortex, etc. It seems that after transplantation, the HUC-MSCs could migrate to the site of hemorrhage [29]. A possible explanation is that the lesions at the site with hemorrhage could stimulate the production of chemokines for migration of the HUC-MSCs.
In this study, the scores of mNSS in the HUC-MSCs transplantation group was significantly lower than that in the ICH group at each indicated time point (p < 0.05). Furthermore, the scores of mNSS in the HUC-MSCs transplantation group were significantly lower than those in the hematoma aspiration group on day 7 and 14 (p < 0.05), while not on day 30. It seems that the short-term effects of HUC-MSCs transplantation are better than the effects of hematoma aspiration. This might be because HUC-MSCs could replace the necrotic neurons which are not reproducible and produce growth factors. However, there was no significant difference in the long-term effects between HUC-MSCs transplantation and hematoma aspiration which accorded with the reports on other nervous system diseases [13-16]. The possible mechanisms of the effects of HUC-MSCs transplantation on ICH are as the follows, 1) HUC-MSCs could differentiate into neurons [30-32], 2) HUC-MSCs could secret neurotrophic factors [33], and 3) HUC-MSCs transplantation could reduce the inflammatory responses caused by ICH [34].
HUC-MSCs transplantation combined with minimally invasive hematoma aspiration for ICH treatment has not been reported. In this study, we performed HUC-MSCs transplantation shortly after minimally invasive hematoma aspiration to avoid more severe injuries induced by transplantation at other time points. Hematoma aspiration could reduce the damage of brain tissues and improve the microenvironment for transplanted HUC-MSCs. There was no significant difference in the efficacy between combination treatment and either treatment on day 1 and 3 after ICH. This might be because transplantation combined with aspiration could lead to more severe extra damage in the brain tissues than either therapy at the early stage after the treatment. However, the scores of mNSS in the combination group were significantly lower than those in either group on day 14 and 30 which indicated that HUC-MSCs transplantation combined with minimally invasive hematoma aspiration may be more beneficial to the recovery of neural functions than either therapy alone.
p53 is an important protein with multiple functions and could promote apoptosis [35-37]. Increased levels of p53 and p53-induced apoptosis could be observed in many nervous system diseases such as Alzheimer’s disease [38-40], ischemic stroke [41,42], Parkinson’s disease [43,44] and Huntington’s disease [45,46]. In this study, p53 expression was detected at 1 day after ICH. And its expression peaked on day 3 then decreased on day 7 after ICH. This was consistent with the time course of apoptosis induction. It seems that there is a correlation between p53 expression and apoptosis induction in the rats with ICH [47]. In this study, the results indicate that HUC-MSCs transplantation combined with minimally invasive hematoma aspiration could significantly reduce p53 expression.
In summary, HUC-MSCs transplantation combined with minimally invasive hematoma aspiration might be more effective than either therapy in rats with ICH and could distinctly reduce the damage of nerve cells and be more beneficial to the recovery of neural function. These results provide valuable information on the investigation of new therapy methods for ICH.
Acknowledgements
This work was supported by The Shandong Provincial Medical and Health Science and Technological Program of China (No. 2013WS0123) and The Development of Science and Technology Plan Projects of Yantai City (Grant N0. 2012077).
Disclosure of conflict of interest
None.
References
- 1.Chen-Roetling J, Lu X, Regan RF. Targeting heme oxygenase after intracerebral hemorrhage. Ther Targets Neurol Dis. 2015;2:474. doi: 10.14800/ttnd.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer SA, Lignelli A, Fink ME, Kessler DB, Thomas CE, Swarup R, Van Heertum RL. Perilesional blood flow and edema formation in acute intracerebral hemorrhage a SPECT study. Stroke. 1998;29:1791–1798. doi: 10.1161/01.str.29.9.1791. [DOI] [PubMed] [Google Scholar]
- 3.Kang DW, Han MK, Kim HJ, Yun SC, Jeon SB, Bae HJ, Kwon SU, Kim JS. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012;79:848–855. doi: 10.1212/WNL.0b013e3182648a79. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015;6:4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 5.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of Aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 6.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 7.Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of Edema Formation After Intracerebral Hemorrhage Effects of Extravasated Red Blood Cells on Blood Flow and Blood-Brain Barrier Integrity. Stroke. 2001;32:2932–2938. doi: 10.1161/hs1201.099820. [DOI] [PubMed] [Google Scholar]
- 8.Wu RD, Dong YX, Liu ZH. Mechanism Study of Secondary Nerve injury of Intracerebral Hemorrhage. Medical Recapitulate. 2007;17:1801–1802. [Google Scholar]
- 9.Liu LJ, Xue ZC, Yang GQ, Zhang XM. Optimal time window for minimally invasive aspiration and drainage of the hematoma in patients with intracerebral hemorrhage. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:544–546. [PubMed] [Google Scholar]
- 10.Sang YH, Liang YX, Liu LG, Ellis-Behnke RG, Wu WT, So KF, Cheung RT. Rat Model of intracerebral hemorrhage permitting hematoma aspiration plus intralesional injection. Exp Anim. 2013;62:63–69. doi: 10.1538/expanim.62.63. [DOI] [PubMed] [Google Scholar]
- 11.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42:2045–2053. doi: 10.1161/STROKEAHA.110.603621. [DOI] [PubMed] [Google Scholar]
- 13.Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B, Xu J, Liu P, Yang S, Wang J, Han Z, Han ZC. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009;87:350–359. doi: 10.1097/TP.0b013e318195742e. [DOI] [PubMed] [Google Scholar]
- 14.Zhang MJ, Sun JJ, Qian L, Liu Z, Zhang Z, Cao W, Li W, Xu Y. Human umbilical mesenchymal stem cells enhance the expression of neurotrophic factors and protect ataxic mice. Brain Res. 2011;1402:122–31. doi: 10.1016/j.brainres.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 15.Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 16.Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 18.Can A, Balci D. Isolation, culture, and characterization of human umbilical cord stroma-derived mesenchymal stem cells. Methods Mol Biol. 2011;698:51–62. doi: 10.1007/978-1-60761-999-4_5. [DOI] [PubMed] [Google Scholar]
- 19.Dolbeare F, Gratzner H, Pallavicini MG, Gray JW. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci. 1983;80:5573–5577. doi: 10.1073/pnas.80.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- 21.Kallmünzer B, Tauchi M, Schlachetzki JC, Machold K, Schmidt A, Winkler J, Schwab S, Kollmar R. Granulocyte colony-stimulating factor does not promote neurogenesis after experimental intracerebral haemorrhage. Int J Stroke. 2014;9:783–8. doi: 10.1111/ijs.12217. [DOI] [PubMed] [Google Scholar]
- 22.Belur PK, Chang JJ, He S, Emanuel BA, Mack WJ. Emerging experimental therapies for intracerebral hemorrhage: targeting mechanisms of secondary brain injury. Neurosurgical Focus. 2013;34:E9. doi: 10.3171/2013.2.FOCUS1317. [DOI] [PubMed] [Google Scholar]
- 23.Altumbabic M, Peeling J, Del Bigio MR. Intracerebral hemorrhage in the rat: effects of hematoma aspiration. Stroke. 1998;29:1917–1923. doi: 10.1161/01.str.29.9.1917. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, Dong Q, Guo J, Li L, Guo J, Xie P. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. 2012;43:2923–2930. doi: 10.1161/STROKEAHA.112.667535. [DOI] [PubMed] [Google Scholar]
- 25.Pountos I, Giannoudis PV, Jones E, English A, Churchman S, Field S, Ponchel F, Bird H, Emery P, McGonagle D. NSAIDS inhibit in vitro MSC chondrogenesis but not osteogenesis: implications for mechanism of bone formation inhibition in man. J Cell Mol Med. 2011;15:525–534. doi: 10.1111/j.1582-4934.2010.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui X, Chopp M, Zacharek A, Roberts C, Lu M, Savant-Bhonsale S, Chen J. Chemokine, vascular and therapeutic effects of combination Simvastatin and BMSC treatment of stroke. Neurobiol Dis. 2009;36:35–41. doi: 10.1016/j.nbd.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao-Fang Z, Hong-Tian Z, Zhi-Nian Z, Yuan-Li H. PKH26 as a fluorescent label for live human umbilical mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2011;47:516–520. doi: 10.1007/s11626-011-9424-5. [DOI] [PubMed] [Google Scholar]
- 28.Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–31. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 29.Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6:e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 31.Fu YS, Shih YT, Cheng YC, Min MY. Transformation of human umbilical mesenchymal cells into neurons in vitro. J Biomed Sci. 2004;11:652–660. doi: 10.1007/BF02256131. [DOI] [PubMed] [Google Scholar]
- 32.Kitada M. Mesenchymal cell populations: development of the induction systems for Schwann cells and neuronal cells and finding the unique stem cell population. Anat Sci Int. 2012;87:24–44. doi: 10.1007/s12565-011-0128-4. [DOI] [PubMed] [Google Scholar]
- 33.Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339–353. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, Yang S, Liu P, Xu J, Wang J, Han Z, Han ZC. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009;24:307–316. doi: 10.1159/000233255. [DOI] [PubMed] [Google Scholar]
- 35.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellamy CO. p53 and apoptosis. Br Med Bull. 1997;53:522–38. doi: 10.1093/oxfordjournals.bmb.a011628. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH. Early Brain Injury or Cerebral Vasospasm. Vol. 110. Springer Vienna; 2011. Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage; pp. 43–48. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax-or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid β peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davenport CM, Sevastou IG, Hooper C, Pocock JM. Inhibiting p53 pathways in microglia attenuates microglial-evoked neurotoxicity following exposure to Alzheimer peptides. J Neurochem. 2010;112:552–563. doi: 10.1111/j.1471-4159.2009.06485.x. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65:520–530. doi: 10.1002/ana.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernández-Jiménez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I, Moro MA. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke. 2013;44:2333–2337. doi: 10.1161/STROKEAHA.113.001715. [DOI] [PubMed] [Google Scholar]
- 43.da Costa CA, Sunyach C, Giaime E, West A, Corti O, Brice A, Safe S, Abou-Sleiman PM, Wood NW, Takahashi H, Goldberg MS, Shen J, Checler F. Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson’s disease. Nat Cell Biol. 2009;11:1370–5. doi: 10.1038/ncb1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- 45.Liang ZQ, Wang XX, Wang Y, Chuang DM, DiFiglia M, Chase TN, Qin ZH. Susceptibility of striatal neurons to excitotoxic injury correlates with basal levels of Bcl-2 and the induction of P53 and c-Myc immunoreactivity. Neurobiol Dis. 2005;20:562–573. doi: 10.1016/j.nbd.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Feng Z, Jin S, Zupnick A, Hoh J, de Stanchina E, Lowe S, Prives C, Levine AJ. p53 tumor suppressor protein regulates the levels of huntingtin gene expression. Oncogene. 2006;25:1–7. doi: 10.1038/sj.onc.1209021. [DOI] [PubMed] [Google Scholar]
- 47.Shi YX. p53, PUMA and MCL-1 Regulation of Apoptotic Pathway. Medical Recapitulate. 2014;20:1923–1926. [Google Scholar]


