Abstract
Biomechanical cues of the microenvironment are recognized as potent regulators of cell behaviors. Skin regeneration induced by tissue expansion has been confirmed by results of experimental and clinical studies. However, it is still unknown whether skin regeneration induced by mechanical factor is the same biological process as skin morphogenesis during embryonic development. In order to explore the potential role of biomechanical force (BioF) in skin regeneration and whether epithelial-mesenchymal transition (EMT) is induced by BioF, continuous mechanical tension (CMT) at 10% elongation was applied to human keratinocytes in vitro for 12, 24, 48 and 72 hours. Cell proliferation and differentiation were analyzed, including the expression of markers of EMT: vimentin, FSP1, E-cadherin and N-cadherin. Normal and mechanical stretched skin specimens collected from mice were examined by immunofluorescence analysis and RT-PCR. We found that BioF promoted the proliferation and inhibited differentiation of keratinocytes in vitro. The expression of markers of EMT vimentin, FSP1, E-cadherin and N-cadherin were transiently up-regulated by BioF. Keratinocytes activation, epidermal thickening and EMT features were also observed in the stretched epidermis of mice, compared to normal mice. Furthermore, the mechanism of BioF induced EMT was found to be the enhanced autocrine effect of TNF-α, in part, and direct activation of the NF-κB pathway. Collectively, BioF promoted the proliferation of keratinocytes by transiently inducing some EMT features. BioF, as a vital biomechanical cue of the microenvironment of skin, was identified to be a novel inducer of EMT, regulating keratinocytes’ proliferation, differentiation and homeostasis of skin tissue.
Keywords: Biomechanical, epithelial-mesenchymal transition, skin expansion, skin regeneration
Introduction
Tissue expansion is a widely applied procedure in plastic surgery that stimulates and promotes skin regeneration through continuous mechanical stretching provided by an underlying silicone tissue expander [1]. Skin regeneration induced by mechanical force has been confirmed by clinical studiesand experimental researches [2-6]. The growing skin exhibits normal histology and cellular architecture without evidence of malignant degeneration [7]. Mechanical force increases keratinocyte growth and protein synthesis and alters cell morphology during tissue expansion [8,9]. The mechanism that mediates keratinocyte proliferation following mechanical stretching involves a network of several integrated cascades, implicating growth factors, cytoskeleton, and signal transduction pathways [10-12]. However, it remains unclear and we wonder whether skin regeneration induced by mechanical factors is based on the same biological process as in skin morphogenesis during embryonic development.
Epithelial-mesenchymal transition (EMT) is a complex process happened in skin morphogenesis by which epithelial cells lose their epithelial characteristics and acquire a mesenchymal-like phenotype [13,14]. EMT was originally described as an important cellular programming that enabled embryonic epithelial cells to gain the ability of migration and transient dedifferentiation [15]. Recently, EMT has been uncovered in carcinogenesis, tissue remodeling, and fibrotic diseases [16,17]. In cutaneous diseases, the migratory activity and reduced intercellular adhesion of keratinocytes exhibited several aspects of EMT during the reepithelialization of acute skin wound healing [18,19]. In hypertrophic scars, EMT-related genes expression was also up-regulated [20]. In vitro, human keratinocytes expressed mesenchymal features such as vimentin and fibroblast specific protein (FSP1), when they were exposed to inflammatory cytokines [19]. This transient transition of keratinocytes was known as a physiological response to injury [21]. Further, we tried to figure out whether EMT was induced in skin expansion: a natural response of epidermis to exogenous mechanical stimulation.
A plethora of biochemical signaling pathways and cytokines were discovered to induce EMT, such as the TGF-β superfamily, Wnts, Notch, TNF-α, and EGF [13,17]. During acute and fibrotic cutaneous wound healing of human skin, the keratinocytes were continuously under the pulling force exerted by the contraction of the wound [19,22]. However, the potential impact of biomechanical cue in EMT was disregarded so much. Biomechanical and architectural cues of the microenvironment are increasingly recognized as potent and pervasive regulators of cell behavior [23-27]. Many pathological dermatological diseases, such as hypertrophic scarring or keloid, muscular dystrophies and cardiovascular diseases were also associated with inappropriate mechanical stimuli [28]. So, in this study, we speculated that biomechanical force (BioF) might activate the EMT features of keratinocytes during the skin expansion procedure. Based on mechanical stretching of keratinocytes in vitro and a murine model of skin expansion, we investigated whether and how EMT contributed to BioF induced skin regeneration.
Materials and methods
Cells culture and mechanical force loading
Primary human keratinocytes were isolated from neonatal or adult foreskin and cultured with Keratinocyte Serum-Free Medium with supplements (KSFM; GIBCO) as described previously [29]. Keratinocytes were plated at the density of 2×105 cells/well (if not mention) in 1ml of medium on six-well flexible silicone rubber BioFlex™ plates coated with collagen type I (Flexcell International Corporation, Hillsborough, NC). Cells were cultured for 24 h before mechanical tension was applied. Cyclic mechanical tension (CMT) at 0.5 Hz sinusoidal curve at 10% elongation was applied using an FX-5000T™ Flexercell® Tension Plus™ unit (Flexcell International Corporation). The cultures were incubated in a humidified atmosphere at 37°C and 5% CO2 while stretching. Cells were harvested immediately after CMT stimulation was applied. Control cells were cultured on the same plates in the same incubator but not subjected to tension.
Cell proliferation assay
For the cell proliferation assay, keratinocytes were seeded on Flexcell® culture plates, followed by 10% CMT treatment. According to the manufacturer’s instructions, cell viability and proliferation were assessed by AlamarBlue® assay (Invitrogen, Carlsbad, CA, USA). The cells were incubated in medium supplemented with 10% (v/v) Alamar Blue fluorescent dye for 2 h before time points respectively at 37°C and 5% CO2. Then 100 μl sample of the medium were transferred and the absorbance at 570 and 590 nm was measured in a 96-well plate using a Multiscan UV visible spectrophotometer (Safire2; TECAN, Mannedorf, Switzerland).
Flow cytometric analysis of cell cycle
Cells were harvested and fixed with 75% ethanol and then stained for total DNA content with a solution containing 50 μg/ml propidium iodide (PI) and 50 μg/ml RNase I in PBS for 30 min at 37°C. Flow cytometry (BD Calibur) was performed on populations of 5000-10,000 cells. Different cell cycle phases were determined with ModFit LT cell-cycle analysis software (Verity Software House, Topsham, US), respectively.
Phalloidin staining
Formalin fixation (4%; Sigma-Aldrich, St. Louis, MO, USA) was performed for 10 min at room temperature. Cells were permeabilized (0.25% Triton X-100; Sigma-Aldrich, St. Louis, MO, USA), and nonspecific binding was blocked (3% bovine serum albumin (BSA)). F-actin was stained with Alexa Fluor 488® conjugated Phalloidin for 30 min at room temperature (1:200; Cytoskeleton, Inc., Denver, CO, USA). Then, Cells were visualized using a confocal microscope (Leica, Solms, Germany).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
The total RNA of cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After reverse transcription reaction, real-time polymerase chain reaction (PCR) was performed by Roche480 system using SYBR® Premix (Takara, Dalian, China) according to the manufacturer’s instructions. The conditions of real-time PCR were as follows: Denaturation at 95°C for 10 s, 40 cycles at 95°C for 10 s, and 60°C for 30 s. Dissociation stage was added to the end of the amplification procedure. No nonspecific amplification was observed, as determined by the dissociation curve. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control. Data was analyzed using the comparison Ct (2-ΔΔCt) method and expressed as fold change compared to the respective control, as previously described [30]. Each sample was analyzed in triplicate. Primers sequences were as follows: for homo sapiens: ΔNp63, 5’-GGAAAACAATGCCCAGACTC-3’ (forward) and 5’-GTGGAATACGTCCAGGTGGC-3’ (reverse); Keratin5, 5’-ATCGCCACTTACCGCAAGCTGCTGGAGGG-3’ (forward) and 5’-AAACACTGCTTGTGACAACAGAG-3’ (reverse); Loricrin, 5’-TCATGATGCTACCCGAGGTTTG-3’ (forward) and 5’-CAGAACTAGATGCAGCCGGAGA-3’ (reverse); Vimentin, 5’-AAAGTGTGGCTGCCAAGAACCT-3’ (forward) and 5’-ATTTCACGCATCTGGCGTTCCA-3’ (reverse); FSP1, 5’-GATGAGCAACTTGGACAGCAA-3’ (forward) and 5’-CTGGGCTGCTTATCTGGGAAG-3’ (reverse); E-cadherin, 5’-ACAACAAGCCCGAATTCACCCA-3’ (forward) and 5’-TCACAGCTGTTGCTGTTGTGCT-3’ (reverse); N-cadherin 5’-TCATTGCCATCCTGCTCTGCAT-3’ (forward) and 5’-AGTTGTTTGGCCTGGCGTTCTT-3’ (reverse); TNF-α, 5’-AAGCCTGTAGCCCATGTTGTA-3’ (forward) and 5’-TCAGCTCCACGCCATTG-3’ (reverse); TNFR1, 5’-GCTGGAGATGCAGAACGGGC-3’ (forward) and 5’-ACGAGGGGGCGGGATTTCTC-3’ (reverse); MMP9, 5’-GGGACGCAGACATCGTCATC-3’ (forward) and 5’-TCGTCATCGTCGAAATGGGC-3’ (reverse); GAPDH, 5’-AGGTCGGTGTGAACGGATTTG-3’ (forward) and 5’-TGTAGACCATGTAGTTGAGGTCA-3’ (reverse). For Mus musculus: E-cadherin, 5’-TACACTGCCCAGGAGCCAGA-3’ (forward) and 5’-TGGCACCAGTGTCCGGATTA-3’ (reverse); Vimentin, 5’-CGTCCACACGCACCTACAG-3’ (forward) and 5’-GGGGGATGAGGAATAGAGGCT-3’ (reverse); FSP1, 5’-TGAGCAACTTGGACAGCAACA-3’ (forward) and 5’-CTTCTTCCGGGGCTCCTTATC-3’ (reverse); GAPDH, 5’-GCACCGTCAAGGCTGAGAAC-3’ (forward) and 5’-TGGTGAAGACGCCAGTGGA-3’ (reverse).
Western blot
For western blot analysis, total proteins were extracted from cultured cells using radioimmunoprecipitation assay (RIPA) lysis buffer. Protein concentrations were determined using a bicinchoninic acid (BCA) assay. Thirty micrograms of each protein lysate was resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Following transfer, membranes were blocked and then incubated with primary antibodies overnight at 4°C. The primary antibodies used were anti-Vimentin, anti-FSP1 and anti-TNFR1 (Abcam, Cambridge, UK) at a dilution of 1:1,000. For normalization of protein loading, GAPDH (Sigma-Aldrich, St. Louis, MO, USA) antibody was used at a dilution of 1:2,000. Immunoreactive bands were quantitatively analyzed in triplicate by normalizing the band intensities to their respective controls on scanned films with ImageJ software.
Tissue expansion model
All animal procedures were conducted according to the guide for the care and use of laboratory animals. All mice were maintained in a pathogen-free environment. All experiments were performed under laminar flow hoods. Biomechanical loading devices were constructed as previously described [31]. BALB/C mice (6 weeks old, 75-85 g in weight) retrieved from Shanghai Experimental Animal Center, Shanghai, China, were anesthetized and then loading devices were carefully secured to the dorsum of mice with 6-0 nylon sutures. Tension was created by carefully distracting the expansion screws by 4 mm every other day thereafter.
Immunofluorescence
After being subjected to CMT for 24 h, keratinocytes were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.25% Triton X-100 for 5 min, and blocked with 3% BSA for 60 min. Anti-E-cadherin and N-cadherin (560061, 561553, BD Biosciences, San Jose, CA, 1:100), anti-NF-κB p65 (ab7970, Abcam, Cambridge, UK, 1:100) were used to incubate cells overnight at 4°C. Expanded skin from mice were routinely fixed with paraformaldehyde and embedded in Tissue Tec OCT Compound. Skin sections (10 µm) were treated with primary antibodies as follows: anti-Vimentin (ab185030, Abcam, Cambridge, UK, 1:50), anti-P63 (ab139929, Abcam, Cambridge, UK, 1:100), anti-FSP1 (ab27957, Abcam, Cambridge, UK, 1:50), anti-E-cadherin (560061, BD Biosciences, San Jose, CA, 1:50), anti-Keratin5 (PRB-160, Covance, Richmond, CA 1:1000), anti-Ki67 (ab15580, Abcam, Cambridge, UK, 1:50). Primary antibodies were visualized using secondary antibodies conjugated to either 488- or 555-nm absorbing fluorophores (Invitrogen, Carlsbad, CA, USA). After the final wash, the nuclei was counterstained by adding 100 µL of a 2-mg/mL solution of 4’,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO, USA) in 1× phosphate-buffered saline (PBS) for 8 min before imaging. Cells were visualized using a confocal microscope (Leica, Solms, Germany).
Statistical analysis
Results are expressed as mean ± SD. Statistical differences were determined by a two-tailed Student’s t-test. P-values less than 0.05 were considered statistically significant.
Results
BioF regulated the proliferation and differentiation of keratinocytes
First, the effect of BioF on the proliferation and differentiation of keratinocytes was examined. After CMT was applied to keratinocytes, flow cytometric analysis of cell cycle showed that the percentage of keratinocytes in the G0/G1 phrase was decreased from 61% to 38%, and the percentage of S phrase was significantly increased from 26% to 40% (Figure 1A, 1B). By alamar blue assay, absorbance was enhanced by CMT loading for 72 hours, representing increased cell proliferation (Figure 1C). RT-PCR with mRNA isolated from mechanical loaded keratinocytes found that the expression of epidermal stem/progenitor cells marker (keratin5) or activated epidermal stem cells marker (ΔNp63) was up-regulated after mechanical stretching for 12 and 24 hours. The expression of late differentiation marker loricrin was inhibited (Figure 1D, 1E). To visualize the effect of mechanical force, F-actin was labeled by phalloidin. After CMT stimulation, the morphology of keratinocytes was transited from polygonal to spindle shapes (Figure 1F). So, BioF promoted the proliferation and inhibited differentiation of keratinocytes.
Figure 1.

BioF regulated the proliferation and differentiation of keratinocytes. A. Flow cytometry was applied to analyze the cell cycle when keratinocytes were mechanically stretched for 24 h at 10% elongation. B. The quantitative analysis of the percentage of keratinocytes in the G0/G1, S and G2 phrase. C. Alamar blue assay of cell proliferation. Absorbance was measured at 570 and 590 nm. D, E. The relative expressions of ΔNP63, loricrin and keratin5 were measured by real-time PCR after keratinocytes were applied to cyclic mechanical tension (CMT) for 24 h. GAPDH expression was used as an internal control. F. The effect of CMT on the cytoskeleton was studied by F-actin staining by phalloidin. Scale bars: 50 μm. NC: negative control. Data were presented as the mean ± standard deviation (SD), n ≥ 3. *p < 0.05
.
EMT features were induced by BioF
After demonstrating the pro-growth effect of BioF on keratinocytes, we evaluated whether mesenchymal features were acquired. RT-PCR with mRNA isolated from mechanical loaded keratinocytes showed that the expression of mesenchymal markers vimentin and FSP1 were regulated in a time-dependent manner. The expression remained unaltered when cells were stretched for 12 h while the expression was upregulated at the time point of 24 h, 48 h and returned to the control level after 48 h (Figure 2A, 2B). Induction of vimentin and FSP1 protein were also measured by western blot analysis, which were consistent with the mRNA levels. The marker of cell-cell adhesion Ecadherin was significantly downregulated when keratinocytes were stretched for 24 h (Figure 2D), whereas the mesenchymal markers of cell-cell adhesion N-cadherin was upregulated (Figure 2D), visualized and confirmed by immunofluorescene experiments (Figure 2E). Expression of MMP9 is another benchmark of EMT to facilitate cell migration, but BioF had no influence on the expression of MMP9 in keratinocytes (Figure 2C), which implicated that these features of EMT status were different from those in wound healing or carcinogenesis.
Figure 2.
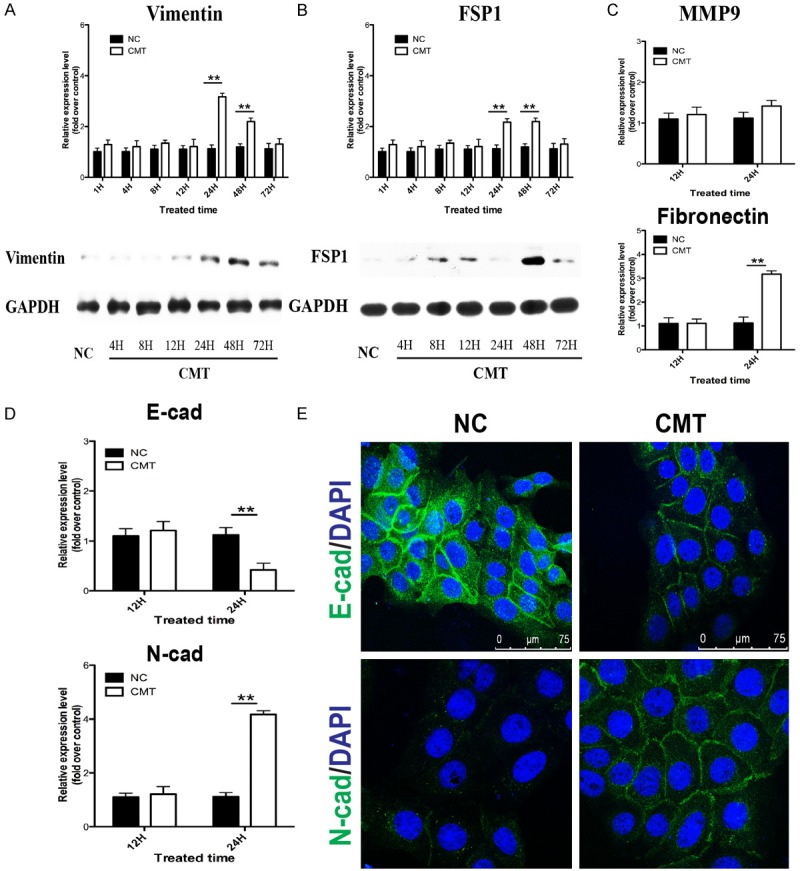
EMT features were induced in keratinocytes by BioF. (A-C) The relative expression of EMT markers vimentin and FSP1, MMP9 over control were measured by real-time PCR and western blot at 1, 4, 8, 12, 24, 48, and 72 h after CMT was applied. The relative expression of cell-cell adhesion markers E-cadherin, N-cadherin (D) were measured by real-time PCR at 12 and 24 h after CMT was applied. GAPDH expression was used as an internal control. (E) The location and expression of E-cadherin and N-cadherin, labeled by Alexa Fluor® 488 conjugated antibody, were visualized by immunofluorescence experiments. Nucleus (blue) was stained with DAPI. NC: negative control. Data were presented as the mean ± SD, n ≥ 3; *p < 0.05, **p < 0.01
.
EMT features were observed in the BioF stretched skin of mice
To confirm these results, we examined skin specimens from mechanical stretched skin and normal skin of mice. Normal and stretched skin of six representative mice were stained by routine hematoxylin-eosin and immunofluorescence staining for vimentin, FSP1, Ki67, Ecadherin and P63 (Figure 3A). We found obviously thickened epidermis in mechanical stretched group, compared to the normal epidermis. Besides, markers of proliferation Ki67 and P63 were also up-regulated in stretched epidermis. However, no discontinuous and apparently disassembled basal membrane was found. This result was different from the EMT features observed in carcinogenesis or wound healing. Both vimentin and FSP1 were positively expressed in the basal cells of the stretched epidermis, illustrating EMT induction. Moreover, cell-cell adhesion marker E-cadherin was down-regulated in stretched epidermis. Then, the expression of EMT features vimentin, FSP1 and E-cadherin in stretched skin and normal skin of mice were examined by qRT-PCR (Figure 3B), which were consistent with the abovementioned results. Together, these data suggested that the epidermal cells of skin gained kind of EMT status under BioF condition.
Figure 3.

EMT features were observed in the BioF stretched skin of mice. A. Normal and BioF stretched skin of mice were stained by routine hematoxylin-eosin and immunofluorescence staining for keratin5, vimentin (green), FSP1 (red), Ki67 (red), E-cadherin (green) and P63 (red). The differences were indicated by white arrowheads. Primary antibodies were visualized using secondary antibodies conjugated to Alexa Fluor® 488 or 555 nm. Nucleus (blue) was stained with DAPI. Scale bars: 50 μm. The illustration of a murine model of skin expansion was shown in the upper right corner. B. The expression of EMT features vimentin, FSP1 and E-cadherin in stretched skin and normal skin of mice were examined by qRT-PCR. NC: negative control. Data were presented as the mean ± SD, n ≥ 3; *p < 0.05, **p < 0.01.
The mechanisms of BioF inducing EMT features
Next, we tried to explore the potential molecular mechanisms of how BioF induced EMT. Previous studies found that the EMT features in wound healing were mediated by inflammatory cytokine TNF-α. So we examined the association between TNF-α and EMT in BioF induced epidermal proliferation. When keratinocytes were stretched for 48 hours, the mRNA expression of TNF-α was upregulated at the time point of 12 h and 24 h (Figure 4A). In Elisa analysis, the secreted TNF-α protein in the culture medium was increased (Figure 4B). The effect of BioF on the expression of TNF-α receptor 1 (TNFR1) was also measured by qRT-PCR and western blot analysis. Consistent with the TNF-α data, the expression of TNFR1 was enhanced after stretching for 12 hours, at the mRNA and protein level (Figure 4C, 4D). It seemed that BioF inducing EMT was mediated by the autocrine effect of TNF-α.
Figure 4.

The mechanisms of BioF inducing EMT in keratinocytes. The relative expression of EMT inducer TNF-α over control was measured by real-time PCR (A) and Enzyme-linked immunosorbent assay (ELISA) (B) at 1, 4, 8, 12, 24 and 48 h after CMT was applied. The expression of TNF-α receptor 1 (TNFR1) was examined by real-time PCR (C) and western blot (D) respectively. GAPDH expression was used as an internal control. NC: negative control. Data were presented as the mean ± SD, n ≥ 3; **p < 0.01.
To demonstrate the causal relationship, inhibitors of NF-κB (BAY11-7082) and p38 (SB-203580) were used, since TNF-α could induce the activation of these pathways. Cells were pretreated with inhibitors for 1 h before mechanical stimulation. Then the expression of vimentin and FSP1 were determined by qRT-PCR and western blot. We found that p38 inhibitor (SB-203580) did not block BioF induced upregulation of vimentin and FSP1 at the mRNA (Figure 5A) and protein level (Figure 5B), whereas NF-κB inhibitor (BAY11-7082) could mostly rescue the EMT induction (Figure 5C). Moreover, NF-κB inhibitor also restored the pro-growth effect of BioF on keratinocytes, as measured the expression of ΔNp63 and keratin5 by qRT-PCR (Figure 5D) and by cell proliferation assay (Figure 5E). In addition, when BioF was removed, the upregulated expression of vimentin and FSP1 returned to the normal level, at the mRNA (Figure 5F) and protein level (Figure 5G). Furthermore, by immunofluorescence examination, we found that BioF could directly activate the NF-κB pathway as the p65 subunit of NF-κB was translocated into nucleus (Figure 5H). Collectively, these results indicated that BioF induced the EMT of keratinocytes by directly activating the NF-κB pathway.
Figure 5.
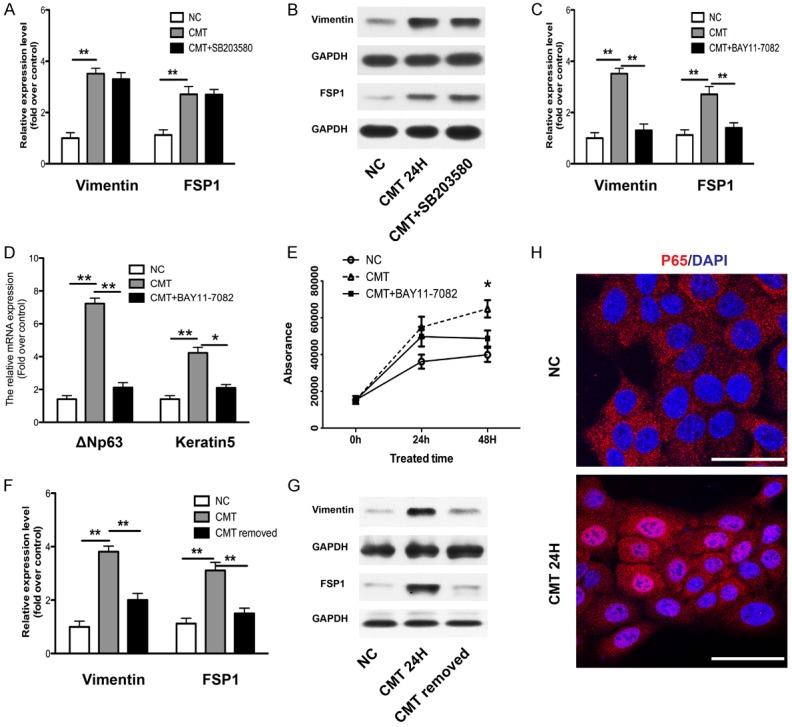
BioF induced EMT features of keratinocytes by directly activating the NF-κB pathway. Keratinocytes were pretreated with inhibitors of NF-κB (BAY11-7082) and p38 (SB-203580) for 1 h respectively followed by stimulation with CMT. The expression of EMT features vimentin and FSP1 were examined by real-time PCR (A, C) and western blot (B). The effect of BAY11-7082 on the proliferation of keratinocytes was shown by the expression of keratin5, ΔNP63 measured by real-time PCR (D) and AlamarBlue® assay (E). Keratinocytes were stimulated with CMT for 24 h, and then the BioF was removed for 24 h. The mRNA (F) and protein (G) were collected for examining the expression of EMT features vimentin and FSP1. (H) After keratinocytes were stimulated with CMT for 12 h, the location and expression of P65 (red), subunit of NF-κB, were analyzed by immunofluorescence staining. Nuclei were visualized with DAPI staining. Scale bars: 50 μm. GAPDH expression was used as an internal control. NC: negative control. Data were presented as the mean ± SD, n ≥ 3; **p < 0.01.
Discussion
Mechanical force induces proliferative signals in vivo, as seen in the expanded skin stimulated by a tissue expander and the abdominal skin of pregnant women, which cover much larger areas than normal. To our knowledge, this is the first study to provide evidence that biomechanical force induced EMT to promote epidermal regeneration. Furthermore, our study suggested that BioF induced the EMT of keratinocytes by directly activating the NF-κB pathway.
EMT has been observed in skin morphogenesis, wound repair, cutaneous fibrosis and skin tumors [18,32]. The misexpression of Snail1, an essential gene for EMT, in the epidermis of transgenic mice led to epidermal hyperproliferation [32]. EMT features were also found to play an important role in both acute and fibrotic cutaneous wound healing of human skin, motivated by cytokines TNF-α [19,33]. Our study found for the first time that EMT was also involved in skin regeneration of the human adult. EMT markers, namely vimentin and FSP1, were induced in keratinocytes in vitro and observed in the epidermis of human skin. However, no discontinuous and apparently disassembled basal membrane was found in our study, which was different with the EMT features observed in wound healing [34]. Skin growth exhibited normal histology and cellular architecture without evidence of malignant transition. So, we speculate that EMT involved in skin regeneration of human adults was a physiological response to BioF.
EMT inducers could be loss of cell-cell adhesion/cell polarity, change of extracellular matrix (ECM) and many pathways triggered by membrane receptors, new intracellular molecules and external pro-inflammatory cytokines [17]. For decades, it was found that biochemical factors such as TGF-β, Notch, EGF could induce the translocation of snails, thus fulfilling important aspects of EMT [35,36]. The NF-κB pathway is also emerging as an important regulator of EMT through the induction of Snail1 transcription [37]. Only few study focused on the impact of biomechanical property of microenvironment in EMT. Both micro-mechanical force exerted by ECM during morphogenesis or tumor formation and macro-mechanical force generated by the contraction of wound healing were non-neglectable cues in EMT [25,26]. In this study, we describe for the first time that BioF was a novel inducer of EMT, inducing the expression of important features of EMT vimentin and FSP1.
Previous studies reported that mechanical stretching induced keratinocyte proliferation, as determined by BrdU incorporation and provoked an antiapoptotic signal [11,12]. Induction of proliferation by mechanical stretching was dependent on EGFR, ERK1/2, and mitogen-activated protein kinase (MAPK) activations [38-41]. In this study, BioF promoted proliferation and induced EMT of keratinocytes through directly activating the NF-κB pathway. BioF induced the autocrine of TNF-α might also function. Since both BioF and TNF-α could activate the NF-κB pathway and induce EMT, we wondered what may be the relationship between biomechanical and biochemical factors. A recent study showed that mechanical stress factors were overarching regulators in multicellular contexts, setting cell responsiveness to Hippo, WNT, and GPCR signaling [42].
Conclusion
Our study provided evidence that by directly activating the NF-κB pathway, biomechanical force transiently induced the expression of EMT features vimentin and FSP1, promoting proliferation and activation of keratinocytes. BioF, as a vital biomechanical cue of the microenvironment, was identified to be a novel inducer of EMT, regulating cell proliferation, differentiation and homeostasis of skin tissue.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 30730092, 30925034) and the National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (No. 2012BAI11B03).
Disclosure of conflict of interest
None.
References
- 1.De Filippo RE, Atala A. Stretch and growth: The molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;109:2450–2462. doi: 10.1097/00006534-200206000-00043. [DOI] [PubMed] [Google Scholar]
- 2.Johnson TM, Lowe L, Brown MD, Sullivan MJ, Nelson BR. Histology and physiology of tissue expansion. J Dermatol Surg Oncol. 1993;19:1074–1078. doi: 10.1111/j.1524-4725.1993.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 3.Duffy JS, Shuter M. Evaluation of Soft-Tissue Properties under Controlled Expansion for Reconstructive Surgical Use. Med Eng Phys. 1994;16:304–309. doi: 10.1016/1350-4533(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 4.Morykwas MJ, Marks MW, Argenta LC. Surface area and tissue volume increases with differential expansion. Ann Plast Surg. 1992;28:311–314. doi: 10.1097/00000637-199204000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 6.Takei T, Mills I, Arai K, Sumpio BE. Molecular basis for tissue expansion: clinical implications for the surgeon. Plast Reconstr Surg. 1998;102:247–258. doi: 10.1097/00006534-199807000-00044. [DOI] [PubMed] [Google Scholar]
- 7.Beauchene JG, Chambers MM, Peterson AE, Scott PG. Biochemical, biomechanical, and physical changes in the skin in an experimental animal model of therapeutic tissue expansion. J Surg Res. 1989;47:507–514. doi: 10.1016/0022-4804(89)90128-5. [DOI] [PubMed] [Google Scholar]
- 8.Takei T, RivasGotz C, Delling CA, Koo JT, Mills I, McCarthy TL, Centrella M, Sumpio BE. Effect of strain on human keratinocytes in vitro. J Cell Physiol. 1997;173:64–72. doi: 10.1002/(SICI)1097-4652(199710)173:1<64::AID-JCP8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Squier CA. The stretching of mouse skin in vivo: effect on epidermal proliferation and thickness. J Invest Dermatol. 1980;74:68–71. doi: 10.1111/1523-1747.ep12519868. [DOI] [PubMed] [Google Scholar]
- 10.Takei T, Han O, Ikeda M, Male P, Mills I, Sumpio BE. Cyclic strain stimulates isoformspecific PKC activation and translocation in cultured human keratinocytes. J Cell Biochem. 1997;67:327–337. doi: 10.1002/(sici)1097-4644(19971201)67:3<327::aid-jcb5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J Invest Dermatol. 2004;122:783–790. doi: 10.1111/j.0022-202X.2004.22328.x. [DOI] [PubMed] [Google Scholar]
- 12.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Activation of Akt by mechanical stretching in human epidermal keratinocytes. Exp Dermatol. 2006;15:356–361. doi: 10.1111/j.0906-6705.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 16.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Tokura Y. Epithelial-mesenchymal transition in the skin. J Dermatol Sci. 2011;61:7–13. doi: 10.1016/j.jdermsci.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N, Han YP. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnoux V, Nassour M, L’Helgoualc’h A, Hipskind RA, Savagner P. Erk5 controls Slug expression and keratinocyte activation during wound healing. Mol Biol Cell. 2008;19:4738–4749. doi: 10.1091/mbc.E07-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 23.Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luis NM, Morey L, Mejetta S, Pascual G, Janich P, Kuebler B, Cozutto L, Roma G, Nascimento E, Frye M, Di Croce L, Benitah SA. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of Cbx4. Cell Stem Cell. 2011;9:233–246. doi: 10.1016/j.stem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Zhou J, Zhang N, Zhang X, Li Q. A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Biophys Res Commun. 2014;450:568–574. doi: 10.1016/j.bbrc.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 32.Jamora C, Lee P, Kocieniewski P, Azhar M, Hosokawa R, Chai Y, Fuchs E. A signaling pathway involving TGF-beta2 and snail in hair follicle morphogenesis. PLoS Biol. 2005;3:e11. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 34.Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2) J Dermatol Sci. 2009;56:19–26. doi: 10.1016/j.jdermsci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Morita T, Mayanagi T, Sobue K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J Cell Biol. 2007;179:1027–1042. doi: 10.1083/jcb.200708174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strippoli R, Benedicto I, Perez Lozano ML, Cerezo A, Lopez-Cabrera M, del Pozo MA. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Model Mech. 2008;1:264–274. doi: 10.1242/dmm.001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol. 2000;278:H521–529. doi: 10.1152/ajpheart.2000.278.2.H521. [DOI] [PubMed] [Google Scholar]
- 39.Yu X, Miyamoto S, Mekada E. Integrin alpha 2 beta 1-dependent EGF receptor activation at cell-cell contact sites. J Cell Sci. 2000;113:2139–2147. doi: 10.1242/jcs.113.12.2139. [DOI] [PubMed] [Google Scholar]
- 40.MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Pulsatile stretch activates mitogenactivated protein kinase (MAPK) family members and focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;259:8–14. doi: 10.1006/bbrc.1999.0720. [DOI] [PubMed] [Google Scholar]
- 42.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]


