Abstract
Norcantharidin (NCTD) has been proven to be able to attenuate renal interstitial fibrosis, but the exact molecular mechanism is still unknown. This study investigated the relationship between the anti-fibrotic effect of NCTD and its inhibition on PP2Ac expression. Here, PP2Ac was found to be positively correlated with extracellular matrix accumulation in the rat unilateral ureteral obstruction (UUO) model. Additional experiments showed that the PP2A inhibitor (okadaic acid) can ameliorate renal interstitial fibrosis by inhibiting the expression of fibronectin (FN) and collagen I (Col-I) and reversing tubular epithelial-to-mesenchymal transition in vivo and in vitro. In vitro experiments also demonstrated that ectopic over-expression of PP2Ac has a profibrotic effect in HK-2 cells. Moreover, NCTD was able to downregulate PP2Ac expression, decrease FN, Col-I, α-SMA expression, and increase E-cadherin expression in a dose-dependent manner both in vivo and in vitro. In particular, it was demonstrated that NCTD induced no evident changes in the expression of FN, Col-I, α-SMA and E-cadherin in HK-2 cells after PP2Ac was knocked down by shRNA. These results indicated that NCTD exerts an anti-fibrosis effect via inhibition of PP2Ac expression. Thus, PP2Ac could be a promising target for intervention in renal interstitial fibrosis.
Keywords: Norcantharidin, renal interstitial fibrosis, protein phosphatase 2, epithelial-mesenchymal transition
Introduction
Renal tubulointerstitial fibrosis is widely regarded as the final common characteristic of many chronic kidney diseases (CKD). Relentless accumulation and dysregulated remodeling of extracellular matrix (ECM) cause destruction of renal architecture and impairment of renal function [1-2]. The epithelial-to-mesenchymal transition (EMT) also plays a critical role in the pathogenesis and progression of interstitial fibrosis [3]. It has been reported that the severity of renal interstitial fibrosis is the most reliable determinant of renal function declination [4]. Therefore, preventing renal interstitial fibrosis could be an effective therapeutic approach to ameliorate, or even reverse, chronic kidney injury. However, there are currently no candidate anti-fibrotic drugs available for clinical use [5]. Prospective therapies, based on new insights into the mechanisms contributing to renal fibrosis, are providing hope for future treatment of Chronic kidney disease [6].
Norcantharidin (NCTD), a synthetic demethylated analog of cantharidin (extracted from Chinese blister beetles (Mylabris)), has been used in the treatment of different kinds of human cancers, such as colorectal, gastric, hepatic and ovarian carcinomas [7-9]. Clinical studies have shown that, compared to cantharidin, NCTD has a significantly reduced nephrotoxicity and displays pro-inflammatory side effects, while maintaining effective anticancer activity [10]. Our previous studies have demonstrated that NCTD can attenuate renal tubulointerstitial fibrosis both in vivo and in vitro. The underlying mechanisms were associated with preventing EMT by suppression activation of TGF-β1/Smad2/3 signaling [11-14]. These findings suggested that NCTD could be exploited as a promising therapeutic agent for renal fibrosis. However, the exact molecular mechanism of NCTD has not been thoroughly elucidated yet.
Protein phosphatase 2A (PP2A) is a major cellular serine-threonine phosphatase, and consists of a catalytic subunit C (PP2Ac), a structural subunit A (PP2Aa), and a highly variable regulatory subunit B (PP2Ab). PP2Ac is essential for the dephosphorylation activity of PP2A [15]. NCTD has been reported to inhibit protein phosphatase, and can thus significantly reduce the expression and activity of PP2A [16,17]. Therefore, we hypothesized that the anti-fibrotic effect of NCTD could be related to its inhibition of PP2A. The significance of PP2A in the pathogenesis of cancer has been widely demonstrated. It has been reported that PP2A is a tumor suppressor whose activity is inhibited in many solid cancers [18], and that restoring PP2A activity may result in inhibition of cancer cell proliferation and EMT [19]. Regarding the kidney, although an earlier study documented that PP2A is of particular importance during renal morphogenesis and development [20], the role of PP2A in renal interstitial fibrosis is still unknown.
In this study, we studied the relationship between PP2Ac and renal interstitial fibrosis, and the effects of PP2Ac over-expression on ECM accumulation and tubular EMT. Furthermore, we investigated the underlying mechanism of NCTD in an obstructive nephropathy model and TGF-β1-induced interstitial fibrosis model. Our results suggested that PP2Ac could act as a novel therapeutic target for renal fibrosis, and that the protective effect of NCTD on renal interstitial fibrosis is related to its inhibition of PP2Ac expression.
Materials and methods
Animal model
Healthy 8-week-old male Sprague-Dawley (SD) rats weighing 200 to 220 g were purchased from the Animal Department at Xiangya School of Medicine, Central South University. All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of Central South University. In the first panel of experiments, 32 SD rats were randomly divided into four groups (n = 8): (1) sham operation, (2) UUO day 3, (3) UUO day 7, and (4) UUO day 14. UUO was performed using an established protocol, as previously described [13]. Rats were sacrificed at 1, 3, 7 and 14 days after UUO, and kidney tissues were harvested. In the second panel of experiments, another 24 SD rats were also randomly divided into three groups (n = 8): (1) sham operation, (2) UUO, and (3) UUO+okadaic acid (OA) (30 μg/kg/day). OA (Sigma, St. Louis, MO) was diluted with 1.8% alcohol and administered to rats through a gastric tube. Rats were sacrificed at 3 days after UUO, and the kidney tissues were harvested. In the third panel of experiments, 20 rats were divided into four groups (n = 5): (1) sham operation, (2) UUO, (3) UUO+NCTD (0.05 mg/kg/day), and (4) UUO+NCTD (0.1 mg/kg/day). NCTD (Beijing Shuang He Biotechnological Limited Company, China) was dissolved in normal saline and administered to rats by intraperitoneal injection at the doses of 0.05 and 0.1 mg/kg/day. Rats were sacrificed at 14 days after UUO, and the kidney tissues were harvested.
Cell culture and treatment
A proximal tubular cell line, HK-2 (ATCC, Rockville, MD), was cultured at 37°C in a humidified atmosphere of 5% CO2 and maintained in a defined medium (1:1 mixture of DMEM and F12 supplemented with 10% FBS). For in vitro experiments, NCTD was dissolved in double-distilled water and OA was added to the solvent DMSO. HK-2 cells were serum-starved for 16 h before treatment with 2.5 μg/ml NCTD or 40 nM OA for 0.5 h, and then incubated with or without recombinant TGF-β1 (Peprotech, Rocky Hill, NJ) at 5 ng/ml for 24 h. Cells were then collected for real-time RT-PCR and Western blot analysis.
Cell viability determination
Cell viability was assessed by trypan blue exclusion and methylthiazol tetrazolium (MTT) assay. After incubation for 24 h with various concentrations (10 to 1000 nM) of OA, HK-2 cells cultured in 24-well plates were harvested. Cell viability was assessed by trypan blue exclusion in triplicate wells. At least 400 cells were counted for each determination, and the number of viable cells was expressed as a percentage of total cells. For the MTT assay, HK-2 cells were seeded in 96-well culture plates at a density of 5 × 103 cells/well with 100 μl of medium containing 10% FBS and then synchronously cultured in serum-free medium for 16 h. Cells were further incubated in medium plus 10% FBS containing 5 ng/ml TGF-β1 with OA (10 to 1000 nM) for 24 h. A volume of 20 μl of methylthiazol tetrazolium was then added to each well. The supernatant was then discarded and DMSO (150 μl) was added to each well to dissolve the blue formazan crystals. The optical density (OD) was measured using a microplate reader (SpectraMax 340, Molecular Devices, Japan) at a wavelength of 490 nm. The arithmetic mean of the OD of each group of wells was calculated, and the OD of the cells in the normal group was assigned a relative value of 100.
Construction of plasmids and transfection
To over-express and deplete PP2Ac, HK-2 cells were transfected with the plasmid pEGFP-N1-PP2Ac and SD11-PP2Ac shRNA (short hairpin RNA) (GeneChem, Shanghai, China) using LIPOFECTAMINE 2000, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). The vector-only plasmid pEGFP-N1 and SD11 were used as the negative controls, respectively. Normal HK-2 cells without plasmid transfection were used for the blank control. After selection of stable transfectants, cells were maintained in the defined medium.
Western blot analysis
Western blot analysis for specific protein expression was performed according to an established procedure [13]. The primary antibodies used were as follows: anti-E-cadherin (sc-7870), anti-fibronectin (sc-18827), anti-PP2Ac (sc-14020) and anti-β-actin (sc-47778) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-α-SMA (ab5694) and anti-collagen I (ab34710) (Abcam, Cambridge, MA).
Immunohistochemistry staining
Paraffin-embedded kidney tissue sections (3-μm thick) were prepared. Sections were dewaxed, rehydrated, and pretreated with citrate buffer (10 mM citric acid, pH 6.0) for microwave antigen retrieval, followed by incubation with blocking serum for 30 min at room temperature to reduce nonspecific background staining. Sections were then incubated with anti-fibronectin, anti-collagen I, anti-E-cadherin, anti-α-SMA, or anti-PP2Ac antibody overnight at 4°C. After being washed with PBS, sections were incubated with HRP-labeled biotinylated goat anti-rabbit IgG or anti-mouse IgG for 1 h at room temperature. As a negative control, the primary antibody was replaced with non-immune IgG, and no staining occurred. All images were semi-quantitatively analyzed using the computer-assisted Image-Pro Plus software, version 6.0 (Media Cybernetics, Bethesda, MD). Values were expressed as average optical density (AOD), which is defined as the ratio of the positive target optical density and the positive area percentage (IOD/Area).
Real-time RT-PCR
Total RNA extraction was performed with TRIZOL (Invitrogen). RNA purity was determined by the A260 to A280 ratio. cDNA was synthesized according to the instructions in the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas). mRNA expression was assessed by real-time PCR using the SYBR green PCR reagent kit (Applied Biosystems, Foster City, CA). PCR thermal cycling parameters consisted of one cycle of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 10 min at 72°C. The mRNA levels of various genes were calculated after normalizing with glyceraldehyde 3-phosphate dehydrogenase or β-actin, and the 2ΔΔCt method was used for analyzing target gene expression level. All the oligonucleotide primers were designed by Shanghai Ying Jun Limited Company (Table 1).
Table 1.
Primer sequences
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Rat fibronectin | 5’-TGACAACTGCCGTAGACCTGG-3’ | 5’-TACTGGTTGTAGGTGTGG CCG-3’ |
| Human fibronectin | 5’-TGGAGGAAGCCGAGGTTT-3’ | 5’-CAGCGGTTTGCGATGGTA-3’ |
| Rat collagen-I | 5’-TGGCAAGAACGGAGATGA-3’ | 5’-AGCTG TTCCAGGCAATCC-3’ |
| Human collagen-I | 5’-CCTGCGTACA GAACGGCCT-3’ | 5’-CTCGTCACAGATCACGTCATCG-3’ |
| Rat E-cadherin | 5’-GTCAAACGGCATCTAA AGC-3’ | 5’-CAAAGACCTCCTGGATAAACT-3’ |
| Human E-cadherin | 5’-CACAGGGGCCTGGACGCTC-3’ | 5’-GGGTCAGTATCAGCCGCTTT-3’ |
| Rat α-SMA | 5’-TCCTGACCCTGAAGTATCCG-3’ | 5’-TCTCCAGAGTCCAGCACAAT-3’ |
| Human α-SMA | 5’-CTGTTCCAGCCATCCTTCATC-3’ | 5’-CCGTGATCTCCTTCTGCATT-3’ |
| Rat PP2Ac | 5’-CGGCATCATGGACGAGAA-3’ | 5’-GCCCATGCACATCTCCACA-3’ |
| Human PP2Ac | 5’-CGCCTACAAGAAGTTCCCCA-3’ | 5’-CGAGGAGATATACCCCAACCA-3’ |
| GAPDH | 5’-GGCACAGTCAAGGCTGAGAATG-3’ | 5’-ATGGTGGTGAAGACGCCAGTA-3’ |
Statistical analysis
The data are presented as mean ± SEM. Measure data were analyzed using one-way analysis of variance (ANOVA) and Student’s t-test was used for multiple comparisons of two sample means. P < 0.05 was considered statistically significant.
Results
PP2Ac is correlated with ECM protein expression
In the first in vivo experiment, we investigated the expression of PP2Ac, fibronectin (FN) and collagen I (Col-I) in rats with unilateral ureteral obstruction (UUO). Immunohistochemical staining showed that in the sham operation group, PP2Ac was weakly expressed in the normal distal and proximal tubule cells. On day 3 after UUO, PP2Ac expression in tubule epithelial cells and the interstitium markedly increased and reached a peak at 14 days after UUO. At the same time, the expression of FN and Col-I was also elevated accompanied with the prolongation of time after ligation of the left ureter (Figure 1A). Linear regression showed that PP2Ac expression was positively correlated with FN and Col-I expression (Figure 1B), suggesting that PP2Ac upregulation in the kidney could be involved in the pathogenesis of interstitial fibrosis. Therefore, we designed further experiments in vivo and in vitro to evaluate the role of PP2Ac in ECM accumulation and tubular EMT.
Figure 1.
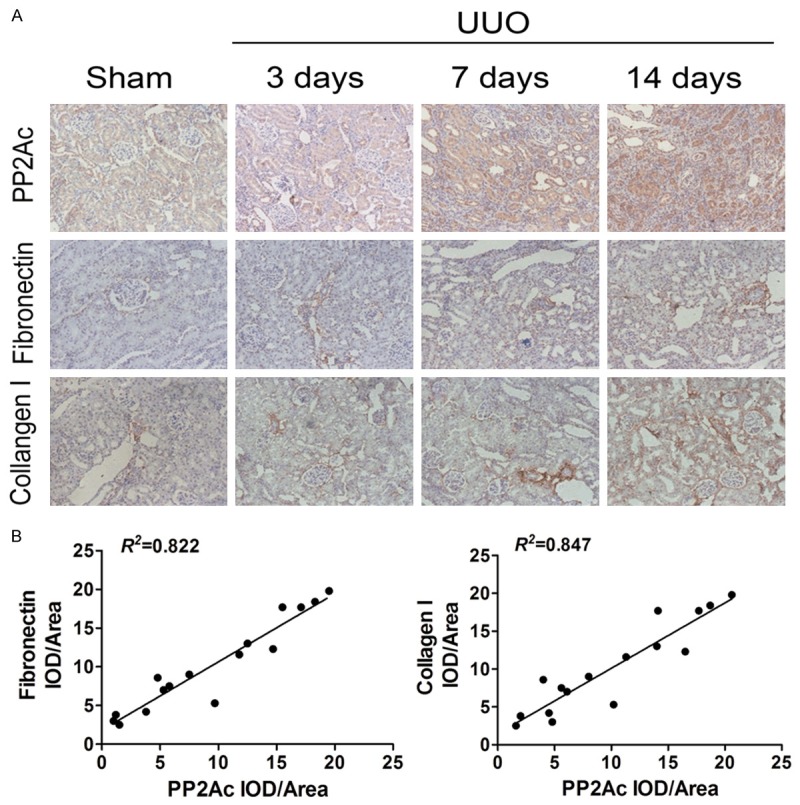
PP2Ac was positively correlated with FN and Col-I expression in an obstructive nephropathy model. A. Immunohistochemical staining showed the localization and expression of PP2Ac, FN and Col-I in the obstructed kidney at different time points after UUO. B. Linear regression showed a close correlation between PP2Ac and FN (or Col-I) expression. The correlation coefficient (R 2) is shown here.
Inhibition of PP2Ac by OA reduces ECM accumulation and reverses tubular EMT in vivo and in vitro
Based on the above results, we hypothesized that inhibiting PP2Ac could cause attenuation of renal fibrosis. To verify this, a phosphatase inhibitor, okadaic acid (OA), was used [21]. OA intervention was able to decrease the expression of PP2Ac, as well as FN and Col-I, in vivo. Meanwhile, there was a decrease in α-smooth muscle actin (α-SMA) expression and an increase in E-cadherin expression, as demonstrated by real-time RT-PCR and Western blot analysis, respectively (Figure 2A and 2B).
Figure 2.

PP2Ac inhibition by OA reduced ECM accumulation and reversed tubular EMT in vivo. A. Real-time RT-PCR analysis demonstrated that OA inhibited PP2Ac mRNA expression, while downregulating FN, Col-I, α-SMA mRNA expression and upregulating E-cadherin mRNA expression in the obstructed kidneys at 3 days after UUO. *P < 0.05 versus sham control; †P < 0.05 versus UUO alone (n = 5). B. Representative Western blots show that OA inhibited PP2Ac protein expression, while decreasing FN, Col-I, α-SMA protein expression and increasing E-cadherin protein expression in the obstructed kidneys at 3 days after UUO.
We then examined the ability of OA to inhibit TGF-β1-induced interstitial fibrosis in cultured HK-2 cells. HK-2 cells were treated with different concentrations of OA. On the basis of the viability and proliferation results of the trypan blue dye exclusion test and the MTT assay (Figure 3A and 3B), we selected 40 nM OA as the optimal concentration. Western blot analysis showed that, compared with the control group, PP2Ac protein expression was markedly increased after treatment with TGF-β1 for 24 h. Moreover, the expression of FN, COL-I and α-SMA proteins was also elevated, while E-cadherin protein expression was decreased. However, these indexes of tubulointerstitial fibrosis and EMT were dramatically blocked by administering OA (Figure 3C). Therefore, it was conceivable that renal interstitial fibrosis and tubular EMT could be attenuated by inhibiting PP2Ac.
Figure 3.
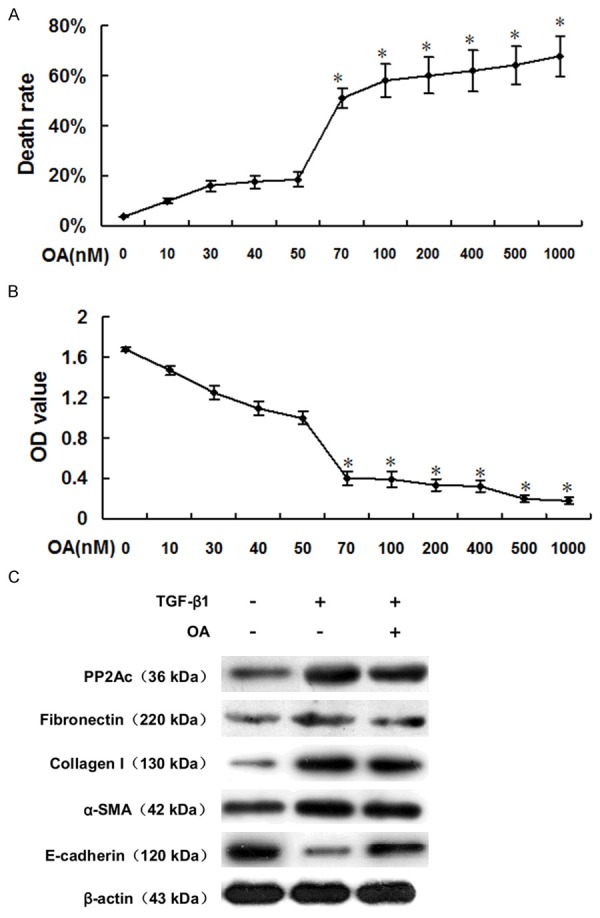
PP2Ac inhibition by OA reduced ECM accumulation and reversed tubular EMT in vitro. A. The trypan blue dye exclusion test showed that OA concentrations over 50 nM were cytotoxic to HK-2 cells. *P < 0.05 versus OA-untreated group. B. The MTT assay indicated that OA concentrations over 50 nM can inhibit TGF-β1-stimulated HK-2 cell proliferation. *P < 0.05 versus OA-untreated group. C. Representative Western blots show that OA reduced PP2Ac protein expression, while decreasing FN, Col-I, α-SMA protein expression and increasing E-cadherin protein expression in TGF-β1-stimulated HK-2 cells.
Ectopic expression of PP2Ac accelerates tubular ECM accumulation and promotes tubular EMT in vitro
To further elucidate the influence of PP2Ac on interstitial fibrosis, we then increased PP2Ac expression by transfecting HK-2 cells with a PP2Ac-overexpression plasmid to detect changes in tubular ECM and EMT. More than 70% of pEGFP-N1-PP2Ac-transfected cells contained intracellular green fluorescence, suggesting a successful introduction of PP2Ac-overexpression plasmid into HK-2 cells (Figure 4A). Real-time RT-PCR and Western blot analysis showed that, compared with the normal control, the expression of PP2Ac mRNA and protein in PP2Ac-overexpressed cells was significantly increased, along with FN, Col-I and α-SMA mRNA and protein expression, while E-cadherin mRNA and protein expression was decreased (Figure 4B and 4C). These results indicated that ectopic overexpression of PP2Ac was able to cause tubular ECM accumulation and EMT. Therefore, PP2Ac could be a promising target for reducing renal interstitial fibrosis.
Figure 4.
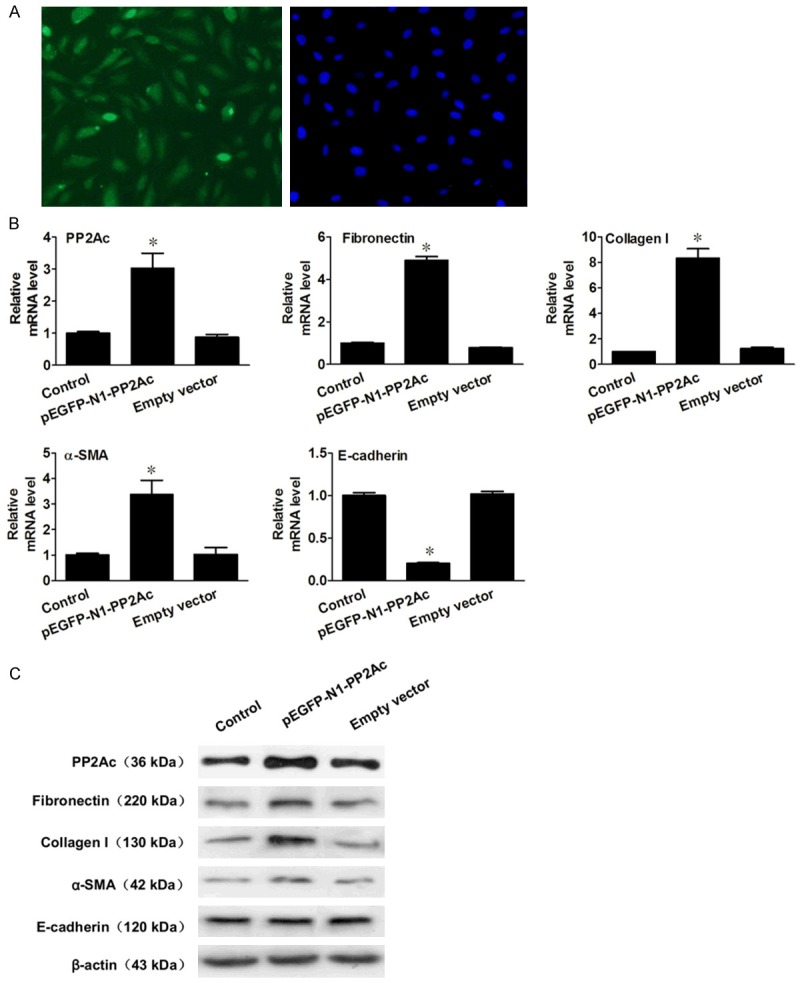
Ectopic expression of PP2Ac elevated FN and Col-I expression and changed the tubular cell phenotype in vitro. A. Representative micrograph shows that more than 70% of HK-2 cells contained intracellular green fluorescence. B. Ectopic expression of PP2Ac promoted induction of FN, Col-I and α-SMA and decrease of E-cadherin, as shown by real-time RT-PCR analysis. *P < 0.05 versus normal control. C. Representative Western blots show that in HK-2 cells transfected with pEGFP-N1-PP2Ac, the protein levels of FN, Col-I and α-SMA was upregulated and E-cadherin was downregulated.
NCTD inhibits PP2Ac expression and ameliorates renal interstitial fibrosis in vivo
NCTD was administered to rats after UUO, via intraperitoneal injection at doses of 0.05 and 0.1 mg/kg/day. Immunohistochemistry staining revealed that, compared with the UUO group, NCTD decreased PP2Ac expression, as well as FN and Col-I, in a dose-dependent manner, in vivo (Figure 5A). We then assessed the effect of NCTD on the expression of the tubular EMT regulatory genes, and we demonstrated that α-SMA expression was decreased and E-cadherin expression was increased (Figure 5A). Consistently, real-time RT-PCR and Western blot analysis results confirmed that NCTD could repress the expression of PP2Ac at both the mRNA and protein levels, while at the same time reducing ECM accumulation and reversing tubular EMT in a dose-dependent manner (Figure 5B and 5C). Taken together, these results suggest that NCTD can inhibit renal interstitial fibrosis and reverse tubular EMT in vivo by inhibiting PP2Ac expression.
Figure 5.
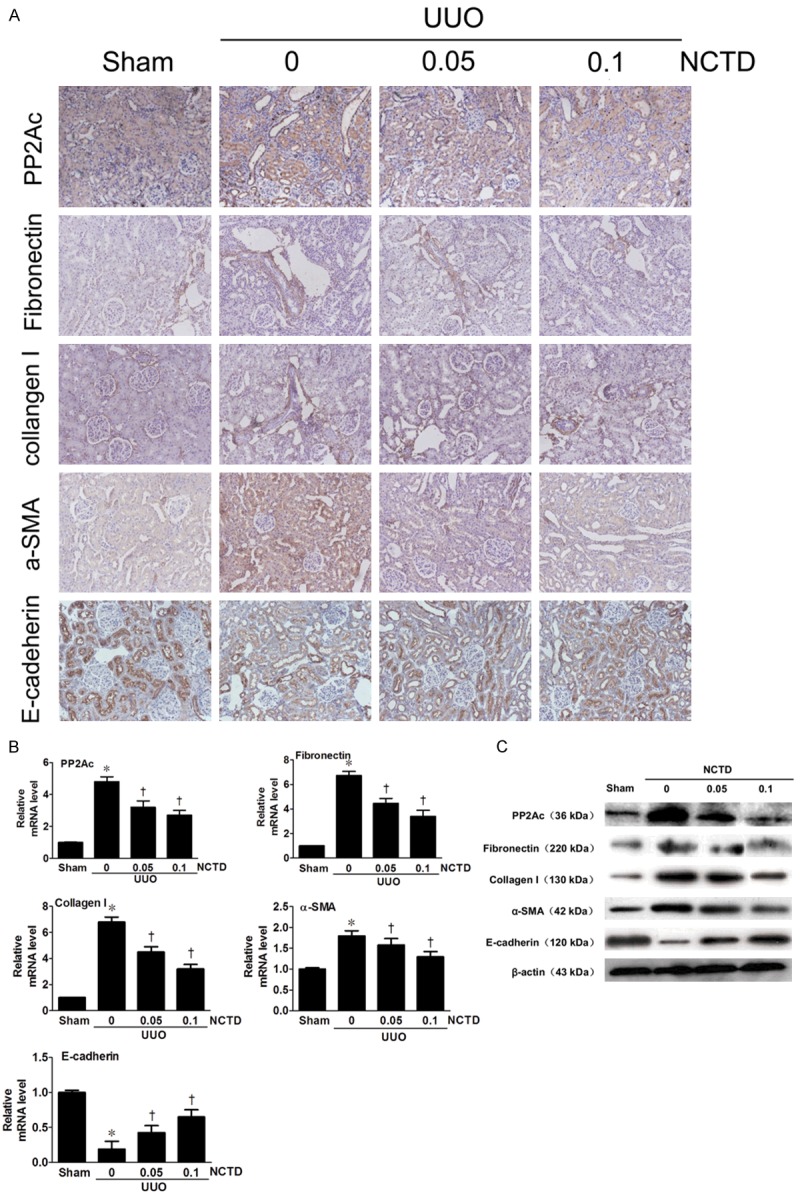
NCTD inhibited PP2Ac expression and ameliorated interstitial fibrosis after UUO. A. Representative micrographs show that NCTD inhibited PP2Ac expression and blocked ECM accumulation and tubular EMT, as illustrated by immunohistochemical staining for PP2Ac, FN, Col-I, α-SMA and E-cadherin in the obstructed kidneys at 14 days after UUO. *P < 0.05 versus sham control; †P < 0.05 versus UUO alone (n = 5). Numbers in brackets (0.05 and 0.1) indicate the dosages of NCTD used (in mg/kg body weight). B. Real-time RT-PCR analysis demonstrated that NCTD decreased the PP2Ac mRNA level, and reduced FN, Col-I, α-SMA mRNA and increased E-cadherin mRNA levels. C. Inhibition of PP2Ac by NCTD decreased FN, Col-I and α-SMA protein expression and increased E-cadherin protein expression in the obstructed kidneys, as shown by Western blot analysis.
Effects of NCTD on renal interstitial fibrosis in PP2Ac-overexpressing HK-2 cells
PP2Ac expression was manipulated in normal HK-2 cells by transfecting with a pEGFP-N1-PP2Ac vector to further elucidate the influence of PP2Ac expression and the protective effect of NCTD on interstitial fibrosis. Compared to the TGF-β1-stimulated cells, the results showed a further increase in PP2Ac expression caused by combined treatment with TGF-β1 and pEGFP-N1-PP2Ac transfection, which led to an aggravated renal fibrosis phenotype. However, the presence of NCTD was able to ameliorate the TGF-β1-induced pro-fibrotic phenotype. Real-time RT-PCR and Western blot analysis showed that NCTD effectively downregulated the expression of PP2Ac, thereby blocking PP2Ac-mediated FN, Col-I and α-SMA induction and E-cadherin suppression (Figure 6A and 6B). Taken together, these results suggested that the inhibitory effect of NCTD on renal interstitial fibrosis was related to its inhibition on PP2Ac expression.
Figure 6.
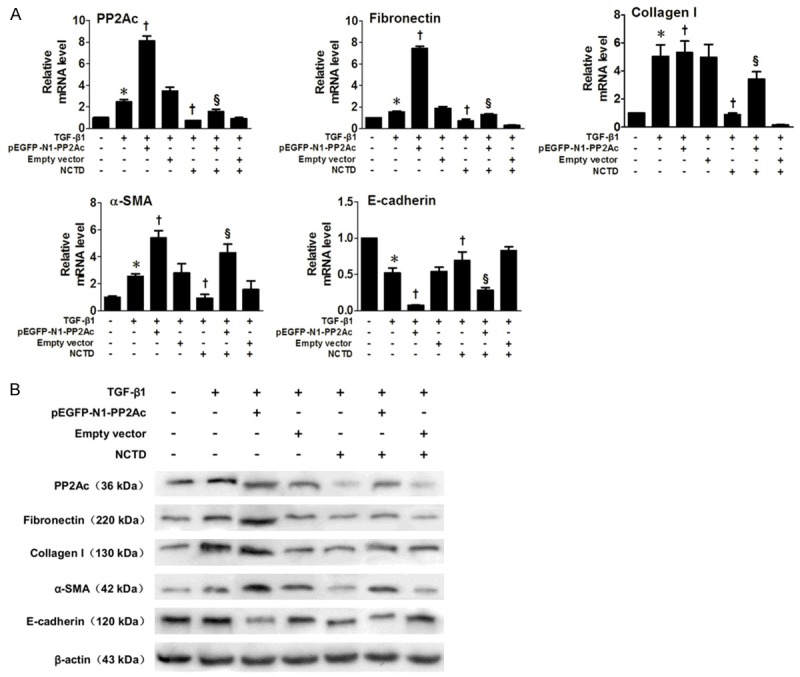
Effects of NCTD on indexes of Interstitial Fibrosis and EMT in PP2Ac-overexpressed HK-2 Cells. A. Real time RT-PCR showed that a further increase of PP2Ac expression, caused by incubation with TGF-β1 after transfection of pEGFP-N1-PP2Ac, led to an increase of ECM and EMT markers. NCTD effectively downregulated the expression of PP2Ac and, moreover, blocked the PP2Ac-mediated FN, Col-I and α-SMA induction and E-cadherin suppression. *P < 0.05 versus normal control; †P < 0.05 versus TGF-β1 stimulated group; §P < 0.05 versus TGF-β1 plus pEGFP-N1-PP2Ac. B. Western blots confirm the results of real time RT-PCR.
Effects of NCTD on renal interstitial fibrosis in PP2Ac shRNA fransfected HK-2 cells
To further clarify the role of PP2Ac inhibition in the anti-fibrotic effect of NCTD, we analyzed the effects of NCTD on HK-2 cells with or without knocking down PP2Ac by SD11-PP2Ac shRNA. We showed that, compared with the HK-2 cells without transfection, or those transfected with an empty vector, PP2Ac expression was clearly depleted by the specific PP2Ac knockdown (Figure 7A-C). Both NCTD and SD11-PP2Ac shRNA inhibited PP2Ac protein expression, and induced subsequent downregulation of FN, Col-I and α-SMA protein expression and upregulation of E-cadherin, indicating an abrogated pro-fibrotic and EMT phenotype induced by TGF-β1. However, compared with single SD11-PP2Ac shRNA transfection, combined-treatment of SD11-PP2Ac shRNA transfection and NCTD incubation showed no further decrease in FN, Col-I and α-SMA protein expression or increase in E-cadherin expression, which indicated that NCTD had no significant anti-fibrotic effect after knockdown of PP2Ac (Figure 7D and 7E). These results demonstrated that NCTD inhibits renal interstitial fibrosis by specifically inhibiting PP2Ac expression.
Figure 7.
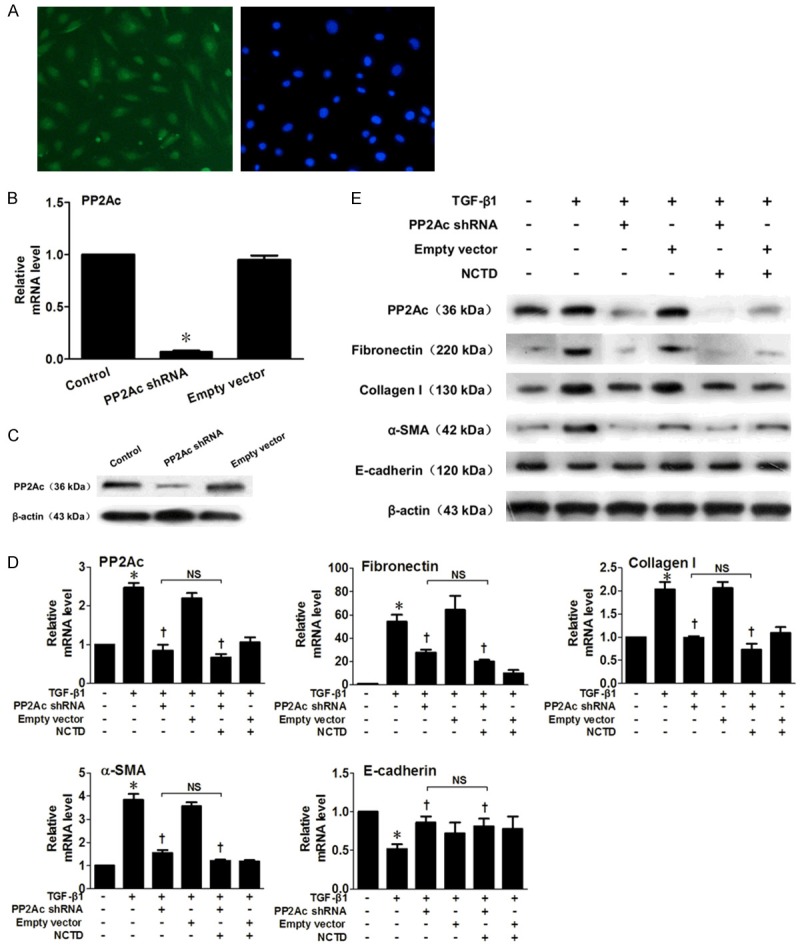
Effects of NCTD on Interstitial Fibrosis and EMT indicators in PP2Ac shRNA HK-2 Cells. A. Representative micrograph shows more than 80% of HK-2 cells contained intracellular green fluorescence. B. The mRNA level of PP2Ac was clearly depleted by SD11-PP2Ac transfection. C. The protein level of PP2Ac was clearly reduced by SD11-PP2Ac transfection. D. Real-time RT-PCR shows that NCTD and SD11-PP2Ac shRNA inhibited PP2Ac mRNA expression, while FN, Col-I and α-SMA mRNA levels were also downregulated, while E-cadherin mRNA expression was upregulated. However, cells transfected with SD11-PP2Ac shRNA and incubated with NCTD showed no further inhibition of FN, Col-I and α-SMA mRNA levels or increase of E-cadherin. *P < 0.05 versus normal control; †P < 0.05 versus TGF-β1 stimulated group; NS, no significant difference. E. Western Blots confirm the results of real-time RT-PCR.
Discussion
The major pathological features of renal interstitial fibrosis include accumulation of ECM, inflammatory cell infiltration, tubular atrophy and interstitial angiogenesis [4]. Recently, the role of renal tubular epithelial cell EMT in the process of renal interstitial fibrosis has been emphasized. Tubular epithelial cells secrete a large amount of ECM after EMT, which can aggravate renal interstitial fibrosis [3]. Therefore, both ameliorating ECM deposition and reversing tubular EMT may be effective ways to inhibit renal interstitial fibrosis. NCTD is the derivative of cantharidin, a traditional Chinese medicine, which is mainly used in the treatment of gastrointestinal tumors. NCTD displays a significantly reduced irritation of the urinary system compared with cantharidin, while maintaining its antitumor activity, along with its unique effect of elevating white blood cells. Some in vitro investigations have observed the effect of NCTD on tumor cells and normal cells, and reported that normal cells can resist the NCTD cytotoxic effect without showing DNA fragmentation, which indicates that the use of NCTD could be extended to the non-tumor treatment area [22,23]. Our previous studies have demonstrated that NCTD effectively reduces renal interstitial fibrosis in rat models of protein overload nephropathy, diabetic nephropathy and obstructive nephropathy. In addition, our in vitro study has found that NCTD inhibits TGF-β/Smad3 signal pathway-mediated EMT [11-14]. However, the underlying molecular mechanism involved in this process is still unclear.
Hill et al. demonstrated- through crystal structure analysis- that NCTD is a protein phosphatase inhibitor, which inhibits serine/threonine protein phosphatases, including PP1, PP2A, and PP2B [24]. We previously demonstrated that the anti-fibrotic effect of NCTD was unrelated to its inhibition on PP2B [25]. Meanwhile, it has been reported that NCTD has a stronger inhibitory effect on PP2A than PP1 (IC50 2.69 vs 10.3 μM) [26]. PP2A plays an important role in cell cycle regulation, cell growth and signal transduction [27]. PP2Ac, the catalytic subunit of PP2A, is translationally regulated to maintain a constant level, and is essential for the dephosphorylation activity of PP2A [28]. PP2Ac is globular in structure, ubiquitously expressed in almost every tissue, with the most abundant levels in the heart and brain. In the adult kidney, PP2A protein is mainly detected in the nuclei of distal tubular cells [20]. However, the physiological function of PP2A in the kidney is not very clear. It has been reported that PP2A is involved in the regulation of Na+-K+-ATPase in cortical collecting duct cells [29], and plays a role in cell death induced by simulated ischemia in renal tubule epithelial cells [30]. Regarding the underlying molecular mechanism of PP2A, it has been reported to be implicated in regulating TGF-β signal transduction [31]. However, the role of PP2A in renal interstitial fibrosis is unknown.
This study detected the expression of PP2Ac in the kidneys of UUO rats at different time points after ureter ligation and found that PP2Ac expression in distal tubule and proximal tubule cells was elevated accompained with prolonged obstruction time. PP2Ac expression was shown to be positively correlated with the expression of FN and Col-I. In vivo and in vitro experiments showed that OA, a specific PP2Ac inhibitor, can ameliorate renal interstitial fibrosis by inhibiting the expression of FN and Col-I and reversing tubular EMT. These results indicated that renal interstitial fibrosis could be attenuated by inhibiting PP2Ac expression. Further in vitro experiments also showed that PP2Ac over-expression had a pro-fibrotic effect in HK-2 cells. These results suggested that PP2Ac may be a promising target for treatment of renal interstitial fibrosis.
In the next investigation, we demonstrated that NCTD could decrease the expression of FN, Col-I and α-SMA expression, in a dose-dependent manner, while increasing the expression of E-cadherin in UUO rats, which was similar to the results of our previous studies [11-14]. In addition, NCTD was also able to inhibit PP2Ac expression in a dose-dependent manner. Moreover, we found that NCTD downregulated FN, Col-I, and α-SMA expression, and upregulated E-cadherin expression in TGF-β1-stimulated and PP2Ac-overexpressed HK-2 cells. In particular, our results showed that there was no difference in the expression of FN, Col-I, α-SMA and E-cadherin between cells treated with NCTD alone and cells treated with NCTD combined with PP2Ac shRNA transfection. These results suggested that the anti-fibrotic effect of NCTD was dependent on its inhibition on PP2Ac.
PP2A is generally considered to be a tumor suppressor gene, which has been found to be genetically altered or functionally inactivated in many solid cancers and leukemias [32,33]. PP2A has also been demonstrated to be involved in the regulation of cell cycle and apoptosis of cancer cells [34]. Restoration of PP2A activity results in inhibition of cell proliferation [32] and increased sensitivity to anti-cancer drugs [35]. Therefore, PP2A is considered to be a protective factor in the pathogenesis of cancer. However, our investigation using this renal fibrosis model demonstrated that the PP2A activation leads to aggravated renal interstitial fibrosis, suggesting that the role of PP2A in renal interstitial fibrosis is different from that in cancer. However, the exact mechanism is unknown. It can be inferred that the effect of PP2A may be cell type- and subunit-dependent due to its complex structure, function and regulation.
On the basis of the data described above, the role of PP2Ac in renal interstitial fibrosis was confirmed. We also demonstrated that NCTD exerts its anti-fibrotic effect by inhibiting PP2Ac. Thus, inhibiting PP2Ac expression by NCTD may represent a specific and effective therapeutic strategy for the treatment of renal interstitial fibrosis. However, further studies are still required to provide additional evidence for the use of NCTD as a potential drug against renal interstitial fibrosis.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81100486 and 81370792), Hunan Provincial Science and Technology Program of China (Grant No. 2013SK3036), and Clinical Special Fund of Chinese Medical Association (Grant No. 13030400425).
Disclosure of conflict of interest
None.
References
- 1.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304:C216–225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7:4. doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decleves AE, Sharma K. Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol. 2014;10:257–267. doi: 10.1038/nrneph.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26:147–162. doi: 10.1016/0378-8741(89)90062-7. [DOI] [PubMed] [Google Scholar]
- 8.Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, Mao YQ, Kan B, Lei S, Wang GS, Jiang Y, Wang QR, Luo F, Zou LQ, Liu JY. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol. 2002;128:223–230. doi: 10.1007/s00432-002-0326-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Ji Q, Liu X, Chen X, Chen Z, Qiu Y, Sun J, Cai J, Zhu H, Li Q. Norcantharidin inhibits tumor angiogenesis via blocking VEGFR2/MEK/ERK signaling pathways. Cancer Sci. 2013;104:604–610. doi: 10.1111/cas.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massicot F, Dutertre-Catella H, Pham-Huy C, Liu XH, Duc HT, Warnet JM. In vitro assessment of renal toxicity and inflammatory events of two protein phosphatase inhibitors cantharidin and nor-cantharidin. Basic Clin Pharmacol Toxicol. 2005;96:26–32. doi: 10.1111/j.1742-7843.2005.pto960104.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu FY, Li Y, Peng YM, Ye K, Li J, Liu YH, Duan SB, Ling GH, Xu XQ, Zhou LT. Norcantharidin ameliorates proteinuria, associated tubulointerstitial inflammation and fibrosis in protein overload nephropathy. Am J Nephrol. 2008;28:465–477. doi: 10.1159/000112850. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Chen Q, Liu FY, Peng YM, Hou T, Duan SB, Li J, Luo JH, Sun L, Ling GH. Norcantharidin attenuates tubulointerstitial fibrosis in rat models with diabetic nephropathy. Ren Fail. 2011;33:233–241. doi: 10.3109/0886022X.2011.553305. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Sun Y, Liu F, Sun L, Li J, Duan S, Liu H, Peng Y, Xiao L, Liu Y, Xi Y, You Y, Li H, Wang M, Wang S, Hou T. Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition. PLoS One. 2013;8:e66356. doi: 10.1371/journal.pone.0066356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Liu FY, Peng YM, Zhan M, Duan SB, Li J, Sun L, Ye K, Luo JH. Norcantharidin inhibits proliferation and fibronectin expression of HK-2 cells induced by albumin in vitro. Cell Biol Int. 2011;35:1239–1241. doi: 10.1042/CBI20100850. [DOI] [PubMed] [Google Scholar]
- 15.Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- 16.Deng L, Tang S. Norcantharidin analogues: a patent review (2006 - 2010) Expert Opin Ther Pat. 2011;21:1743–1753. doi: 10.1517/13543776.2011.629190. [DOI] [PubMed] [Google Scholar]
- 17.Kadioglu O, Kermani NS, Kelter G, Schumacher U, Fiebig HH, Greten HJ, Efferth T. Pharmacogenomics of cantharidin in tumor cells. Biochem Pharmacol. 2014;87:399–409. doi: 10.1016/j.bcp.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Peng B, Li Y, Lei N, Li W, Zhang JY. p90/CIP2A mediates breast cancer cell proliferation and apoptosis. Mol Biol Rep. 2014;41:7471–7478. doi: 10.1007/s11033-014-3635-2. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj A, Singh S, Srivastava SK, Arora S, Hyde SJ, Andrews J, Grizzle WE, Singh AP. Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. Br J Cancer. 2014;110:2000–2010. doi: 10.1038/bjc.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett AD, Xue C, Stoops T. Developmental expression of protein phosphatase 2A in the kidney. J Am Soc Nephrol. 1999;10:1737–1745. doi: 10.1681/ASN.V1081737. [DOI] [PubMed] [Google Scholar]
- 21.Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Köhler A, Glossmann H, Schneider R, Sutherland C, Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, Lee MS, Hsiao M, Lin SK. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol. 2003;39:19–26. doi: 10.1016/s1368-8375(01)00129-4. [DOI] [PubMed] [Google Scholar]
- 23.Liu XH, Blazsek l, Comisso M, Legras S, Marion S, Quittet P, Anjo A, Wang GS, Misset JL. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer. 1995;31A:953–963. doi: 10.1016/0959-8049(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 24.Hill TA, Stewart SG, Gordon CP, Ackland SP, Gilbert J, Sauer B, Sakoff JA, McCluskey A. Norcantharidin analogues: synthesis, anticancer activity and protein phosphatase 1 and 2A inhibition. Chem Med Chem. 2008;3:1878–1892. doi: 10.1002/cmdc.200800192. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Chen Q, Liu FY, Peng YM, Wang S, Li J, Li J, Duan SB, Sun L, Ling GH, Luo JH. Norcantharidin inhibits the expression of extracellular matrix and TGF-beta1 in HK-2 cells induced by high glucose independent of calcineurin signal pathway. Lab Invest. 2011;91:1706–1716. doi: 10.1038/labinvest.2011.119. [DOI] [PubMed] [Google Scholar]
- 26.Stewart SG, Hill TA, Gilbert J, Ackland SP, Sakoff JA, McCluskey A. Synthesis and biological evaluation of norcantharidin analogues: towards PP1 selectivity. Bioorg Med Chem. 2007;15:7301–7310. doi: 10.1016/j.bmc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pais SM, Tellez lnon MT, Capiati DA. Serine/threonine protein phosphatases type 2A and their roles in stress signaling. Plant Signal Behav. 2009;4:1013–1015. doi: 10.4161/psb.4.11.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blot-Chabaud M, Coutry N, Laplace M, Bonvalet J, Farman N. Role of protein phosphatase in the regulation of Na+-K+-ATPase by vasopressin in the cortical collecting duct. J Membr Biol. 1996;153:233–239. doi: 10.1007/s002329900126. [DOI] [PubMed] [Google Scholar]
- 30.Tsao CC, Nica AF, Kurinna SM, Jiffar T, Mumby M, Ruvolo PP. Mitochondrial protein phosphatase 2A regulates cell death induced by simulated ischemia in kidney NRK-52E cells. Cell Cycle. 2007;6:2377–2385. doi: 10.4161/cc.6.19.4737. [DOI] [PubMed] [Google Scholar]
- 31.Van Berlo JH, Vonckken JW, Kubben N, Broers JL, Duisters R, van Leeuwen RE, Crijns HJ, Ramaekers FC, Hutchison CJ, Pinto YM. A-type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum Mol Genet. 2005;14:2839–2849. doi: 10.1093/hmg/ddi316. [DOI] [PubMed] [Google Scholar]
- 32.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, Roy DC, Valtieri M, Bruner-Klisovic R, Caligiuri MA, Bloomfield CD, Marcucci G, Perrotti D. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABLregulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Janghorban M, Farrell AS, Allen-Petersen BL, Pelz C, Daniel CJ, Oddo J, Langer EM, Christensen DJ, Sears RC. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc Natl Acad Sci U S A. 2014;111:9157–9162. doi: 10.1073/pnas.1317630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang CY, Chao TT, Chang FY, Chen YL, Tsai YT, Lin HI, Huang YC, Shiau CW, Yu CJ, Chen KF. CIP2A mediates erlotinib-induced apoptosis in non-small cell lung cancer cells without EGFR mutation. Lung Cancer. 2014;85:152–160. doi: 10.1016/j.lungcan.2014.05.024. [DOI] [PubMed] [Google Scholar]


