Abstract
A novel membrane for guided bone regeneration (GBR), constituting silk fibroin (SF) nanofiber from native silk nanofibril solution, was prepared by electrospinning process. Another barrier membrane, a collagen-type membrane (Bio-Gide®), was used as a comparative sample. Twelve healthy male Sprague-Dawley rats were used in this study. Bilateral round defects were created in the calvarial bone. The bone regenerative efficacy was evaluated in rat calvarial defects. Animals were killed at 4 and 12 weeks. Bone regeneration was analyzed using micro-computed tomography and histological analysis. The SF nanofibrous membrane showed superior results with regard to mechanical tensile properties. At 4 weeks, the bone volume and collagen I positive areas in the SF group were greater than in the Bio-Gide group. At 12 weeks, the defect had completely healed with new bone in both the groups. In conclusion, the SF nanofibrous membranes showed satisfactory mechanical stability, good biocompatibility, slow degradability, and improved new bone regeneration without any adverse inflammatory reactions. Considering the low cost and low risk of disease transmission, the SF nanofibrous membrane is a potential candidate for GBR therapy compared with the widely used collagen membranes.
Keywords: Silk fibroin nanofiber, guided bone regeneration (GBR), rat calvarial defects
Introduction
Guided bone regeneration (GBR) is a widely used strategy to preserve and reconstruct alveolar bony defects. The GBR technique uses a membrane that serves as a barrier to resist the migration of fast growing epithelial and connective tissues into the bony defect. Meanwhile, the membrane maintains a secluded space to allow the necessary time for osteogenic cell proliferation and new bone formation [1]. The ideal GBR membrane needs to satisfy the following criteria such as biocompatibility, space creation and maintenance, ability to exclude epithelial and connective tissues, osteogenesis, proper rate for degradation, clinical manageability, and cost-effectiveness [2].
In general, two types of GBR membranes (resorbable and nonresorbable) are available based on their degradation characteristics. The nonresorbable membranes (e.g. expanded polytetrafluoroethylene) have desirable correlation between the level of bone regeneration and maintenance of space. However, they need a secondary surgical procedure for removing the membrane, which may result in additional discomfort, infection, and increased economic burden [3]. To overcome these problems, all types of resorbable membranes have been developed. These membranes are usually made of polyglycolic acid, polylactic acid, polycaprolactone, and their copolymers or tissue-derived collagens [4]. Although these polymeric membranes show positive results in clinical studies, their poor osteogenesis and host immune reactivity are still major drawbacks [5]. In contrast, collagen membranes show better bone regenerative results due to their excellent biocompatibility and cell affinity. However, the collagen derived from animal sources may have problems such as disease transmission, increased cost, and ethical and cultural issues. Moreover, its low mechanical strength and variable rate of degradation are the concerns of many clinicians [6]. Because of these shortcomings or defects in currently used membranes, new composite biomaterial with better properties is required.
Silk fibroin (SF) is a promising substrate for bone tissue engineering because of its useful properties such as biocompatibility, biodegradability, good oxygen and water vapor permeability, high tensile strength, and noninflammatory characteristics [7-9]. Recently, some researchers have investigated the SF as a membrane for GBR, and the SF showed some encouraging results [10,11], suggesting that SF can be a promising biomaterial for GBR membranes. To improve the biological performance, silk has been processed into nanofibrous membrane by electrospinning, which significantly improved cell adhesion and proliferation in vitro and bone defect regeneration in vivo [12].
In spite of the numerous advantages of SF nanofibers as biomedical materials, its application is largely restricted due to its poor mechanical properties resulting from a breakdown of the peptide chain by specific solvent [13]. Recently, a new strategy to fabricate SF nanofibers with improved mechanical properties by dissolving silk in calcium chloride-formic acid (CaCl2-FA), which features preservation of nanofibrils, has been reported [14,15]. Therefore, it was hypothesized that SF nanofibrous membranes prepared by this method would have enough mechanical stability to maintain the space for bone regeneration and similar or better efficacy than widely used collagen membranes such as Bio-Gide® (Geistlich Pharma AG, Wolhusen, Switzerland). Bio-Gide is a type of commercial collagen membrane that has been successfully used in GBR techniques.
Thus, in this study, the effectiveness of the novel SF nanofibrous membrane derived from CaCl2-FA as GBR membrane was evaluated in a rat model of bone defect repair. Meanwhile, Bio-Gide membrane was used as a positive control. Later, surface morphology and mechanical properties were characterized and the efficacy of GBR of SF nanofibrous membranes and Bio-Gide membranes was compared in rat calvarial defect models.
Materials and methods
SF nanofibrous membranes
SF nanofibrous membranes were prepared as per our previous study [14,15]. Briefly, SF electrospin solution was prepared by dissolving degummed silk in 98% FA containing 2% lithium bromide (LiBr) at a concentration of 10% w/v. This SF-LiBr-FA solution was used for electrospinning with the following electrospin parameters: needle spinneret diameter 0.42 mm, rate of injection 1 mL/h, working distance 10 cm, voltage 15 kV. After 20 h preparation to obtain 0.4-mm-thick SF nanofibrous membranes, the collected membranes were treated with 75% v/v ethanol to induce structural transition. Finally, SF nanofibrous membranes were sterilized using gamma irradiation at standard dose of 25 kGy for animal experiment.
Morphological observation
The morphology of electrospun SF nanofibrous membranes and Bio-Gide membranes (the spongy layer) was observed using a scanning electron microscope (SEM) (S-4800; Hitachi, Japan) at 20°C, 60% relative humidity (RH).
Structural analysis
The structure of electrospun SF was observed using a Magna spectrometer (NicoLET 5700). The spectra were obtained in the spectral region of 400-4000 cm-1. The powder of electrospun SF nanofibrous membrane was pressed into potassium bromide pellets prior to collection of data.
Mechanical properties
The mechanical properties of electrospun SF nanofibrous membranes and collagen membranes (Bio-Gide) (25 mm × 13 mm) were obtained using a universal texting machine (Instron 3365; Instron, Norwood, MA) (gauge length: 20 mm; cross-head speed: 0.2 mm/s) at 25±0.5°C, 60±5% RH. At least five measurements for each sample were performed in the testing.
Animal surgery for in vivo test
Twelve healthy male Sprague-Dawley rats with a mean weight of 250 g (≈7-8 weeks) were used in this study. This entire study protocol was approved by the Animal Care and Experiment Committee of Institute of Soochow University (Suzhou, China). Rats were provided general anesthesia using celiac injections of 4% chloral hydrate (1 mL/100 g body weight; Sigma-Aldrich). Once fully anesthetized, the site of surgery was shaved and disinfected with povidone-iodine, and a longitudinal incision was made in the skull from the nasal to the occipital region. After separating the skin and muscle, the calvarial surface on both sides of the midline was exposed. A dental-trephine bur (5 mm in diameter; Dentium, Korea) was used to treat bilateral full-thickness calvarial defects under sterile saline irrigation (Figure 3A). During the punching process, great care was taken to avoid perforation of the dura mater. Later the SF and Bio-Gide membranes were trimmed into squares (6 mm × 6 mm in size) to fit the calvarial defects well. The defects on the left were covered with SF nanofibrous membranes, and those on the right were covered with Bio-Gide (Figure 3B). The pericranium and skin were sutured in layers with 3-0 silk sutures. After surgery, the rats were caged and received food and water individually. At different time points of healing (4 or 12 weeks), the animals were killed by injecting an overdose of pentobarbital sodium, and then the calvarial samples including both the defects, the membranes, and the surrounding tissue were removed from the bodies. These samples were fixed with 4% paraformaldehyde for 24 h at room temperature.
Figure 3.
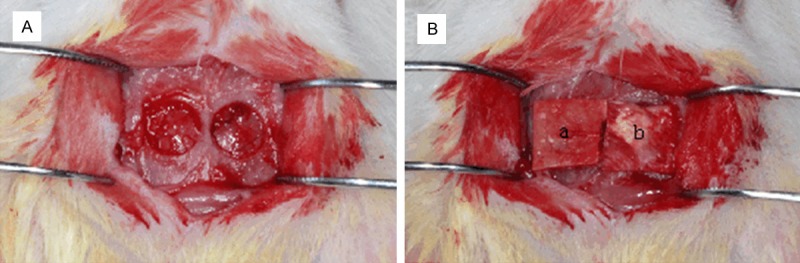
Establishment of rat calvarial defect model. (A) Before covering with GBR membranes; (B) SF nanofibrous membranes (a) and Bio-Gide® membranes (b) covering the bone defect regions. GBR, guided bone regeneration; SF, silk fibroin.
Micro-computed tomography analysis
The prepared samples were scanned using a micro-computed tomography (CT) (SkyScan 1176; Bruker-microCT, Kontich, Belgium). The scanning conditions were set at voltage 65 kV, current 100 μA, duration of exposure 600 ms, and Al filter 1 mm. The width of scanning was 50 mm and the axis of ray was vertical to the bone defect surface. The system software was used to reconstruct three-dimensional images. The upper and lower threshold values for bone were 255 and 85 grey. Because the initial bone defect was round and 5 mm diameter, the region of interest (ROI) was selected to reflect the initial shape. The ROI of each sample was analyzed for bone volume (BV) and bone mineral density (BMD).
Histological and immunohistochemical staining
Following micro-CT testing, samples were decalcified in 10% ethylenediaminetetraacetic acid for 2-4 weeks and dehydrated in a graded series of ethanol. Later samples were embedded in paraffin and cut into 5-µm sections from the central area of the bony defects using a microtome (Lecia RM2235 microtome; Lecia, Wetzlar, Germany). For histological staining, the sections were stained with hematoxylin and eosin (H&E) and then were evaluated using a microscope. For immunohistochemical staining, the sections were incubated with primary rabbit anti-rat collagen I antibody (Biosynthesis Biotechnology, Beijing, China) at 5 µg/mL for 60 min. Later, the sections were washed with phosphate-buffered saline three times. The secondary antibody was accomplished using the Vectastain Elite ABC kit (Vector, Burlingame, CA). These stained sections were also evaluated under microscope (Axioveter 40CFL; Zeiss, Germany). The percentage of positive areas in each section was calculated using the Image-J software.
Statistical analysis
All quantitative data were expressed as mean±standard deviation. Comparisons of the data between the groups over time were performed using analysis of variance tests with Tukey’s post hoc test. Statistical significance was noted at P<0.05.
Results
Characterization of electrospun SF nanofibrous membrane
SEM images of electrospun SF nanofibrous membranes and Bio-Gide membranes are shown in Figure 1. The SF nanofibrous membrane comprises randomly oriented nanofibers. The nanofibrous diameter was 200 nm to 600 nm. It has a porous structure, wide distribution of pore size, and a large surface area to volume ratio. The spongy layer of Bio-Gide membrane was denser and less porous than the SF nanofibrous membrane.
Figure 1.
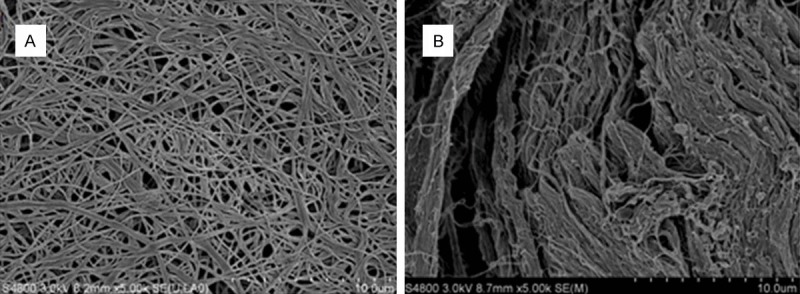
SEM images of electrospun SF nanofibrous membranes (A) and Bio-Gide® membranes (B). SEM, scanning electron microscope; SF, silk fibroin.
The results of the Fourier transform infrared spectroscopy spectra are shown in Figure 2. The as-spun SF nanofibrous membrane showed three absorption peaks at 1655 cm-1 (amide I), 1543 cm-1 (amide II), and 1250 cm-1 (amide III), which indicates the random coil or helical conformation.
Figure 2.
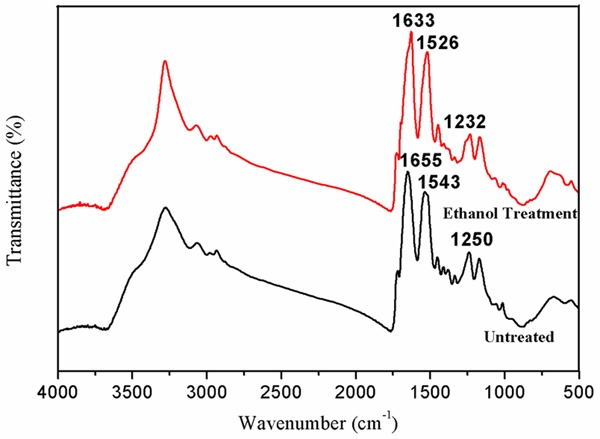
FTIR spectra of SF nanofibrous electrospun membrane. FTIR, Fourier transform infrared spectroscopy; SF, silk fibroin.
However, after treatment with 75% v/v, the structure of the SF nanofibrous membrane transferred to Silk II with the absorption peaks at 1633 cm-1 (amide I), 1526 cm-1 (amide II), and 1232 cm-1 (amide III), which could improve the stability and mechanical properties.
The results of the tensile testing are shown in Table 1. The breaking elongation and strength of the SF nanofibrous membrane (ethanol treated) were 46.5% and 9.4 Mpa, respectively, compared with 31.35% and 7.78 Mpa in the Bio-Gide group. The SF nanofibrous membranes had higher tensile strength than Bio-Gide (P<0.05).
Table 1.
Mechanical Properties of SF nanofibrous membrane and Bio-Gide® membrane
| Breaking elongation (%) | Breaking strength (Mpa) | |
|---|---|---|
| SF nanofibrous membrane | 46.5 | 9.4 |
| Collagen membranes (Bio-Gide®) | 31.35 | 7.78 |
SF, silk fibroin.
General observation of animals
All rats recovered well from the surgery and remained in good health until the end of the study. No significant weight reduction, rejection of the membranes, and other postoperative infection were noted.
Micro-CT analysis
Data and images from micro-CT analysis are presented in Figures 4 and 5. Obvious bone regeneration was found in both groups at 4 and 12 weeks after surgery. At 4 weeks, the BV value in the SF group was 3.52±0.68 mm3, which was greater than (2.23±0.89 mm3) that in the Bio-Gide group. The difference was statistically significant (P<0.05). At 12 weeks, the BV value in the SF group was 9.38±2.52 mm3 and in the Bio-Gide group it was 10.56±2.08 mm3. However, the difference was not statistically significant (P>0.05). The BMD values in both groups at 4 and 12 weeks were almost the same. No statistically significant difference was noted (P>0.05).
Figure 4.
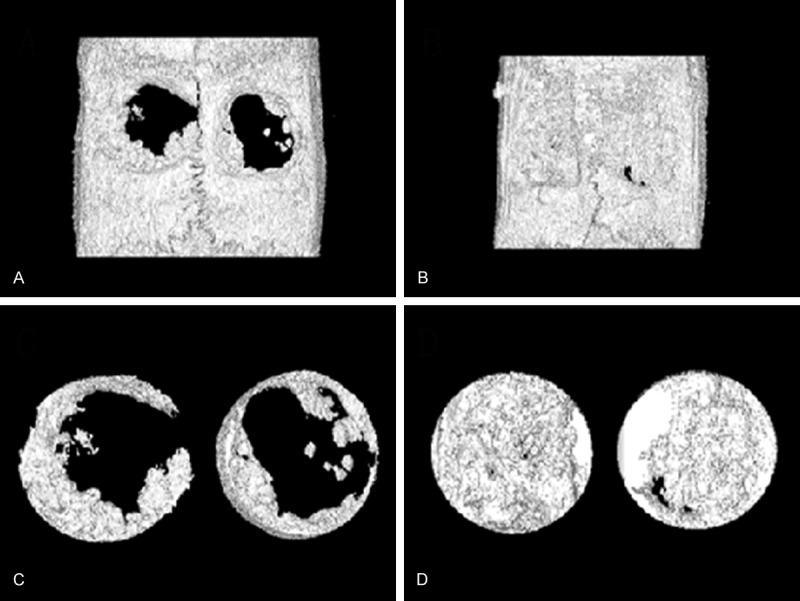
Reconstructions of bone defects using micro-CT. Bone defects were covered by SF nanofibrous membranes (left) and Bio-Gide® membranes (right). (A) The front image of operated specimen at 4 weeks; (B) The front image of operated specimen at 12 weeks; (C) The ROI image of operated specimen at 4 weeks. (D) The ROI image of operated specimen at 12 weeks. CT, computed tomography; ROI, region of interest; SF, silk fibroin.
Figure 5.
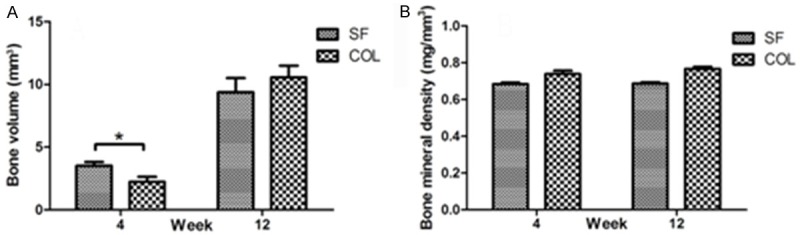
Analysis of new bone formation using micro-CT at 4 weeks and 12 weeks postoperatively. (A) Bone volume; (B) Bone mineral density. Data are mean±SD (n=6, *P<0.05). CT, computed tomography; SD, standard deviation.
Histological evaluation
H&E staining of rat calvarial defects covered by the SF or Bio-Gide membranes is shown in Figure 6. At 4 weeks after surgery, a considerable amount of new bone was found from the margins of calvarial defects in both the groups. Additionally, the bone formation seemed to increase with time, and completely bridged the defect after 12 weeks. No sign of abnormal tissue response was observed.
Figure 6.
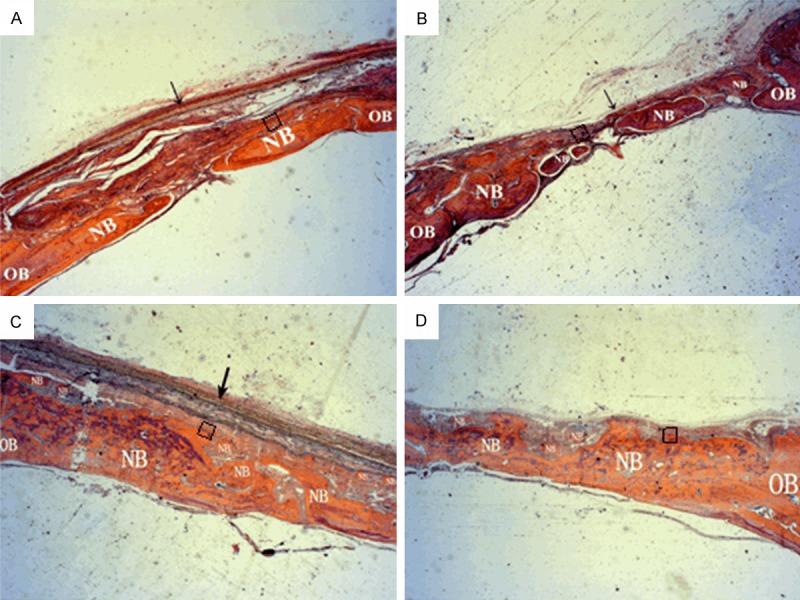
H&E histological staining of rat calvarial defects covered by SF nanofibrous membranes or Bio-Gide® membranes after 4 and 12 weeks. (A) SF nanofibrous membrane at 4 weeks postoperatively; (B) Bio-Gide membranes at 4 weeks postoperatively; (C) SF nanofibrous membrane at 12 weeks postoperatively; (D) Bio-Gide membranes at 12 weeks postoperatively (magnification × 25). Arrows: membranes; H&E, hematoxylin and eosin; NB: new bone; OB: original bone; SF, silk fibroin.
The results of collagen I antibody immunostaining are shown in Figures 7 and 8. At 4 weeks after surgery, moderate collagen I positive signals were found in newly formed woven bone. The percentage of collagen I positive areas in the SF group was significantly higher than those in the Bio-Gide group (P<0.05). After 12 weeks, strong collagen I signals were found in the both groups. These two types of staining results were consistent with micro-CT analysis.
Figure 7.
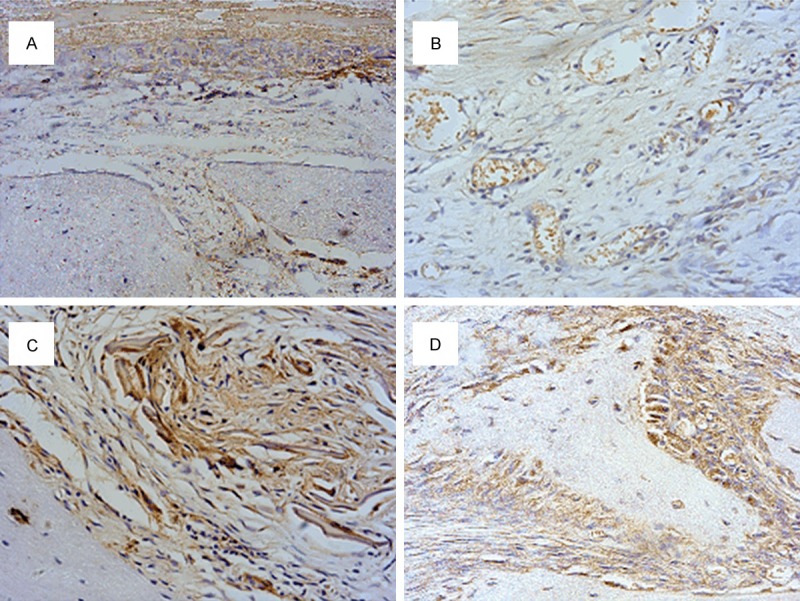
Collagen I antibody immunohistological staining of rat calvarial defects covered by SF nanofibrous membranes or Bio-Gide® membranes after 4 and 12 weeks. (A) SF nanofibrous membrane at 4 weeks postoperatively; (B) Bio-Gide membranes at 4 weeks postoperatively; (C) SF nanofibrous membranes at 12 weeks postoperatively; (D) Bio-Gide membranes at 12 weeks postoperatively (magnification, × 400). SF, silk fibroin.
Figure 8.
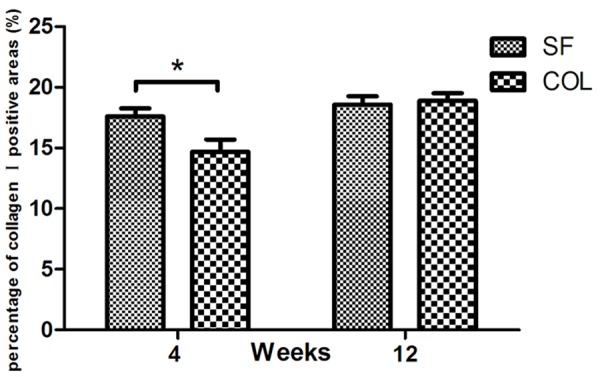
Percentage of collagen I positive areas at 4 and 12 weeks postoperatively. Data are the mean±SD (n=6, *P<0.05). SD, standard deviation.
The degradation of the two membranes was totally different. The Bio-Gide membranes showed clear resorptive sign at 4 weeks, and fully degraded at 12 weeks. In contrast, the SF nanofibrous membranes could maintain morphological integrity by the end of 12 weeks.
Discussion
SF is an attractive biomaterial and has been widely used as bone graft scaffolds [16] and used as membranes for GBR because of its desirable properties. These properties include good biocompatibility, low inflammation, and immunogenicity as well as slow rate of degradation and remarkable mechanical properties. Recently, electrospun nanofibers have attracted considerable interest in tissue regeneration including bone reconstruction [17]. SF nanofiber prepared by electrospinning supported mesenchymal stem cell attachment, proliferation, and deposition of extracellular matrix (ECM), and significantly improved new bone formation at the defect area, showing a potential application for GBR. However, electrospun SF nanofibers usually suffer from poor tensile strength, which limited its clinical application. The present study reported a novel strategy to fabricate SF nanofibrous membrane with significantly improved mechanical properties by preserving silk nanofibril structure during dissolving process [14,18], which could be an ideal GBR membrane for bone defect repair.
A successful biomaterial membrane for GBR used in bone defect repair should not only promote bone regeneration, but also alleviate infection and inflammation. In this study, the above novel electrospun SF nanofibrous membrane with higher strength and slower degradation was employed for GBR to repair rat calvarial defects. Rat calvarial defect model is often used to test bone-regenerating materials in vivo. A critical defect size of 5 mm in rat calvarial has been defined as the smallest bone defect that does not heal spontaneously in its lifetime [19]. In the present study, the CT and histology showed that the defects covered by the SF or Bio-Gide membranes demonstrated obvious new bone formation at 4 weeks, and almost completely healed the defect after 12 weeks. These results confirmed earlier studies that SF could be used as a GBR membrane in vivo [10-12,20,21]. Meanwhile, at first it was found that the BV value and collagen I positive areas in the SF group were greater than that in the Bio-Gide group at 4 weeks after surgery (P<0.05). It is indicated that the novel electrospun SF nanofibrous membrane may promote early osteogenesis and may have better GBR efficacy than collagen membranes at early stage after surgery.
In addition to the biomaterials used, the preparation technique is another important factor in affecting the properties and biocompatibility of membranes [22]. Usually, GBR membranes are prepared via dynamic filtration [23], particulate leaching, and film casting [24]. Recently, a new technique called electrospinning has become popular for producing nanofiber membranes [25,26]. In this study, the electrospun SF nanofibrous membrane (the diameter of the nanofibers ranged from 200 nm to 600 nm) was fabricated. Moreover, it had a porous structure, wide distribution of pore size, and a large surface area to volume ratio. Thus, the electrospun SF nanofibrous membrane can provide a biomimetic cellular environment by mimicking the dimensions of the ECM, which is important for cellular growth [27,28]. Kim et al. had reported that osteoblastic cells attached and proliferated well on the electrospun SF nanofibrous membrane, and presented more ALP activity than on culture plate [12]. Other studies have shown that SF could induce bone tissue growth when seeded with bone cells or stem cells [29,30]. Based on these earlier results, it was deduced that the novel electrospun SF nanofibrous membrane promoted bone formation at early stage likely due to cell adhesion and proliferation.
Furthermore, previous studies have demonstrated that the pore size of the electrospun membranes is smaller than the mean cell size, which can inhibit the invasion of fibroblast but allow efficient exchange of nutrients and growth factors [26,31]. This feature is able to satisfy the demand of GBR membranes excellently. Interestingly, another study indicated that increasing the porosity of the membrane may promote early osteogenesis, but may not increase the final amount of new bone [32]. This conclusion was consistent with the results of the present study, suggesting that porosity of the membrane is an essential factor for GBR in early healing stage but that the final BV obtained is almost the same.
The mechanical stability of GBR membranes is an essential factor for the clinical success of GBR therapy. Moreover, the extent and rate of degradation may influence the new bone formation by changing the mechanical stability of the membranes used [33]. In previous studies, SF had been fabricated into types of membranes by casting [10,11] or electrospinning [12,20] for GBR. However, they were fragile, easily dissolved, and had no data for comparing the mechanical stability between SF and collagen membranes. In this study, it was found that the tensile strength of electrospun SF nanofibrous membrane can be significantly improved by preserving native silk nanofibrils. Furthermore, the electrospun SF nanofibrous membrane showed significant higher tensile strength than Bio-Gide. In addition, in histological study, no distortion was observed in the SF nanofibrous membranes at 12 weeks. They could maintain morphological integrity, which provided the space for bone formation, and prevented invasion of soft tissue. However, Bio-Gide membranes showed resorptive sign at 4 weeks, and fully degraded at 12 weeks, which may decrease the space available for bone regeneration. The SF nanofibrous membranes prepared in this study show satisfactory mechanical stability, structural integrity as well as low immune reactivity, and thus have potential for clinical application of GBR.
Conclusion
A novel SF nanofibrous membrane was fabricated from native silk nanofibril solution by the electrospinning process. In rat calvarial defect model study, the SF nanofibrous membranes showed satisfactory mechanical stability, good biocompatibility, slow degradability, and improved new bone regeneration without any adverse inflammatory reactions. Considering the low cost and low risk of disease transmission, the SF nanofibrous membrane is a potential candidate for GBR therapy compared with the widely used collagen membranes.
Acknowledgements
The study was supported by National Natural Science Foundation of China (81200936), Science Foundation of The Health Department of Jiangsu Province (JZ201401), and Science and Technology Development Program of Suzhou (SYSD2014070).
Disclosure of conflict of interest
None.
References
- 1.Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672–676. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, Janowski GM. Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective. Dent Mater. 2012;28:703–721. doi: 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Becker W, Dahlin C, Lekholm U, Bergstrom C, van Steenberghe D, Higuchi K, Becker BE. Five-year evaluation of implants placed at extraction and with dehiscences and fenestration defects augmented with ePTFE membranes: results from a prospective multicenter study. Clin Implant Dent Relat Res. 1999;1:27–32. doi: 10.1111/j.1708-8208.1999.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 4.Gentile P, Chiono V, Tonda-Turo C, Ferreira AM, Ciardelli G. Polymeric membranes for guided bone regeneration. Biotechnol J. 2011;6:1187–1197. doi: 10.1002/biot.201100294. [DOI] [PubMed] [Google Scholar]
- 5.Dupoirieux L, Pourquier D, Picot MC, Neves M. Comparative study of three different membranes for guided bone regeneration of rat cranial defects. Int J Oral Maxillofac Surg. 2001;30:58–62. doi: 10.1054/ijom.2000.0011. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzmann M. Use of collagen membranes for guided bone regeneration: a review. Implant Dent. 2000;9:63–66. doi: 10.1097/00008505-200009010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Wray LS, Hu X, Gallego J, Georgakoudi I, Omenetto FG, Schmidt D, Kaplan DL. Effect of processing on silk-based biomaterials: reproducibility and biocompatibility. J Biomed Mater Res B Appl Biomater. 2011;99:89–101. doi: 10.1002/jbm.b.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65:457–470. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 10.Song JY, Kim SG, Lee JW, Chae WS, Kweon H, Jo YY, Lee KG, Lee YC, Choi JY, Kim JY. Accelerated healing with the use of a silk fibroin membrane for the guided bone regeneration technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e26–33. doi: 10.1016/j.tripleo.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Yang BE, Ahn JH, Park SO, Shim HW. Comparable efficacy of silk fibroin with the collagen membranes for guided bone regeneration in rat calvarial defects. J Adv Prosthodont. 2014;6:539–546. doi: 10.4047/jap.2014.6.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, Kim TI, Park YJ, Seol YJ, Lee YM, Ku Y, Rhyu IC, Han SB, Chung CP. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol. 2005;120:327–339. doi: 10.1016/j.jbiotec.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Zhang B, Shi L, Zhong J, Zhu J, Yan J, Wang P, Cao C, He D. Regenerated silk fibroin films with controllable nanostructure size and secondary structure for drug delivery. ACS Appl Mater Interfaces. 2014;6:21813–21821. doi: 10.1021/am502278b. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Lu Q, Ming J, Dou H, Liu Z, Zuo B, Qin M, Li F, Kaplan DL, Zhang X. Silk dissolution and regeneration at the nanofibril scale. Journal of Materials Chemistry B. 2014;2:3879. doi: 10.1039/c3tb21582b. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, You X, Dou H, Liu Z, Zuo B, Zhang X. Facile Fabrication of Robust Silk Nanofibril Films via Direct Dissolution of Silk in CaCl2-Formic Acid Solution. ACS Appl Mater Interfaces. 2015;7:3352–3361. doi: 10.1021/am508319h. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Kim UJ, Kim HS, Li C, Wada M, Leisk GG, Kaplan DL. Bone tissue engineering with premineralized silk scaffolds. Bone. 2008;42:1226–1234. doi: 10.1016/j.bone.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Zhang F, Ming J, Bie S, Li J, Zuo B. Preparation of electrospun silk fibroin nanofibers from solutions containing native silk fibrils. Journal of Applied Polymer Science. 2015:132. [Google Scholar]
- 19.Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Microcomputed tomographic and histomorphometric analyses of novel titanium mesh membranes for guided bone regeneration: a study in rat calvarial defects. Int J Oral Maxillofac Implants. 2014;29:826–835. doi: 10.11607/jomi.3219. [DOI] [PubMed] [Google Scholar]
- 20.Seok H, Kim MK, Kim SG, Kweon H. Comparison of silkworm-cocoon-derived silk membranes of two different thicknesses for guided bone regeneration. J Craniofac Surg. 2014;25:2066–2069. doi: 10.1097/SCS.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, Kim SG, Song JY, Kweon H, Jo YY, Lee KG, Kang SW, Yang BE. Silk fibroin and 4-hexylresorcinol incorporation membrane for guided bone regeneration. J Craniofac Surg. 2013;24:1927–1930. doi: 10.1097/SCS.0b013e3182a3050c. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro C, Sencadas V, Areias AC, Gama FM, Lanceros-Mendez S. Surface roughness dependent osteoblast and fibroblast response on poly(l-lactide) films and electrospun membranes. J Biomed Mater Res A. 2015;103:2260–2268. doi: 10.1002/jbm.a.35367. [DOI] [PubMed] [Google Scholar]
- 23.Teng SH, Lee EJ, Yoon BH, Shin DS, Kim HE, Oh JS. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J Biomed Mater Res A. 2009;88:569–580. doi: 10.1002/jbm.a.31897. [DOI] [PubMed] [Google Scholar]
- 24.Milella E, Barra G, Ramires PA, Leo G, Aversa P, Romito A. Poly(L-lactide)acid/alginate composite membranes for guided tissue regeneration. J Biomed Mater Res. 2001;57:248–257. doi: 10.1002/1097-4636(200111)57:2<248::aid-jbm1165>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005;26:4139–4147. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Both SK, Yang X, Walboomers XF, Jansen JA. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009;5:3295–3304. doi: 10.1016/j.actbio.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 28.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 29.Vetsch JR, Paulsen SJ, Muller R, Hofmann S. Effect of fetal bovine serum on mineralization in silk fibroin scaffolds. Acta Biomater. 2015;13:277–285. doi: 10.1016/j.actbio.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Gholipourmalekabadi M, Mozafari M, Bandehpour M, Salehi M, Sameni M, Caicedo HH, Mehdipour A, Hamidabadi HG, Samadikuchaksaraei A, Ghanbarian H. Optimization of nanofibrous silk fibroin scaffold as a delivery system for bone marrow adherent cells: in vitro and in vivo studies. Biotechnol Appl Biochem. 2014 doi: 10.1002/bab.1324. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 32.Zellin G, Linde A. Effects of different osteopromotive membrane porosities on experimental bone neogenesis in rats. Biomaterials. 1996;17:695–702. doi: 10.1016/0142-9612(96)86739-1. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009;10:1514–1524. doi: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]


